Abstract
Summary
Ovariectomized (OVX) rats with tibial fracture received vehicle, ZA, PTH, or ZA plus PTH treatment for 4 and 8 weeks. Bone metabolism, callus formation, and the mass of undisturbed bone tissue were evaluated by serum analysis, histology, immunohistochemistry, radiography, micro-computerized tomography, and biomechanical test.
Introduction
Previous studies have demonstrated the effect of ZA or PTH on osteoporotic fracture healing. However, reports about effects of ZA plus PTH on callus formation of osteoporotic fracture were limited. This study was designed to investigate the impact of combined treatment with ZA and PTH on fracture healing in OVX rats.
Methods
Twelve weeks after bilateral ovariectomy, all rats underwent unilateral transverse osteotomy on tibiae. Animals then randomly received vehicle, ZA (1.5 μg/kg weekly), PTH (60 μg/kg, three times a week), or ZA plus PTH until death at 4 and 8 weeks. The blood and bilateral tibiae of rats were harvested for evaluation.
Results
All treatments increased callus formation and strength other than the control; ZA + PTH showed the strongest effects on percent bone volume (BV/TV), trabecular thickness, total fluorescence-marked callus area, and biomechanical strength. Additionally, inhibited RANKL and enhanced osteoprotegerin expression were observed in the ZA + PTH group. But no difference in bone mineral density and BV/TV of the contralateral tibiae was observed between treated groups.
Conclusion
Findings in this study suggested an additive effect of ZA and PTH on fracture healing in OVX rats, and this additive effect was specific to callus formation, not to undisturbed bone tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to excessive bone resorption and deficient bone formation, osteoporotic bodies with fracture are usually subject to depressed callus quality and prolonged healing time, even nonunion [1–4]. Consequently, there has been interest in treatments that could enhance callus formation and shorten healing time on osteoporotic fracture. Since imbalanced bone turnover is the main reason for deteriorated fracture healing in osteoporotic bodies, various anti-resorptive or anabolic drugs have been investigated to inhibit excessive bone resorption or promote new bone formation during fracture healing, such as estrogen, selective estrogen receptor modulators, calcitonin, bisphosphonates (BPs), and intermittently administered human parathyroid hormone (PTH) [5–8]. Although the exact mechanism of how osteoporosis influences fracture healing is still unclear, anti-catabolic drugs have been demonstrated to increase callus volume, bone mineral density (BMD), and biomechanical strength at the early healing period based on the inhibition of bone resorption [9, 10]. The anabolic drug PTH could increase bone remodeling with a greater effect on bone formation than bone resorption and lead to increased bone mass and improved bone microarchitecture [11, 12], and it has also been reported to enhance fracture healing in both intact and ovariectomized (OVX) rats [13, 14], and in postmenopausal women [15].
PTH, which could directly stimulate bone formation, is currently the only Food and Drug Administration-approved anabolic agent for osteoporosis treatment. But the anabolic effect of PTH may reach its plateaus over time, and the withdrawal of PTH is reported to cause bone resorption in rats [16, 17], while the anti-resorptive drug BPs, although potent in preserving bone mass, also eventually reduce bone formation because of overall bone turnover decreases [18]. Thus, both anti-resorptive and anabolic compounds seem to be oversimplified, and it leads to the plausible hypothesis that combined use of anabolic PTH and anti-resorptive BPs may have additive effects on callus formation in osteoporotic fracture, namely to inhibit excessive bone resorption and stimulate new bone formation simultaneously.
The purpose of this study is to observe the effects of combined treatment with zoledronic acid (ZA) and PTH on fracture healing in OVX rats. Four and 8 weeks after different drug treatments, the systemic bone metabolism condition and local callus healing were evaluated by serum analysis, histology, immunohistochemistry, fluorescent labeling, radiology, and micro-computed tomography (micro-CT) assessment. The undisturbed tibiae were also analyzed by histology, dual energy X-ray absorptiometry (DXA), and micro-CT to determine whether the effects of different treatments were specific to callus formation or to all bone tissue.
Materials and methods
Experimental animals
One hundred forty-five female Sprague Dawley rats, aged 3 months with the body weight of 250 ± 33 g, were included in this study. Every four animals were kept in one cage with climate-controlled conditions (25°C, 55% humidity, 12 h of light alternating with 12 h of darkness). Free access to tap water and standard laboratory diet containing 1.56% calcium, 0.8% phosphorus, and 800 IU/kg vitamin D were permitted. All the animal experiments were conducted in accordance with international standards on animal welfare as well as being compliant with the Animal Research Committee of the university.
