Abstract
Background
Activated leukocytes may contribute to the development and progression of heart failure (HF). We investigated the predictive value of circulating levels of stable and readily detectable markers reflecting both monocyte/macrophage and T-cell activity, on clinical outcomes in HF patients with reduced ejection fraction (HFrEF).
Methods
The association between baseline plasma levels of soluble CD163 (sCD163), macrophage migration inhibitory factor (MIF), granulysin, soluble interleukin-2 receptor (sIL-2R), and activated leukocyte cell adhesion molecule (ALCAM) and the primary endpoint of death from any cause or first hospitalization for worsening of HF was evaluated using multivariable Cox proportional hazard models in 1541 patients with systolic HF and mild to moderate anemia, enrolled in the Reduction of Events by darbepoetin alfa in Heart Failure (RED-HF) trial. Modifying effects and interaction with darbepoetin alfa treatment were also assessed.
Results
All leukocyte markers, except granulysin, were associated with the primary outcome and all-cause death in univariate analysis (all p < 0.01) and remained significantly associated in multivariable analysis adjusting for conventional clinical variables (e.g. age, gender, BMI, NYHA class, creatinine, LVEF, etiology) and CRP. However, after final adjustment for TnT and NT-proBNP no associations were found with outcomes. No interaction with darbepoetin alpha treatment was observed for any marker.
Conclusions
Leukocyte activation markers sCD163, MIF, sIL-2R, and ALCAM were associated with adverse outcome in patients with HFrEF, but add little as prognostic markers on top of established biochemical risk markers.
Clinical Trial Registration
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is a progressive disorder with an element of immune activation and a persistent low grade inflammation [1,2,3,4]. Activated monocytes/macrophages and T cells release a variety of inflammatory cytokines that have direct effects on cardiac cells, promoting hypertrophy, fibrosis, and apoptosis [3, 5, 6]. Cytokine secretion from monocytes/macrophages, T cells as well as other leukocyte subpopulations and myocardial cells initiate further leukocyte mobilization and recruitment to the myocardium, which creates a vicious circle, further promoting the development of HF [3].
Multiple circulating cytokines are increased in HF patients systemically but they frequently circulate at relatively low levels which limits their analytical performance and discriminatory properties beyond established clinical markers of HF [7]. Some markers reflecting general or more specific leukocyte activation, circulate at readily detectable levels, but have been scarcely evaluated in large HF populations, with a few exceptions. Circulating myeloperoxidase, an enzyme primarily secreted by activated granulocytes, gave no prognostic information in adjusted analysis in 1513 HF patients with reduced ejection fraction (HFrEF) [8]. In contrast, galectin 3, which may partly reflect macrophage activation, has received wide attention in predicting adverse outcomes and response to therapy in HF patients potentially reflecting its ability to reflect both extracellular matrix (ECM) and inflammation [9].
Anemia is frequently observed in HF and associated with poor prognosis [10,11,12]. Based on the established role of persistent leukocyte activation in anemia [13] and its emerging role in the progression of HF, we hypothesized that plasma levels of stable and readily detectable markers of leukocyte activation, reflecting both monocyte/macrophage (soluble [s] CD163 [14], macrophage migration inhibitory factor [MIF] [15]) and/or T-cell (soluble interleukin 2 receptor [sIL-2R] [16], activated leukocyte cell adhesion molecule [ALCAM] [17], granulysin [18]) activity could represent clinically relevant inflammatory biomarkers in HF patients. We therefore evaluated if they could predict clinical outcomes in 1588 anemic patients with HFrEF in the Reduction of Events by darbepoetin alfa in Heart Failure (RED-HF) trial [19]. In general, the intervention with darbepoetin alfa did not show any effects on the primary end-point, but a secondary aim in this study was to evaluate if these markers could identify subgroups of patients who may still benefit from darbepoetin alfa treatment.
