Abstract
Objective
This study aimed to investigate the effect exerted by teriparatide on the repair of femoral metaphyseal defect in ovariectomized rats.
Method
Female Sprague–Dawley rats were ovariectomized and after 3 months a critically sized defect of 3 mm in diameter—a through-hole bone defect—was drilled into each distal femur of the ovariectomized rats. The rats were injected with teriparatide (30 μg/kg) parathyroid hormone (PTH) in the peritoneum three times per week. After 4 and 8 weeks the animals were killed and the blood and bilateral femora were harvested for biochemical analysis, histopathological observation, and micro-computed tomography (CT) examination.
Results
The PTH group and control group were compared 4 and 8 weeks after surgery. PTH increased bone formation in the defect area. Moreover, PTH showed the strongest effects on bone volume per total volume, trabecular number, trabecular thickness, trabecular separation, and total fluorescence-marked new bone area. Additionally, the PTH treatment group showed inhibited serum concentrations of C-terminal telopeptide of type I collagen and enhanced expression of calcium, phosphorus, and bone alkaline phosphatase.
Conclusion
Our findings suggest a positive effect of PTH on defect healing in ovariectomized rats.
Zusammenfassung
Ziel
Ziel war die Untersuchung der Wirkung von Teriparatid auf die Heilung bei einem Femurmetaphysendefekt ovarektomierter Ratten.
Methode
Bei seit 3 Monaten ovarektomierten weiblichen Sprague-Dawley(SD)-Ratten wurde ein Defekt kritischer Größe von 3 mm Durchmesser mit einer Durchgangsbohrung in jedem distalen Femur der ovarektomierten Ratten erzeugt. Den Ratten wurde 3-mal pro Woche PTH-Teriparatid (30 μg/kg) in das Peritoneum injiziert. Nach 4 bzw. 8 Wochen wurden die Tiere getötet, Blut und die Femora beidseits wurden für biochemische und histopathologische Untersuchungen sowie für eine Mikro-CT-Untersuchung entnommen.
Ergebnisse
Die Parathormon(PTH)-Gruppe und die Kontrollgruppe wurden 4 bzw. 8 Wochen nach der Operation verglichen. Durch PTH konnte die Knochenbildung im Bereich des Defekts gesteigert werden; dabei wies PTH die stärksten Wirkungen auf das Knochenvolumen pro Gesamtvolumen („bone volume per total volume“, BV/TV), die Trabekelanzahl, Trabekeldicke, Trabekelseparation sowie den gesamten fluoreszenzmarkierten Bereich der Knochenneubildung auf. Außerdem waren eine Hemmung der Expression von CTX (C-terminales Telopeptid vom Typ-I-Kollagen) sowie eine Verstärkung der Expression von Kalzium (Ca2+), Phosphat (P) und alkalischer Knochenphosphatase („bone alkaline phosphatase“, B-ALP) in der PTH-Therapie-Gruppe festzustellen.
Schlussfolgerung
Den Befunden der vorliegenden Studie zufolge ist von einem positiven Effekt von PTH auf die Defektheilung bei ovarektomierten (OVX-)Ratten auszugehen.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an emerging health-care issue and socioeconomic threat worldwide because of the risk of skeletal fragility and susceptibility to fractures resulting from the reduction in bone mass, poor bone strength, and microarchitectural deterioration in the trabecular and cortical skeleton. Common osteoporotic fractures occur in the hip and spine, which are associated with an increasing mortality rate of 10–20 % [1, 2]. Owing to postmenopausal estrogen deficiency, the balance between osteoblastic bone formation and osteoclastic bone resorption is broken [3, 4]. To date, clinical and experimental studies have consistently demonstrated that bone healing in postmenopausal osteoporotic women and estrogen depletion-induced osteoporotic animals is remarkably delayed or impaired [5–7]. Although much emphasis has been placed on the treatment of osteoporosis and on defect prevention by using biomaterials, little research has been conducted on the therapeutic effect of drugs during osteoporotic defect regeneration.