Establishment of osteoporotic model
After bilateral ovariectomy (n = 140) or sham operation (n = 5) according to a previous report [19], 12 weeks were allowed to pass before fracture surgery for the establishment of standard osteoporotic animal models. Then five randomly selected OVX rats and the five sham-operated ones were euthanized, and the tibial metaphysis was harvested for BMD, micro-CT, and histological evaluation to confirm the establishment of osteoporotic animal model.
Fracture surgery
All the OVX rats left underwent unilateral transverse osteotomy on the proximal tibiae. The operation was performed under general anesthesia and sterile conditions as previously described [20]. Briefly, a transverse osteotomy was made at the proximal diaphysis at the junction of the proximal one third and distal two thirds of the right tibia (Fig. 1a (a)); then the patella was deflected laterally, and a hole was drilled through the intercondylar eminence of the tibia. Afterwards, fracture fragments were contacted, and a stainless steel wire (1.0 mm in diameter) was inserted through the hole across the fracture ends (Fig. 1a (b)). X-ray analysis (RadSpeed M CH with FPD; SHIMADZU Corporation) was performed to assess the location and quality of fractures immediately after surgery (Fig. 1a (c)). All the animals received an intramuscular antibiotic and analgesic injection for 3 postoperative days. Unrestricted activity was allowed after the rats woke up from anesthesia.
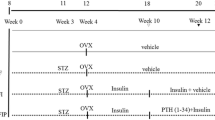
A Transverse osteotomy was made at the proximal one third of the right tibia before fixation. The black arrows show the fracture gap (a) and fracture line after fixation using the intramedullary pin (b), and the white arrow indicates the intramedullary pin used for fracture fixation (a). The location and quality of fracture were assessed by radiography immediately after surgery, and the arrow shows the fracture line (c). B Scheme of the study design and animal number at different experimental stages. c Histological sections (hematoxylin and eosin staining; magnification, ×100; the scale bar represents 200 μm) through the sagittal plane and micro-CT images from 2 mm below the growth plate of the contralateral tibial metaphysis; the scale bar represents 1 mm
Pharmaceutical treatment
After fracture operation, all animals were randomly divided into four groups, and the pharmaceutical interventions commenced from the first postoperative day through the following subcutaneous injections: vehicle (control); ZA (Novartis Pharma AG, Switzerland), 1.5 μg/kg, weekly (group ZA); human PTH 1-34 (Bachem, Torrance, CA), 60 μg/kg, three times a week (group PTH); ZA (1.5 μg/kg, weekly) plus PTH (60 μg/kg, three times a week) (group ZA + PTH). The doses of PTH and ZA were determined according to previous experiments where they showed protective effects in OVX rodent models [21, 22]. During treatment, the body weight of the animals was measured weekly, and the drug dosage was adjusted accordingly.
Fluorochrome labeling
In order to obtain dynamic parameters of callus formation and remodeling, alizarin red (Sigma–Aldrich Inc., Saint Louis, MO, USA; 20 mg/kg) and calcein green (Sigma–Aldrich Inc., 20 mg/kg) were injected subcutaneously at 4 and 7 weeks post-fracture, respectively. All fluorescent agents were prepared immediately before injection and filtered through a 0.45-μm filter.
Animal sacrifice and specimen collection
At the end of the observation time (4 or 8 weeks after fracture), animals were euthanized by cardiac puncture under general anesthesia in the early morning. The blood was collected, and serum was stored at −80°C until use for biochemical assays. After complete excision of soft tissues, the intramedullary pins were removed from fractured tibiae before evaluation. Eight weeks after fracture, the contralateral tibiae were also harvested for assessment.
Micro-CT examination
For micro-CT analysis, the fractured tibiae (n = 6/group) were prepared into 10-mm-long blocks with callus and kept in 70% ethanol. These specimens were scanned on a μCT system (μCT 80 scanner, Scanco Medical, Bassersdorf, Switzerland) and reconstructed with an isotropic voxel size of 10 μm for a detailed qualitative and quantitative evaluation. The scanning system was set to 55 kV, 145 μA, and 700 ms integration time, and the scanned region included 1,000 images with a resolution of 2,048 × 2,048 pixels. The constrained 3-D Gaussian filter (σ = 1.2, support = 1) was used for partial image noise suppression. The callus volume of interest (VOI) was defined as the newly formed bone tissues, and the medullary canal volume and the original bone tissue were excluded from evaluation according to previous reports [23]. After segmentation, the following parameters were quantified within the VOI: bone volume (BV), percent bone volume (BV/TV), and mean trabecular thickness (Tb.Th).