Materials and methods
Patients and study procedures
The study design and baseline characteristics of the RED-HF trial have been reported in detail previously [20, 21]. Patients were eligible for the study if they had the New York Heart Association (NYHA) functional class II–IV; systolic HF with left ventricular ejection fraction (LVEF) ≤ 40%; a hemoglobin level of 9.0–12.0 g per dL and were receiving guideline-recommended HF therapy. Major exclusion criteria were a transferrin saturation of less than 15%, evidence of bleeding or other correctable causes of anemia, a serum creatinine level of more than 3 mg per dL (265 µmol per L), and a blood pressure of more than 160/100 mm Hg. Patients were randomly assigned in a 1:1 ratio to receive either darbepoetin alfa or placebo. The study drug was administered subcutaneously, with doses adjusted according to hemoglobin level, which was measured in a blinded fashion. Patients in the darbepoetin alfa group received a starting dose of 0.75 µg per kg of body weight once every 2 weeks until a hemoglobin level of 13.0 g per dL was reached on two consecutive visits. Thereafter, patients received monthly injections, according to an algorithm [20] that was designed to maintain a hemoglobin level of 13.0 g per dL (but not exceeding 14.5 g per dL). Patients in the placebo group received dose adjustments to mimic those in the darbepoetin alfa group. The trial complied with the Declaration of Helsinki and was approved by the Ethics Committees of the participating hospitals. All patients provided a written informed consent.
Study outcomes and definitions
The primary predefined outcome was a composite of death from any cause or first hospitalization for worsening of HF. The prespecified adjudicated secondary outcomes were (1) composite of death from cardiovascular (CV) causes or first hospitalization for worsening of HF, (2) death from any cause, (3) CV death. Details on the definition and adjudication of all outcomes have been described previously [19].
Blood sampling and biochemical analysis
All blood samples were non-fasting and all reported measurements, except for markers of leukocyte activation, were performed at a central laboratory, including N-terminal pro-brain natriuretic peptide (NT-proBNP) and high sensitivity assays for C-reactive protein (hsCRP) and troponin T (hsTnT) (Medical Research Laboratories, Zaventem, Belgium). Blood samples for the measurement of the cytokines were collected in pyrogen-free EDTA tubes; centrifuged and isolated plasma was stored at − 80 °C until analyses. All samples were thawed an equal number of times (< 3 times) and were on the bench < 2 h when preparing analysis. Circulating sCD163, MIF, granulysin, sIL-2R, and ALCAM were analyzed by enzyme immunoassays from R&D Systems (Minneapolis, MN, USA) with intra- and inter-assay coefficients of variation < 10%. The effect of postprandial and diurnal variation, time-delay on bench and in fridge and freeze–thaw cycles is presented in Supplemental Table 1, while marker levels according to inclusion year and time of day are presented in Supplemental Fig. 1.
Statistical analysis
Trends across tertiles of the leukocyte markers were tested using Kruskal–Wallis H test, one-way ANOVA or Chi square depending on distribution and variable type (i.e. categorical or continuous). For comparing treatment effects on the markers, the Mann–Whitney U test was used on change values, while Wilcoxon matched pairs test was used to assess longitudinal changes within groups. Stepwise linear regression was used to identify the most important predictors of the leukocyte markers. Kaplan–Meier curves were constructed to visualize and evaluate (log-rank test) differences in survival. A restricted cubic spline analysis with three knots was undertaken on the primary outcome to assess linearity of risk. Survival analyses were performed using the Cox proportional hazard regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the leukocyte activation markers as a log-transformed continuous variables at baseline, which included mainly clinical variables at step one (age, gender, NYHA class, hospitalization for HF within 6 months, log serum creatinine, LVEF, etiology, body mass index (BMI), left bundle-branch block, history of atrial fibrillation or flutter, systolic blood pressure). At step two, log-transformed serum concentrations of hsCRP were included and finally, NT-proBNP and hsTnT were included at step three. A two-sided p value < 0.05 was considered to be significant. All statistical analyses were performed with the use of SAS software, version 9.2.
Results
Stability of T cell and monocyte/macrophage activation markers
As shown in Supplemental Fig. 1, there was no association between time (i.e. year) or time of isolation during the day and marker level, except sCD163 was higher at midday. The distribution of marker level was similar at all time-points assessed. As shown in Supplemental Fig. 1, no effect of fasting on biomarker levels was observed although there did appear to be some fluctuation over the day with increased levels of sCD163, Granulysin, ALCAM at noon (non-fasting). As also shown in Supplemental Table 1, time delay experiments, achieved by leaving samples on the bench or in the fridge for up to 24 h, revealed no large major loss over time, although sCD163 and ALCAM tended to decline after 4 h on bench and in the fridge and all markers was lower after 24 h in the fridge. Finally, the effect of freeze thaw cycles had minor effects on marker levels.