An exception to this is teriparatide. Teriparatide, which is a recombinant form of parathyroid hormone 1–34 (rh PTH 1–34, PTH), is the only FDA-approved medicine for curing osteoporosis and stimulating new bone formation. PTH can precipitate bone formation; it improves the quantity, quality, and density of bone by increasing the volume of trabecular bone in the hip [8]. Moreover, it helps to increase the quantity and density of centrum, to enhance bone strength, and to fortify the quantity of the femur shaft, the cortical thickness, and the maximum compressive load thereby notably reducing the chance of bone fracture for patients suffering from osteoporosis [9–11]. PTH has been shown in studies to enhance: (a) structural allograft healing by anabolic effects on new bone formation via small-vessel angiogenesis; (b) inhibition of angiopoietin-2-mediated arteriogenesis; and (c) enhancement of osteogenic differentiation via bone morphogenetic protein signaling [12].
The purpose of this study was to observe the effects of teriparatide on the healing of bone defects in ovariectomized (OVX) rats.
Materials and methods
Experimental animals
Forty female Sprague–Dawley rats, aged 3 months and with a body weight of 230 ± 26 g, were included in this study. Animals were kept in cages (four animals per cage) under climate-controlled conditions (25 °C, 55 % humidity, alternating cycle of 12 h light/12 h dark). Free access to tap water and a standard laboratory diet containing 1.56 % calcium (Ca2+), 0.8 % phosphorus (P), and 800 IU/kg vitamin D was permitted. All the animal experiments were conducted in accordance with international standards on animal welfare as well as being compliant with the Animal Research Committee of the university.
Surgery and treatment
A bilateral ovariectomy (n = 35) or sham operation (n = 5) was performed on the animals according to a previous report; 12 weeks later fracture surgery was carried out for the establishment of standard osteoporotic animal models [13, 14]. Five randomly selected OVX rats and five sham-operated rats were then euthanized, and the distal femoral condyles were harvested for bone mineral density (BMD) evaluation to confirm the establishment of the osteoporotic animal model. Subsequently, the rats were divided into two groups: the control group and the PTH group. Afterward, femoral cylindrical defects were created, which were standardized at 3 mm in diameter and 5 mm in length, penetrated internally and externally, and lay above the distal epiphyseal growth plate, as previously described [13, 14]. After the bone defect operation, all animals from the treatment group were injected with PTH (1–34; 30 μg/kg, three times a week) from the first postoperative day. The dose of PTH was determined according to previous experiments in rats [13, 15]. After each operation, all rats received a subcutaneous injection of buprenorphine (0.06 mg/kg, twice a day) as analgesic treatment for three postoperative days.
Fluorochrome labeling and specimen collection
In order to obtain the dynamic parameters of callus formation and remodeling, all animals were labeled fluorescently with calcein green subcutaneously (Sigma–Aldrich Inc., 20 mg/kg) 13 and 23 days before sacrifice. All fluorescent agents were prepared immediately before injection and filtered through a 0.45-μm filter. At the end of the observation time (4 or 8 weeks after defect), animals were euthanized by cardiac puncture under general anesthesia in the early morning. The blood was collected, and serum was stored at − 80 °C until use for biochemical assays. After complete excision of soft tissues, the bilateral femora were collected. The left femora were frozen at − 80 °C and wrapped in gauze soaked in isotonic saline until micro-computed tomography (CT) examination.
Micro-CT examination
A micro-CT imaging system (Micro-CT 50, Scanco Medical, Bassersdorf, Switzerland) was used to evaluate the osteogenesis within the defect region. A consistent volume of interest (VOI), located in the central 2.5-mm-diameter region of the 3-mm-diameter defect was defined to evaluate the level of bone regeneration. The mineralized bone tissue was differentially segmented with a fixed low threshold (value = 184), and the bone volume fractions (BV/TV), trabecular number (Tb-N), trabecular thickness (Tb-Th), trabecular separation (Tb-Sp), and BMD of new bone in the VOI were automatically collected and analyzed using the built-in software of the micro-CT according to previous reports [13, 14].