Fracture site BMD measurement
After micro-CT evaluation, the BMD (milligrams per square centimeter) of callus (n = 6/group) was assessed using Lunar iDXA (GE Healthcare Lunar, USA), with the hand regional high-resolution and small-animal scan mode. The fractured tibiae were placed in deionized water with the same position for X-ray scan. The region of interest (ROI) was chosen as a longitudinal rectangle covering the whole callus area, with the transverse fracture line right-angled in center. After scanning, BMD values were obtained for statistical analysis.
Histological and fluorescent analysis
After radiological analysis, the fractured tibiae (n = 6/group) were used for undecalcified histological sections. After fixation in 70% alcohol for 3 days, specimens were dehydrated in graded ethanol (80–100%) and embedded in methylmethacrylate (Technovit 7200 VCL; Exact Apparatbau, Nordenstedt, Germany) without decalcification. Five- and 10-μm-thick sections were prepared along the coronal plane of tibiae using a diamond saw (Leica SP1600, Germany). Finally, the 5-μm-thick sections through the central portion of the calluses were stained in 1% toluidine blue. The 10-μm-thick slices were kept unstained for fluorescent observation under a laser confocal scanning microscope (LCSM; Zeiss LSM 510, Germany). The fluorescence-marked callus area and relative mineral apposition rate (MAR) were analyzed by ZEN 2009 Light Edition software (Zeiss, Germany). The relative MAR was calculated as the relative ratio of calcein green- and alizarin red-marked callus areas [24, 25].
Biomechanical testing
Before biomechanical test, the fractured tibiae (n = 6/group) were taken out of the freezer and allowed to thaw completely at room temperature. Three-point bending test on the fractured calluses was performed by a commercial material testing system (Instron 4302; Instron, Norwood, MA, USA) as previously described [26]. Briefly, the tibia was placed on two lower support bars 1.5 cm apart in a repeated position, and the central part of the callus was defined as the testing area. The compressing force was then exerted on the callus at the speed of 1 mm/min until breakage. The ultimate load at failure (newtons, maximum force that the tibia could bear) and the stiffness (newtons per millimeter, slope of the load-deflection curve from the linear part) were calculated from the load-deflection curve recorded by a connected computer.
Serum analysis
Serum measurements of osteocalcin (OCN) and tartrate-resistant acid phosphatase (TRAP) 5b were performed to study the serum levels of osteoblastic and osteoclastic markers. Eight serum samples from each group at 4 or 8 weeks were analyzed. Serum levels of OCN were assayed using a rat OCN immunoradiometric assay kit (Immutopics, San Clemente, CA), and the TRAP 5b was measured by ELISA (SBA Sciences) according to a previous report and the manufacturer's instructions [27].
Immunohistochemistry
For immunohistochemical evaluation of osteoprotegerin (OPG) and receptor activator of nuclear factor kappa B ligand (RANKL) expression, specimens (n = 3/group) were fixed in 4% buffered formaldehyde for 2 days at room temperature, then decalcified in ethylenediaminetetraacetic acid (changed every 3 days) for 4 weeks. Decalcified tissues were then washed, dehydrated in gradient alcohol, embedded in paraffin wax, and cut into 4-μm-thick sections along the coronal plane of the tibia. Immunohistochemical localization of OPG and RANKL was carried out using commercially available antibodies according to the manufacturer's suggested protocol (Abcam, Hong Kong). Negative controls were obtained by omitting the primary antibody. Stained sections were examined qualitatively under light microscopy (Eclipse 80i, Nikon, Japan) with a digital camera.
Assessments of the contralateral tibiae
In order to investigate whether the effects of different treatments were specific to callus formation or to all bone tissue, the contralateral tibial metaphysis was analyzed by iDXA, micro-CT, and histology. For iDXA examination (n = 10/group), the ROI was defined as a longitudinal rectangle covering 1 cm of the tibial metaphysis. In micro-CT evaluation (n = 10/group), the VOI was chosen as 500 slices (10 μm/slice) from the growth plate, and BV/TV was analyzed quantitatively. For histological assessment (n = 5/group), sagittal decalcified sections through the central portion of the tibial metaphysis were made and stained with hematoxylin and eosin.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using the statistics package SPSS 13.0 (SPSS, Chicago, IL, USA). Multiple comparisons between groups were carried out using one-way ANOVA and Tukey's post hoc test. The significance level of 0.05 was applied for all analyses.