Plasma levels of T cell and monocyte/macrophage activation markers in the RED-HF cohort
Of the 2278 patients enrolled in the RED-HF study, samples for measurement of the T cell and monocyte/macrophage activation markers were available for 1582 (69%). The median plasma level of sCD163 at baseline in the overall population was 0.64 µg/mL (IQR 0.41–1.00 µg/mL); MIF 1.7 (1.1–2.5 ng/mL); granulysin 1.5 ng/mL (1.1–2.0 ng/mL); sIL-2R 277 pg/mL (189–409 pg/mL); ALCAM (175 ng/mL) 136–661 ng/mL. Clinical characteristics of the study population are given in Table 1. Clinical characteristics according to the tertiles of leukocyte markers are shown in Supplementary Table 2. Several demographic and clinical variables were associated with most of these markers, such as race, kidney function, hsCRP, hsTnT and NT-proBNP. None or few associations were found with NYHA class, LVEF and BMI. Of relevance to this patient population that also had anemia, high sIL-2R and ALCAM levels were associated with low hemoglobin levels, and high sIL-2R and sCD163 also correlated with low transferrin saturation.
Supplemental Table 3 shows predictors of levels of the monocyte/macrophage/T cell markers identified by stepwise linear regression. The four strongest significant predictors of the markers were, for sCD163: race, hsTnT, platelet count and hsCRP; for MIF: heart rate, hsTnT and age; for granulysin: eGFR, race, gender and previous MI; for sIL-2R: creatinine, NT-proBNP, hsCRP and race; for ALCAM: creatinine, race, hsTnT and sex.
Association between baseline levels of T cell and monocyte/macrophage activation markers and outcome in RED-HF trial
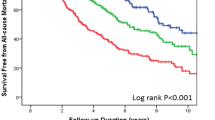
During a mean follow-up of 2.3 years (range 0.0–6.0 years), 798 patients reached the primary end point, 716 patients reached the composite of death from cardiovascular (CV) causes or first hospitalization for worsening of HF, while 649 patients died and of these, 543 due to CV causes. Kaplan–Meier estimates for the primary endpoint according to baseline tertiles of the measured leukocyte markers are presented in Fig. 1. A moderate association with the primary outcome was observed for all markers, with a more defined tertile effect for sIL-2R, than for the other proteins. Also, for granulysin, the lowest outcome was observed in tertile 2, suggesting a non-linear U-shaped association with outcome, which was confirmed by a restricted cubic spline analysis (Supplementary Fig. 2). For the other markers, the cubic spline analysis against the primary endpoint revealed a linear increase in the first and second tertile, followed by flattening of the curve around tertile 3 (Supplementary Fig. 2).
When investigating the associations between log-transformed values of the inflammatory markers and outcome, univariate Cox proportional hazard models including the markers separately showed that all of them except granulysin were significantly associated with the primary outcome (Fig. 2a), with the strongest association for sIL-2R. These markers remained significantly associated with the primary outcome and all-cause death in multivariable analysis, adjusting for a wide range of conventional clinical variables (i.e. age, gender, BMI, NYHA class, HF hospitalization last 6 months, creatinine, LVEF, etiology, left bundle-branch block, history of atrial fibrillation or flutter, systolic blood pressure (BP) and study drug) (Step 1). sCD163, MIF, and sIL-2R were only marginally affected and still significantly associated with both outcomes after further adjustment for hsCRP (Step 2). ALCAM remained significantly associated with all-cause death, but not with the primary outcome. However, associations with outcomes were markedly attenuated after the last adjustment for hsTnT and NT-proBNP showing no significant associations with outcome (Step 3). Similar, but less prominent trends were seen for the secondary endpoints of CV death or first hospitalization for worsening of HF and CV death, with the strongest association for MIF and sIL-2R (Table 2).