Histological and fluorescent analyses
Following micro-CT scanning, femoral samples were decalcified in 10 % EDTA and embedded in paraffin with the long axis parallel to the base plane. Serial sections of 5 μm were cut and mounted on poly-l-lysine-coated slides and then subjected to H&E staining to be used for undecalcified histological sections. After fixation in 70 % alcohol for 3 days, specimens were dehydrated in graded ethanol (80–100 %) and embedded in methyl methacrylate (Technovit 7200 VCL; Exact Apparatbau, Nordenstedt, Germany) without decalcification. Following this, 10-μm-thick sections were prepared along the internal portions of the bone defect using a diamond saw (Leica SP1600, Germany). The 10-μm-thick slices were kept unstained for fluorescent observation under a laser confocal scanning microscope (LCSM; Zeiss LSM 510, Germany). The fluorescence-marked callus area and relative mineral apposition rate (MAR) were analyzed using ZEN 2009 Light Edition software (Zeiss, Germany). The relative MAR was calculated as the relative ratio of calcein green-marked callus areas.
Serum analysis
The serum concentrations of Ca2+ and P were determined with a Hitachi 7080 biochemical automatic analyzer (Hitachi, Tokyo, Japan), and the serum concentrations of bone alkaline phosphatase (B-ALP) and C-terminal telopeptide of type I collagen (CTX) were measured using rat enzyme-linked immunoassay kits. All the samples were run in the same assay unless an individual value needed to be obtained again.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using the statistics package SPSS 19.0 (SPSS, Chicago, Ill.). Multiple comparisons between groups were carried out using one-way ANOVA and Tukeyʼs post hoc test. The significance level was set at 0.05 for all analyses.
Results
Animals
Three rats were excluded from analysis owing to anesthetic accident, infection, or inaccurate defect site. Thus, there were 27 animals left for evaluation in the two groups at each observation time, two rats belonging to the PTH group and one belonging to the control group. The BMD of the distal femurs was measured by dual-energy X-ray absorptiometry (Lunar Prodigy Advance, GE Lunar, Madison, Wis.). The BMD of the distal femurs from the sham-treated and OVX groups was 232.47 ± 31.14 (mg/cm2) and 169.65 ± 27.65 (mg/cm2), respectively. In quantitative analysis, the BMD of the distal femurs from sham-operated rats was 37.5 % higher than from OVX animals (p < 0.05). These results indicate the successful establishment of osteoporosis in the OVX rats.
Body weight
The body weight of rats in each group before surgery is shown in Fig. 1a. There was no statistically significant difference in the body weights of rats from both group 13 weeks before surgery and after the first surgery (p > 0.05). As shown in Fig. 1b, 4 weeks after the second surgery, the body weight of the two groups increased. Compared with the control group, the PTH group had a smaller weight increase, showing a statistically significant difference (p < 0.05). It could be concluded that the rats’ weight increased with time, and the control group had a more obvious weight increase.
a, b Body weight changes over time during the study period in the control and PTH groups. Data are expressed as mean ± SD; error bars in the figure are presented as SD, N = 7 specimens/group. *p < 0.05, **p < 0.001 vs. control group (by one-way ANOVA and Tukeyʼs post hoc test). PTH parathyroid hormone, W weeks
Micro-CT analysis of osteogenesis in femoral defects
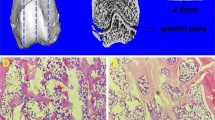
A rat model was used to investigate the potential of PTH to promote bone healing. Defects of critical size were drilled into the distal femora; PTH was used in one group (Fig. 2C, c, D, d) while no drug was used in the control group (Fig. 2A, a, B, b). The bone-healing process was monitored by micro-CT at 4 and 8 weeks after surgery. The PTH group showed evidence of newly formed bone around the defect after 4 weeks (Fig. 2, C, c), which was continuously replaced by newly formed bone after 8 weeks (Fig. 2, D, d). By contrast, only marginal new bone formation was observed in the control group even after 8 weeks (Fig. 2, B, b).