Results
Animals
Fifteen rats in total were excluded from analysis due to anesthetic accident, infection, fracture fixation, or inaccurate fracture site, and there were 15 animals left for evaluation in each group at each observation time (Fig. 1b).
Confirmation of osteoporosis
The histological and micro-CT images of the tibial metaphysis were presented in Fig. 1c. Compared to sham-operated animals, the bone mass was reduced, and the trabecular microarchitecture was destroyed markedly in OVX rats. In quantitative analysis, the BMD and BV/TV of proximal tibiae from sham-operated rats were 27.5% and 35.6% higher than those of OVX rats (t test, p < 0.05, data not shown). These results confirmed the establishment of osteoporosis in OVX rats.
Micro-CT evaluation
The coronal and transverse micro-CT slices through the central part of the callus clearly depicted the effects of different treatments on callus formation qualitatively (Fig. 2a). The quantitative results were expressed as BV, BV/TV, and Tb.Th (Fig. 2b). At 4 weeks, ZA and ZA + PTH showed increased BV than the control and PTH (p < 0.05), while no difference in BV/TV was observed between treated groups (p > 0.05). At 8 weeks, ZA revealed the highest values of BV (p < 0.05), while ZA + PTH exhibited the strongest effect on BV/TV (p < 0.05). Moreover, ZA + PTH treatment presented the strongest effect on Tb.Th at both 4 and 8 weeks (p < 0.05).
A The coronal and transverse micro-CT slices through the center of the callus; groups under different treatments and the observation time were labeled in the figure. The scale bar represents 2 mm. B Quantitative results of micro-CT analysis expressed as BV, BV/TV, and Tb.Th. C Result of BMD by iDXA examination. Data were expressed as mean ± SD; error bars in the figure are presented as SD, n = 6 specimens/group. *p < 0.05 vs. control group, # p < 0.05 vs. ZA group, $ p < 0.05 vs. PTH group (by one-way ANOVA and Tukey's post hoc test)
Fracture site BMD assessment
Results of callus BMD by iDXA were presented in Fig. 2c. All the treated groups showed significantly increased BMD than the control at 4 weeks post-fracture (p < 0.05), but no significant difference was found between treated groups. At 8 weeks after fracture, callus BMD from the control was still the lowest; ZA and ZA + PTH showed similar callus BMD, but both were higher than that of PTH (p < 0.05).
Histological and fluorescent analysis
Undecalcified histological images were exhibited in Fig. 3a. At 4 weeks, more calluses were observed in the ZA + PTH group compared to ZA or PTH alone; at 8 weeks, callus size was similar in the control, PTH, and ZA + PTH group, but that in the ZA group seemed to be larger.
A Undecalcified histological sections, stained with toluidine blue, of the coronal plane through the center of calluses (magnification, ×40); the scale bar represents 2 mm. B Undecalcified sections observed under a laser confocal scanning microscope showing the alizarin red (injected at 4 weeks after fracture)- and calcein green (injected at 7 weeks post-fracture)-marked callus (magnification, ×50); the scale bar represents 2 mm
In fluorescent analysis (Fig. 3b and Fig. 4a), ZA showed the largest alizarin red-marked callus area (95.3% higher than the control, p < 0.05), but ZA + PTH exhibited the highest values of calcein green- and total fluorescence-marked callus areas (p < 0.05). ZA + PTH and PTH alone showed similar relative MAR, which was increased by 60% and 48% compared to the control, respectively (p < 0.05). ZA alone revealed the lowest relative MAR, which was decreased by 41% compared to that of the control (p < 0.05).
A Quantitative results of the alizarin red- and/or calcein green-marked callus areas and relative MAR, n = 6 specimens/group. B Biomechanical results expressed as ultimate load force and stiffness, n = 6 specimens/group. C Results of the serum analysis of osteocalcin and TRAP 5b, n = 8 samples/group. Data were expressed as mean ± SD; error bars in the figure indicate SD. *p < 0.05 vs. control group, # p < 0.05 vs. ZA group, $ p < 0.05 vs. PTH group (by one-way ANOVA and Tukey's post hoc test)
Biomechanical test
Results of biomechanical test of fractured tibiae were expressed as ultimate load and stiffness (Fig. 4b). From 4 to 8 weeks post-fracture, the biomechanical strength increased with time in all groups. At each observation time, the strongest effects on strength of fractured tibiae were observed in the ZA + PTH group (p < 0.05). Compared to the control, ZA + PTH increased the ultimate load by 2.6- and 1.3-fold, and the stiffness by 1.1- and 0.6-fold at 4 and 8 weeks post-fracture, respectively (p < 0.05).