Association between baseline levels of leukocyte markers and a primary outcome or b all-cause death in the RED-HF study. Hazard ratios (HRs) and 95% confidence interval (CI) per SD change with p values are shown for univariate (UNI) analysis, when adjusted for clinical and biochemical variables (S1), further adjusted for hsCRP (S2) and lastly for hsTnT and NT-proBNP (S3)
As shown in Supplemental Table 2, there was a tendency for higher frequency of patients with high levels of leukocyte markers in patients with diabetes, significant for sCD163 and sIL-2R, while granulysin was lower. Based on the high frequency of diabetes in RED-HF we evaluated risk of adverse outcome in patients with (HbA1c mean ± SD: 7.39 ± 1.61%) and without (5.75 ± 0.64%) diabetes separately, restricting analysis to fully adjusted models. As shown in Supplementary Table 4, only sCD163 displayed a trend of increased risk according to diabetes status with a higher risk in non-diabetic patients which was significant for all-cause death.
Effect of darbepoetin alfa on markers of T cell and monocyte/macrophage activation
Table 3 shows levels of the leukocyte markers at baseline and after 6 months treatment with darbepoetin alfa or placebo. There were no significant differences in the levels of the measured inflammatory markers between placebo and darbepoetin alfa groups at baseline. MIF increased after treatment with darbepoetin alfa giving significantly higher levels at 6 months and a significant difference in change between the treatment groups, while sIL-2R decreased. Leukocyte markers did not change in placebo group. No association between change in any of the markers (i.e. decrease: < − 15%, no change: 15–15%, increase: > 15% or evaluated as tertile change) and any outcome was observed (p values between 0.08 and 0.92). No interaction between baseline levels or change of any of the markers and darbepoetin alpha on any outcome was observed (p values for interaction 0.07–0.99).
Discussion
In 1582 patients with HFrEF and mild to moderate anemia, circulating markers reflecting monocyte/macrophage and T cell activation were associated with adverse outcomes following adjustment for a wide range conventional risk variables, including hsCRP, supporting a role for leukocyte activation in the progression of HF. However, the associations were markedly attenuated after further adjustment for hsTnT and NT-proBNP, limiting their clinical usefulness as biomarkers in HF. Darbepoetin alpha treatment increased MIF levels, but no interaction with baseline levels of any of the leukocyte markers and treatment on outcomes was observed.
The systemic immune activation in HF as reflected by high levels of various inflammatory cytokines in plasma or serum could at least partly reflect systemic activation of various leukocyte subpopulations such as T cell and monocyte/macrophages. This activation of leukocyte subpopulations could again be mirrored by soluble markers such as those investigated in this study. Our evaluation of these markers with regard to long-term stability (i.e. association between marker level and inclusion year) and bench stability, suggests they are robust markers with adequate pre-analytical characteristics. In addition to systemic inflammation, however, these markers could also reflect myocardial inflammation. Indeed, linear regression identified hsTnT as a strong predictor for most leukocyte markers. Since leukocyte activation and migration from the circulation to areas of myocardial inflammation may contribute to the tissue injury in chronic HF [3, 5, 6], circulating levels of these markers could therefore also reflect ongoing myocardial inflammation. Recently, Ptaszynska-Kopczynska found higher serum levels of sCD163 in 79 patients with HFrEF [22]. As for sIL-2R, we and others have reported increased circulating sIL-2R levels in HF [23, 24], associated with a more aggressive clinical course [24]. Apart from these smaller studies, we could find no reports on our selected markers as predictors of adverse outcomes in large HF populations. However, we have previously shown in the present RED-HF population that although CRP is associated with poor prognosis following adjustment for conventional risk factors (i.e. step 1 in our adjustment strategy), it does not add information on top of NT-proBNP and TnT. Thus, our finding that high plasma levels of sCD163, MIF, sIL-2R and ALCAM were associated with the primary endpoint and all-cause mortality after adjustment for conventional risk factors and only modestly affected by adjustment for hsCRP supports that T cell and monocyte/macrophage activation markers may reflect ongoing myocardial inflammation and could be of importance in the progression of HF. However, the strong association between all leukocyte markers and NT-proBNP and hsTnT in RED-HF could contribute to the strong attenuation of risk in multivariable analysis when these established risk markers were included, limiting the clinical usefulness of soluble markers of T cell and monocyte/macrophage activation.