The overall state of bone regeneration exhibited on micro-CT images and three-dimensional reconstruction of the defect area from the PTH(C, D, c, d) and control groups (A, B, a, b) at 4 and 8 weeks after surgery. The red circle represents the original extent of the surgical defect. PTH parathyroid hormone, W weeks
The BMD of the VOI area continued to increase in the treatment group, while there was relatively no change in the control group (see Table 1). At 4 weeks, the BMD of the treatment group and of the control group increased by 12.39 % (p = 0.020); however, at 8 weeks, the BMD of the treatment group had increased by 35.76 % (p < 0.001).
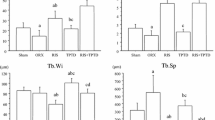
At 4 and 8 weeks after the second surgery, the volume fraction (BV/TV), thickness (Tb-Th), quantity (Tb-N), and degree of separation (Tb-Sp) of the upper trabecular bone of the right femur were measured (see Fig. 3). The BV/TV, Tb-Th, and Tb-N values of the PTH group were all higher than those of the control group both at 4 and at 8 weeks after surgery. The Tb-Sp of the trabecular bone in the PTH group was lower than that of the control group (statistically significant difference, p < 0.05).
Micro-CT analysis of bone volume per total volume (BV/TV), trabecular number (Th.N), trabecular thickness (Tb.Th), and trabecular separation (Th.Sp) of newly formed bone in the distal femur defect at 4 and 8 weeks after treatment in the PTH and control groups. Data are expressed as mean ± SD; error bars in the figure represent SD (N = 7 specimens/group). *p < 0.05, **p < 0.001 vs. control group (by one-way ANOVA and Tukeyʼs post hoc test). PTH parathyroid hormone, W weeks
Histological observation and analysis
The morphology of bone healing conducted by PTH was further investigated via a detailed qualitative histological analysis as shown in Fig. 4. The treatment group showed new woven bone growth following the surrounding defect originating from the existing trabecular bone at the bottom and lateral wall of the defect (Fig. 4A, a). At this stage of healing, most of the defect still existed. Figure 4B and b show the results of healing in the two groups after 8 weeks. In the control group (Fig. 4b), most sections showing bone formation were limited and confined to the margins of the defect with no sections showing any bone formation in the center of the defect. Furthermore, all sections showed (Fig. 4B) that new bone extended from the margins of the defect and into the middle of the defect. Undecalcified specimens were observed for new bone mineralization in each group (Fig. 4C, c). The rate of new bone mineralization of each group is shown in Fig. 4. The distance strip marked by yellow and green in the PTH group was greater than the control group. Results showed that the mineralization rate of the PTH group was higher than that of the control group. This indicates that PTH can strengthen the mineralization of bone tissue in zones of defect.
Serum analysis of bone metabolic markers
Serum levels of Ca2+, P, B-ALP, and CTX were measured to evaluate bone formation and resorption activity under PTH treatment (Table 2). At 4 and 8 weeks, PTH treatment increased Ca2+ levels by 11.8 and 14.8 %, P levels by 11.9 and 14.6 %, and B-ALP levels by 17.9 and 61.7 %, respectively; PTH treatment decreased CTX levels by 27.3 and 33.5 %, respectively (p < 0.05), when compared with the control group (p > 0.05).
Discussion
Owing to their fast generation and low cost, their easy and safe handling, their reliable reproducibility, and their similarities to pathophysiological responses in postmenopausal cancellous bone loss, OVX rats were often used for osteoporotic models to represent the process that occurs in the presence of cytokines or hormonal factors [16, 17]. A study using a successfully developed rat model examined parameters of bone change and reported that changes in the femur are the most obvious [18]. Therefore, the femur was selected as a subject in experiments. Currently, the treatment of bone defects involves implantation of bone tissues or biomaterials. Although good results have been achieved with this method, osteoporosis continues to pose a problem, making surgery inevitable and causing considerable damage for the patient. For these patients, it is important to use a method that heals the defect while at the same time treating the osteoporosis and promoting repair of the bone defects. Some studies show that PTH increases bone deposition and increases the amount and thickness of the trabecula by improving the quantity of recreation units. All these findings are based on intramembranous ossification [19, 20].