Serum analysis of bone metabolic markers
Serum levels of OCN and TRAP 5b were measured to provide an evaluation of bone formation and resorption activity after fracture under PTH and/or ZA treatment (Fig. 4c). At 4 and 8 weeks, PTH increased OCN levels by 38.9% and 49.6%, respectively (p < 0.05); ZA decreased OCN levels by 37.3% and 36.9%, respectively (p < 0.05), while ZA + PTH displayed similar OCN levels when compared to the control (p > 0.05). For analysis of TRAP 5b levels, ZA decreased them by 56.7% and 59.7%, and ZA + PTH reduced them by 53.3% and 54.8% at 4 and 8 weeks compared to the control (p < 0.05). PTH alone showed similar TRAP 5b levels to the control at 4 weeks, but 40.3% higher TRAP 5b levels than the control at 8 weeks (p < 0.05).
Immunohistochemical evaluation of OPG and RANKL
Immunohistochemical reactivity for OPG and RANKL was detected in the cytoplasm and plasma membranes of osteoblasts, bone marrow lining cells, newly embedded osteocytes in the callus, especially in osteoblasts on the surface of newly formed trabeculae (Fig. 5). No positive staining was observed when the primary antibody was omitted (data not shown). Strong reactivity of RANKL was observed in the control and PTH groups, which was slightly (4 weeks) or markedly (8 weeks) inhibited in the ZA + PTH group, and almost completely inhibited in the ZA group. At 4 and 8 weeks, PTH and ZA + PTH showed much more enhanced reactivity of OPG than the control and ZA. This result indicated that ZA + PTH revealed inhibited RANKL but enhanced OPG expression simultaneously.
Assessments of the contralateral tibiae
Eight weeks after different treatments, all treatments increased BMD and BV/TV of the contralateral tibial metaphysis, but no significant difference was observed between treated groups (Fig. 6a). The micro-CT images (Fig. 6b) and histological sections (Fig. 6c) from proximal tibiae were also exhibited.
A Results of BMD by iDXA and percent bone volume (BV/TV) by micro-CT from the contralateral tibial metaphysis 8 weeks after different treatments, n = 10 specimens/group. Data were expressed as mean ± SD; error bars in the figure indicate SD. *p < 0.05 vs. control group (by one-way ANOVA and Tukey's post hoc test). B Micro-CT images from 2 mm below the growth plate of the contralateral tibial metaphysis; the scale bar represents 1 mm. C Histological sections through the central part of tibiae from the sagittal plane (hematoxylin and eosin staining; magnification, ×100; the scale bar represents 200 μm)
Discussion
In this study, ZA + PTH showed stronger effects on fracture healing than either monotherapy in OVX rats, with the highest values of BV/TV (8 weeks) and Tb.Th (4 and 8 weeks) in micro-CT evaluation, the retained OCN and decreased TRAP 5b levels in serum analysis, the strongest effects on total fluorescence-marked callus area in fluorescent analysis, the enhanced OPG and inhibited RANKL expression in immunohistochemistry, and the strongest effects on callus strength in biomechanical test. Moreover, ZA + PTH showed no difference between treated groups in assessments of the contralateral tibial metaphysis by iDXA, histology, and micro-CT. These results seemed to indicate that combined treatment with ZA and PTH had an additive effect on fracture healing in OVX rats, and this effect was specific to callus formation, not to all bone tissue.
Concurrent administration of PTH and BPs has been extensively reported for treatment of osteoporosis or prevention of fracture in different animal models [28–31] and postmenopausal women [32, 33]. Results in these studies indicated that combined treatment did not induce additive or synergistic effect on bone mass and BMD. In addition, the anabolic effect of PTH seemed to be attenuated by BPs when used concurrently, and the effects of combined treatment were similar to those of monotherapy, or even less than PTH alone. A similar phenomenon was observed in the present study in the analysis of serum bone metabolic markers and contralateral tibiae. ZA + PTH showed similar OCN levels and decreased TRAP 5b levels, while PTH alone showed increased OCN and TRAP 5b levels compared to the control. This result seemed to demonstrate that ZA + PTH inhibited bone resorption but did not promote bone formation, namely the effects of ZA and PTH did not add up on systemic bone metabolism. Moreover, ZA + PTH showed no significant difference between treated groups in assessments of the contralateral tibial metaphysis by iDXA, histology, and micro-CT, which indicated that ZA + PTH showed no additive effect on undisturbed bone tissue.