Patients with HF and diabetes have particularly poor outcome and increased inflammatory burden [12, 25] as also observed for sCD163 and sIL-2R in diabetes patients in the present population. However, we found no evidence of increased risk in diabetic patients. In contrast, in patients without diabetes, high sCD163 levels were associated with enhanced risk of all-cause mortality, also in adjusted analysis. Consequently, enhanced immune activation, as reflected by the leukocyte markers assessed in the present study, does not seem to confer additional risk of adverse outcomes in the presence of diabetes, at least in HF patients with anemia. Similarly, sIL-2R and ALCAM were also associated with ischemic etiology and sIL-2R with stroke and MI the last 6 months, which also carries an ischemic component. However, all markers were more strongly correlated with both NT-proBNP and TnT levels suggesting that myocardial inflammation per se is more impactful than that of ischemic etiology in patients with anemia and advanced chronic HF. Possibly, the burden of anemia masks relevant associations and our results cannot be generalized to HF patients without anemia or other inflammatory markers.
The cause of anemia in patients with HF may be related to an absolute or relative deficiency of, or resistance to, erythropoietin (EPO), to which inflammation may contribute through bone marrow suppression [26, 27]. EPO administration has been shown to both reduce and augment inflammatory responses in circulating leukocytes [28,29,30]. Interestingly, darbepoetin alpha treatment was associated with an increase in MIF, which helps to sustain macrophage activation and is an important mediator of anemic complications and bone marrow suppression during malaria infection [31, 32], and could therefore also contribute to resistance to EPO. Furthermore, in patients with chronic kidney disease, non-responders to EPO treatment are characterized by enhanced activation of both monocytes [33] and T cells [34]. Thus, although the RED-HF trial failed to detect a benefit of an erythropoiesis stimulating agent on clinical outcomes, leukocyte activation markers could be potentially identify patients that could have advantage of EPO treatment. However, no interaction between darbepoetin alpha treatment and baseline or change in levels during follow-up of any of the leukocyte markers on outcome was observed.
The strengths of the present investigation include the large number of patients studied with a high event rate and adjustment for multiple relevant confounders. On the other hand, a randomized trial may not necessarily reflect the “real-world” HF population and the use of composite endpoints has an inborn limitation. In addition, the trial populations had mild to moderate anemia, a high frequency of renal dysfunction and all markers were strongly influenced by race. The baseline association between circulating markers and outcome may therefore not apply to all HF patients. Finally, our interpretations are limited to our choice of markers, which represent less studied markers in the context of HF. Other markers of monocyte/macrophage activation such as neopterin and soluble CD14 have been associated with HF progression following ACS [35] and all-cause mortality in elderly [36] are possible candidates.
In conclusion, although our data support activation of monocytes/macrophages and T cells in the progression of HF, the lack of independence from hsTnT and NT-proBNP limits their clinical usefulness as prognostic markers in this population. However, our findings do not rule out that other leukocyte activation markers may prove useful.