The bone remodeling process in this osteoporotic defect model remains unclear. A recent study showed that an osseous femur defect created in osteoporotic mice resulted in bone healing primarily by intramembranous ossification [21]. Histological results revealed that trabecular bone tissue surrounded with abundant osteoblastic cells and traces of cartilage matrix mainly locates at the peripheral regions of defects treated with PTH. The results of the control group demonstrated an increase in oval vacuolar adipocytes. This finding may suggest an activated remodeling process involving both intramembranous and endochondral ossification [22]. In osteoporosis, bone resorption outweighs bone formation. In this study, H&E, micro-CT, and tissue specimens were examined and it was found that PTH can stimulate recreation of callus and formation of new cortical bone so that bone repair is accelerated.
It was verified by our experiments that intermittent injection with a low dose of PTH not only treats osteoporosis but also improves repair of bone defects. It has been statistically shown that when disuse osteoporosis occurs, PTH–Vitamin D3 (Vit D3) is restrained, while PTH, 1,25(OH)2Vit D3, and renal cAMP concentrations drop. Surprisingly, osteoporosis does not happen to animals living through long hibernation; studies show that the PTH level does not drop in this course [23, 24].
Bone histomorphometry is the gold standard for the quantitative analysis and description of bone microstructure including the trabecular area, trabecular thickness, trabecular separation, trabecular number, and the number of pores, etc. [25, 26]. In this study, we used micro-CT through three-dimensional reconstruction of the defect area to observe the situation of new bone tissue more clearly. Our results show that there was abundant formation of new trabecular bone as well as higher BV/TV, Tb-N, and Tb-Th and lower Tb-Sp in the treatment of bone defects after PTH, suggesting there is a close link between the two. The control group, on the other hand, had a small amount of trabecular bone contact and no close link with the other bone parameters, similar to the findings of previous studies of osteoporotic fractures in rats [7, 27]. This may be why the healing of bone damage is closely related to the quantity of trabecular bone.
Conclusion
The results of this study indicate that PTH had a strong effect on defect healing in OVX rats. PTH produced the strongest effects on defects in BV/TV, Tb-N, Tb-Th, and Tb-Sp (at 4 and 8 weeks), as well as on Ca2+, P, B-ALP, and CTX. Histological and micro-CT investigations showed differences between the PTH group and the control group. Although our study did not examine whether an intermittent supply of low-dose PTH can accelerate bone defect repair from a biochemical point of view, our findings indicate that PTH can facilitate repair of bone defects in osteoporosis and it may be a promising method for treating this condition.
Reference
Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA (2002) Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. In: Seminars in cell & developmental biology, vol 2. Elsevier, pp 121–128
Riggs BL, Melton LR (1995) The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17(5):S505–S511
Pei L, Tontonoz P (2004) Fat’s loss is bone’s gain. J Clin Invest 113(6):805
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289(5484):1508–1514
Moazzaz P, Gupta MC, Gilotra MM, Gilotra MN, Maitra S, Theerajunyaporn T, Chen JL, Reddi AH, Martin RB (2005) Estrogen-dependent actions of bone morphogenetic protein—7 on spine fusion in rats. Spine 30(15):1706–1711
Yingjie H, Ge Z, Yisheng W, Ling Q, Hung W, Kwoksui L, Fuxing P (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41(4):631–638
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28(1):80–86
Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT Jr (1980) Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J 280(6228):1340–1344