However, when bone regeneration at the fracture sites was assessed in the present study, ZA + PTH produced stronger effects on fracture healing than either monotherapy in OVX rats, which seemed to suggest the additive effects of ZA + PTH on callus formation. In this study, ZA induced the most alizarin red (injected at 4 weeks)-marked callus but the least calcein green (injected at 7 weeks)-marked callus, which meant that ZA retained the most callus formed at 4 weeks, but inhibited callus remodeling at 7 weeks. On the other hand, ZA + PTH produced the strongest effect on the calcein green- and total fluorescence-marked callus, which indicated that ZA did not inhibit the effect of PTH when applied concurrently. The inhibited RANKL and enhanced OPG expression in immunohistochemistry and the strongest effects on callus strength in biomechanical test also suggested the additive effect of ZA plus PTH treatment.
Why did the PTH plus BP treatment show different effects on bone mass preservation in undisturbed conditions and callus formation at fracture sites? The most plausible explanation may be that the inhibition of overall bone turnover impairs the anabolic activity of PTH in osteoporotic subjects. Previous studies have indicated that PTH primarily acts to enhance the function and life span of mature osteoblasts and might also act to promote osteoblast differentiation [12, 34]. However, there is little evidence that PTH can increase the amount of precursor cells. Thus, when used together with BPs which inhibit bone turnover and reduce bone formation (directly or indirectly), the PTH would be less effective because there are fewer osteoblasts available for it to act on [18]. The inhibited bone resorption by BPs reduced the number of bone remodeling units, which is essential for the anabolic action of PTH. However, situations in bone sites with trauma after fracture were totally different, where a large amount of osteoblasts resided in the wound sites. Shirley et al. have demonstrated that some osteoblasts involved in fracture healing were systemically mobilized and recruited to the fracture site from remote bone marrow places, rather than passively leaked into the circulation and to the bone trauma locality [35]. Due to the large amount of osteoblasts existing in the wound sites after fracture, there were adequate target cells for PTH to work on. In addition, the application of ZA inhibits the active bone resorption during callus remodeling. Consequently, ZA + PTH in this study induced an additive effect on fracture healing in OVX rats. This result was consistent with a previous study about additive effects of PTH and BPs on bone healing response to metaphyseal implants in intact rats [36].
It is an interesting question about what contributes to the increase of fracture healing strength under ZA plus PTH treatment. At 4 weeks, the fracture gap was filled with callus, so callus mass and quality should contribute to the strength at the fracture site. While ZA and ZA + PTH showed similar callus BV, BV/TV, and BMD at 4 weeks, ZA + PTH presented stronger healing strength than ZA. In order to clarify this issue, we assessed callus microarchitecture in micro-CT analysis and found that ZA plus PTH treatment showed higher Tb.Th than ZA. Thus, it maybe concluded that callus mass, density, and microarchitecture contributed to fracture healing strength jointly in the ZA + PTH group at 4 weeks. However, the situation was different at 8 weeks after fracture when bone union had formed. Although ZA alone presented the highest BV value, ZA + PTH still showed the strongest fracture healing strength, which was related to the highest value of BV/TV, Tb.Th, and total fluorescence-marked callus area. This seemed to indicate that callus density, microarchitecture, and ossification contributed to the fracture healing strength at 8 weeks after fracture, but callus mass did not at this stage.
The dose rate at 60 μg/kg (three times a week) of PTH in this study was similar to that routinely used in rodent models [37, 38] but much higher than therapeutic dose in patients (40–100 μg/day) [32, 33]. Thus, the dose of PTH used in rodent animals is generally 20–100 times higher than the therapeutic dose used in humans based on body weight. However, there are very few reports demonstrating that a dose of PTH similar to a human therapeutic dose could induce any significant effects on BMD or bone quality in animals. On the contrary, higher doses of PTH are generally found to exert significant effects on bone mass or serum calcium levels [39, 40]. The dose rate at 1.5 μg/kg weekly of ZA in this study was determined according to a previous report in OVX rats where excessive bone resorption was effectively inhibited [21, 41]. For the application of ZA, a 5-mg once yearly infusion through intravenous injection was reported for treatment of postmenopausal osteoporosis in clinical trials [42, 43]. This dose rate was equal to 1.9 μg/kg weekly by mathematical computation, which was slightly higher than 1.5 μg/kg weekly used in the present study. However, weekly injection of ZA in this study is different from clinical treatment with a single infusion, so these two different application methods of ZA may produce different effects on bone tissue.