References
Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11(5):255–265. https://doi.org/10.1038/nrcardio.2014.28
Vaduganathan M, Greene SJ, Butler J, Sabbah HN, Shantsila E, Lip GY, Gheorghiade M (2013) The immunological axis in heart failure: importance of the leukocyte differential. Heart Fail Rev 18(6):835–845. https://doi.org/10.1007/s10741-012-9352-9
Wrigley BJ, Lip GY, Shantsila E (2011) The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 13(11):1161–1171. https://doi.org/10.1093/eurjhf/hfr122
Radenovic S, Loncar G, Busjahn A, Apostolovic S, Zdravkovic M, Karlicic V, Veskovic J, Tahirovic E, Butler J, Dungen HD (2018) Systemic inflammation and functional capacity in elderly heart failure patients. Clin Res Cardiol 107(4):362–367. https://doi.org/10.1007/s00392-017-1195-x
Hofmann U, Frantz S (2015) Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 116(2):354–367. https://doi.org/10.1161/circresaha.116.304072
Swirski FK, Nahrendorf M (2013) Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339(6116):161–166. https://doi.org/10.1126/science.1230719
Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET (2014) Inflammatory cytokines as biomarkers in heart failure. Clin Chimica Acta Int J Clin Chem. https://doi.org/10.1016/j.cca.2014.09.001
Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, Goldberg LR, Jessup M, Cappola TP (2012) Multiple biomarkers for risk prediction in chronic heart failure. Circulation Heart Fail 5(2):183–190. https://doi.org/10.1161/CIRCHEARTFAILURE.111.965020
Chen YS, Gi WT, Liao TY, Lee MT, Lee SH, Hsu WT, Chang SS, Lee CC (2016) Using the galectin-3 test to predict mortality in heart failure patients: a systematic review and meta-analysis. Biomarkers Med 10(3):329–342. https://doi.org/10.2217/bmm.15.121
Gil VM, Ferreira JS (2014) Anemia and iron deficiency in heart failure. Rev Port Cardiol 33(1):39–44. https://doi.org/10.1016/j.repc.2013.06.003
von Haehling S, Gremmler U, Krumm M, Mibach F, Schon N, Taggeselle J, Dahm JB, Angermann CE (2017) Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP registry. Clin Res Cardiol 106(6):436–443. https://doi.org/10.1007/s00392-016-1073-y
Riedel O, Ohlmeier C, Enders D, Elsasser A, Vizcaya D, Michel A, Eberhard S, Schlothauer N, Berg J, Garbe E (2018) The contribution of comorbidities to mortality in hospitalized patients with heart failure. Clin Res Cardiol 107(6):487–497. https://doi.org/10.1007/s00392-018-1210-x
Nairz M, Theurl I, Wolf D, Weiss G (2016) Iron deficiency or anemia of inflammation? Differential diagnosis and mechanisms of anemia of inflammation. Wiener Medizinische Wochenschrift 166(13–14):411–423. https://doi.org/10.1007/s10354-016-0505-7
Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM (2002) Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol 72(4):711–717
Calandra T, Bernhagen J, Mitchell RA, Bucala R (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 179(6):1895–1902
Rubin LA, Kurman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, Nelson DL (1985) Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol 135(5):3172–3177
Bowen MA, Patel DD, Li X, Modrell B, Malacko AR, Wang WC, Marquardt H, Neubauer M, Pesando JM, Francke U et al (1995) Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med 181(6):2213–2220
Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, Clayberger C, Oppenheim JJ (2010) Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood 116(18):3465–3474. https://doi.org/10.1182/blood-2010-03-273953
Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ, Committees R-H, Investigators R-H (2013) Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 368(13):1210–1219. https://doi.org/10.1056/NEJMoa1214865
McMurray JJ, Anand IS, Diaz R, Maggioni AP, O’Connor C, Pfeffer MA, Polu KR, Solomon SD, Sun Y, Swedberg K, Tendera M, van Veldhuisen DJ, Wasserman SM, Young JB (2009) Design of the reduction of events with darbepoetin alfa in heart failure (RED-HF): a phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail 11(8):795–801. https://doi.org/10.1093/eurjhf/hfp098
McMurray JJ, Anand IS, Diaz R, Maggioni AP, O’Connor C, Pfeffer MA, Solomon SD, Tendera M, van Veldhuisen DJ, Albizem M, Cheng S, Scarlata D, Swedberg K, Young JB, Investigators R-HC (2013) Baseline characteristics of patients in the reduction of events with darbepoetin alfa in heart failure trial (RED-HF). Eur J Heart Fail 15(3):334–341. https://doi.org/10.1093/eurjhf/hfs204