Mosekilde L, Gaard HC, Mcosker EJ, Wronski JT (1994) PTH has a more pronounced effect on vertebral bone mass and biomechanical competence than antiresorptive agents (estrogen and bisphosphonate) &; assessed in sexually mature, ovariectomized rats, vol 4. Elsevier, Amsterdam
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18(1):9–17. doi:10.1359/jbmr.2003.18.1.9
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441. doi:10.1056/NEJM200105103441904
Dhillon RS, Xie C, Tyler W, Calvi LM, Awad HA, Zuscik MJ, OʼKeefe RJ, Schwarz EM (2013) PTH‐enhanced structural allograft healing is associated with decreased angiopoietin‐2–mediated arteriogenesis, mast cell accumulation, and fibrosis. J Bone Miner Res 28(3):586–597
Tao ZS, Zhou Q, Tu KK, Huang ZL, Xu H, Sun T, Lv YX, Cui W (2015) Treatment study of distal femur for parathyroid hormone (1–34) and beta-tricalcium phosphate on bone formation in critical size defects in rats. J Biomater Appl. doi:10.1177/0885328215592854
Wei L, Ke J, Prasadam I, Miron RJ, Lin S, Xiao Y, Chang J, Wu C, Zhang Y (2014) A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporos Int 25(8):2089–2096. doi:10.1007/s00198-014-2735-0
Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng G, Hu J (2012) The effects of combined human parathyroid hormone (1–34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int 23(4):1463–1474. doi:10.1007/s00198-011-1751-6
Egermann M, Goldhahn J, Schneider E (2005) Animal models for fracture treatment in osteoporosis. Osteoporos Int 16(Suppl 2):S129–S138. doi:10.1007/s00198-005-1859-7
Turner AS (2001) Animal models of osteoporosis—necessity and limitations. Eur Cell Mater 1:66–81
Comelekoglu U, Bagis S, Yalin S, Ogenler O, Yildiz A, Sahin NO, Oguz I, Hatungil R (2007) Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol 26(3):380–384. doi:10.1007/s10067-006-0367-2
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26(5):688–703. doi:10.1210/er.2004-0006
Hock JM (2001) Anabolic actions of PTH in the skeletons of animals. J Musculoskelet Neuronal Interact 2(1):33–47
He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, Chan CW, Lee KM, Cao YP, Li G, Wei L, Hung LK, Leung KS, Qin L (2011) Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: a drill-hole defect model. Bone 48(6):1388–1400. doi:10.1016/j.bone.2011.03.720
Cheng N, Dai J, Cheng X, Li S, Miron RJ, Wu T, Chen W, Zhang Y, Shi B (2013) Porous CaP/silk composite scaffolds to repair femur defects in an osteoporotic model. J Mater Sci Mater Med 24(8):1963–1975. doi:10.1007/s10856-013-4945-y
Uebelhart D, Bernard J, Hartmann DJ, Moro L, Roth M, Uebelhart B, Rehailia M, Mauco G, Schmitt DA, Alexandre C, Vico L (2000) Modifications of bone and connective tissue after orthostatic bedrest. Osteoporos Int 11(1):59–67. doi:10.1007/s001980050007
Lentle RG, Kruger MC (2005) Changes in mineralization and biomechanics of tibial metaphyses in splinted rats. J Appl Physiol 99(1):173–180. doi:10.1152/japplphysiol.00845.2004
Cortet B, Colin D, Dubois P, Delcambre B, Marchandise X (1995) Methods for quantitative analysis of trabecular bone structure. Rev Rhum 62(11):781–793
Boyd SK, Davison P, Muller R, Gasser JA (2006) Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 39(4):854–862. doi:10.1016/j.bone.2006.04.017
Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, Pei FX (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41(4):631–638. doi:10.1016/j.bone.2007.06.006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Z-S. Tao, Y-X Lv, W. Cui, Z-L. Huang, K-K. Tu, Q. Zhou, T. Sun, and L. Yang state that there are no conflicts of interest.
All national guidelines on the care and use of laboratory animals have been followed and the necessary approval was obtained from the relevant authorities.
Rights and permissions
About this article
Cite this article
Tao, ZS., Lv, YX., Cui, W. et al. Effect of teriparatide on repair of femoral metaphyseal defect in ovariectomized rats. Z Gerontol Geriat 49, 423–428 (2016). https://doi.org/10.1007/s00391-015-0949-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-015-0949-1