Up to now, the mechanism of how osteoporosis impairs fracture healing has not been completely identified. Although ZA + PTH in this study produced stronger effects than either monotherapy on fracture healing in OVX rats within 8 weeks, longer observation time was needed for evaluation of the long-term efficiency of combined treatment. Furthermore, the physiological condition is different between OVX rats and postmenopausal women, so prediction of effects of PTH plus BP treatment on fracture healing in humans via results from OVX rats should be made cautiously.
In summary, results from this study indicated that ZA + PTH held stronger effects on fracture healing than either monotherapy in OVX rats. ZA + PTH produced strongest effects on callus BV/TV (at 8 weeks), Tb.Th (4 and 8 weeks), total fluorescence-marked callus area, and callus strength. ZA + PTH exerted locally inhibited RANKL and enhanced OPG expression in callus by immunohistochemistry. Moreover, ZA + PTH showed no difference between treated groups in the assessment of the contralateral tibial metaphysis by iDXA, histology, and micro-CT. These results seemed to indicate that ZA plus PTH had an additive effect on fracture healing in OVX rats, but this effect was specific to callus formation, not to all bone tissue.
References
Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, Kitajima I, Semba I, Hirasawa Y (1999) Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol 68:197–202
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28:80–86
McCann RM, Colleary G, Geddis C, Clarke SA, Jordan GR, Dickson GR, Marsh D (2008) Effect of osteoporosis on bone mineral density and fracture repair in a rat femoral fracture model. J Orthop Res 26:384–393
Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, Pei FX (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41:631–638
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H (2002) Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17:2237–2246
Li X, Luo X, Yu N, Zeng B (2007) Effects of salmon calcitonin on fracture healing in ovariectomized rats. Saudi Med J 28:60–64
Jahng JS, Kim HW (2000) Effect of intermittent administration of parathyroid hormone on fracture healing in ovariectomized rats. Orthopedics 23:1089–1094
Nozaka K, Miyakoshi N, Kasukawa Y, Maekawa S, Noguchi H, Shimada Y (2008) Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone 42:90–97
Nagashima M, Sakai A, Uchida S, Tanaka S, Tanaka M, Nakamura T (2005) Bisphosphonate (YM529) delays the repair of cortical bone defect after drill-hole injury by reducing terminal differentiation of osteoblasts in the mouse femur. Bone 36:502–511
Stuermer EK, Sehmisch S, Rack T, Wenda E, Seidlova-Wuttke D, Tezval M, Wuttke W, Frosch KH, Stuermer KM (2010) Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbecks Arch Surg 395:163–172
Kneissel M, Boyde A, Gasser JA (2001) Bone tissue and its mineralization in aged estrogen-depleted rats after long-term intermittent treatment with parathyroid hormone (PTH) analog SDZ PTS 893 or human PTH (1-34). Bone 28:237–250
Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK (2008) Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone 42:806–818
Komatsubara S, Mori S, Mashiba T, Nonaka K, Seki A, Akiyama T, Miyamoto K, Cao Y, Manabe T, Norimatsu H (2005) Human parathyroid hormone (1-34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone 36:678–687
Komrakova M, Stuermer EK, Werner C, Wicke M, Kolios L, Sehmisch S, Tezval M, Daub F, Martens T, Witzenhausen P, Dullin C, Stuermer KM (2010) Effect of human parathyroid hormone hPTH (1-34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 47:480–492
Aspenberg P, Genant HK, Johansson T, Nino AJ, See K, Krohn K, García-Hernández PA, Recknor CP, Einhorn TA, Dalsky GP, Mitlak BH, Fierlinger A, Lakshmanan MC (2010) Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 25:404–414
Girotra M, Rubin MR, Bilezikian JP (2006) The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord 7:113–121
Johansson HR, Skripitz R, Aspenberg P (2008) Bisphosphonates can block the deterioration in implant fixation after withdrawal of intermittent doses of parathyroid hormone. J Bone Joint Surg Br 90:400–404
Khosla S (2003) Parathyroid hormone plus alendronate. A combination that does not add up. N Eng J Med 349:1277–1279