Ptaszynska-Kopczynska K, Marcinkiewicz-Siemion M, Lisowska A, Waszkiewicz E, Witkowski M, Jasiewicz M, Miklasz P, Jakim P, Galar B, Musial WJ, Kaminski KA (2016) Alterations of soluble TWEAK and CD163 concentrations in patients with chronic heart failure. Cytokine 80:7–12. https://doi.org/10.1016/j.cyto.2016.02.005
Gullestad L, Ueland T, Brunsvig A, Kjekshus J, Simonsen S, Froland SS, Aukrust P (2001) Effect of metoprolol on cytokine levels in chronic heart failure—a substudy in the metoprolol controlled-release randomised intervention trial in heart failure (MERIT-HF). Am Heart J 141(3):418–421. https://doi.org/10.1067/mhj.2001.112785
Limas CJ, Hasikidis C, Iakovou J, Kroupis C, Haidaroglou A, Cokkinos DV (2003) Prognostic significance of soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Eur J Clin Invest 33(6):443–448
Suthahar N, Meijers WC, Brouwers FP, Heerspink HJL, Gansevoort RT, van der Harst P, Bakker SJL, de Boer RA (2018) Heart failure and inflammation-related biomarkers as predictors of new-onset diabetes in the general population. Int J Cardiol 250:188–194. https://doi.org/10.1016/j.ijcard.2017.10.035
Anand IS (2008) Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol 52(7):501–511. https://doi.org/10.1016/j.jacc.2008.04.044
O’Meara E, Murphy C, McMurray JJ (2004) Anemia and heart failure. Curr Heart Fail Rep 1(4):176–182
Kristal B, Shurtz-Swirski R, Shasha SM, Manaster J, Shapiro G, Furmanov M, Hassan K, Weissman I, Sela S (1999) Interaction between erythropoietin and peripheral polymorphonuclear leukocytes in hemodialysis patients. Nephron 81(4):406–413. https://doi.org/10.1159/000045324
Sela S, Shurtz-Swirski R, Sharon R, Manaster J, Chezar J, Shkolnik G, Shapiro G, Shasha SM, Merchav S, Kristal B (2001) The polymorphonuclear leukocyte—a new target for erythropoietin. Nephron 88(3):205–210. https://doi.org/10.1159/000045991
Pesce M, Felaco P, Franceschelli S, Speranza L, Grilli A, De Lutiis MA, Ferrone A, Sirolli V, Bonomini M, Felaco M, Patruno A (2014) Effect of erythropoietin on primed leucocyte expression profile. Open Biol 4(6):140026. https://doi.org/10.1098/rsob.140026
Martiney JA, Sherry B, Metz CN, Espinoza M, Ferrer AS, Calandra T, Broxmeyer HE, Bucala R (2000) Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect Immun 68(4):2259–2267
McDevitt MA, Xie J, Ganapathy-Kanniappan S, Griffith J, Liu A, McDonald C, Thuma P, Gordeuk VR, Metz CN, Mitchell R, Keefer J, David J, Leng L, Bucala R (2006) A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med 203(5):1185–1196. https://doi.org/10.1084/jem.20052398
Pereira R, Costa E, Goncalves M, Miranda V, do Sameiro Faria M, Quintanilha A, Belo L, Lima M, Santos-Silva A (2010) Neutrophil and monocyte activation in chronic kidney disease patients under hemodialysis and its relationship with resistance to recombinant human erythropoietin and to the hemodialysis procedure. Hemodial Int Symp Home Hemodial 14(3):295–301. https://doi.org/10.1111/j.1542-4758.2010.00450.x
Cooper AC, Breen CP, Vyas B, Ochola J, Kemeny DM, Macdougall IC (2003) Poor response to recombinant erythropoietin is associated with loss of T-lymphocyte CD28 expression and altered interleukin-10 production. Nephrol Dialysis Transplant 18(1):133–140
Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, Braunwald E (2007) Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation 115(24):3071–3078. https://doi.org/10.1161/CIRCULATIONAHA.106.666511
Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP (2013) Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arteriosclerosis Thromb Vascular Biol 33 (1):158–164. https://doi.org/10.1161/ATVBAHA.112.300421
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Inder Anand, John J. V. Mcmurray, Dirk J. van Veldhuisen and James Young are members of the RED-HF Executive Committee—no payments in the last 12 months. John J. V. Mcmurray has received travel and accommodation costs paid by Cytokinetics/Amgen in relation to advisory board and clinical trial meetings about omecamtiv mecarbil.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abraityte, A., Aukrust, P., Kou, L. et al. T cell and monocyte/macrophage activation markers associate with adverse outcome, but give limited prognostic value in anemic patients with heart failure: results from RED-HF. Clin Res Cardiol 108, 133–141 (2019). https://doi.org/10.1007/s00392-018-1331-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1331-2