Varkey M, Kucharski C, Doschak MR, Winn SR, Brochmann EJ, Murray S, Matyas JR, Zernicke RF, Uludag H (2007) Osteogenic response of bone marrow stromal cells from normal and ovariectomized rats treated with a low dose of basic fibroblast growth factor. Tissue Eng 13:809–817
Li YF, Luo E, Feng G, Zhu SS, Li JH, Hu J (2010) Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int 21:1889–1897
Glatt M, Pataki A, Evans GP, Hornby SB, Green JR (2004) Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporos Int 15:707–715
Skripitz R, Johansson HR, Ulrich SD, Werner A, Aspenberg P (2009) Effect of alendronate and intermittent parathyroid hormone on implant fixation in ovariectomized rats. J Orthop Sci 14:138–143
Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF, Einhorn TA (2009) Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 24:196–208
Matsuzaka K, Shimono M, Inoue T (2001) Characteristics of newly formed bone during guided bone regeneration: observations by immunohistochemistry and confocal laser scanning microscopy. Bull Tokyo Dent Coll 42:225–234
Lu H, Qin L, Fok P, Cheung W, Lee K, Guo X, Wong W, Leung K (2006) Low-intensity pulsed ultrasound accelerates bone-tendon junction healing: a partial patellectomy model in rabbits. Am J Sports Med 34:1287–1296
Wang JW, Xu SW, Yang DS, Lv RK (2007) Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporos Int 18:1641–1650
Iida-Klein A, Hughes C, Lu SS, Moreno A, Shen V, Dempster DW, Cosman F, Lindsay R (2006) Effects of cyclic versus daily hPTH(1-34) regimens on bone strength in association with BMD, biochemical markers, and bone structure in mice. J Bone Miner Res 21:274–282
Delmas PD, Vergnaud P, Arlot ME, Pastoureau P, Meunier PJ, Nilssen MH (1995) The anabolic effect of human PTH (1-34) on bone formation is blunted when bone resorption is inhibited by the bisphosphonate tiludronate-is activated resorption a prerequisite for the in vivo effect of PTH on formation in a remodeling system? Bone 16:603–610
Mashiba T, Tanizawa T, Takano Y, Takahashi HE, Mori S, Norimatsu H (1995) A histomorphometric study on effects of single and concurrent intermittent administration of human PTH (1-34) and bisphosphonate cimadronate on tibial metaphysis in ovariectomized rats. Bone 17:273S–278S
Li M, Mosekilde L, Søgaard CH, Thomsen JS, Wronski TJ (1995) Parathyroid hormone monotherapy and cotherapy with antiresorptive agents restore vertebral bone mass and strength in aged ovariectomized rats. Bone 16:629–635
Zhang L, Endo N, Yamamoto N, Tanizawa T, Takahashi HE (1998) Effects of single and concurrent intermittent administration of human PTH (1-34) and incadronate on cancellous and cortical bone of femoral neck in ovariectomized rats. Tohoku J Exp Med 186:131–141
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ, PaTH Study Investigators (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Eng J Med 349:1207–1215
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Eng J Med 349:1216–1226
Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446
Shirley D, Marsh D, Jordan G, McQuaid S, Li G (2005) Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res 23:1013–1021
Aspenberg P, Wermelin K, Tengwall P, Fahlgren A (2008) Additive effects of PTH and bisphosphonates on the bone healing response to metaphyseal implants in rats. Acta Orthop 79:111–115
Friedl G, Turner RT, Evans GL, Dobnig H (2007) Intermittent parathyroid hormone (PTH) treatment and age-dependent effects on rat cancellous bone and mineral metabolism. J Orthop Res 25:1454–1464
Hashimoto T, Shigetomi M, Ohno T, Matsunaga T, Muramatsu K, Tanaka H, Sugiyama T, Taguchi T (2007) Sequential treatment with intermittent low-dose human parathyroid hormone (1-34) and bisphosphonate enhances large-size skeletal reconstruction by vascularized bone transplantation. Calcif Tissue Int 81:232–239
Tanizawa T, Yamamoto N, Takano Y, Mashiba T, Zhang L, Nishida S, Endo N, Takahashi HE, Fujimoto R, Hori M (1998) Effects of human PTH (1-34) and bisphosphonate on the osteopenic rat model. Toxicol Lett 102–103:399–403
Johnston S, Andrews S, Shen V, Cosman F, Lindsay R, Dempster DW, Iida-Klein A (2007) The effects of combination of alendronate and human parathyroid hormone (1-34) on bone strength are synergistic in the lumbar vertebra and additive in the femur of C57BL/6J mice. Endocrinology 148:4466–4474
Perilli E, Le V, Ma B, Salmon P, Reynolds K, Fazzalari NL (2010) Detecting early bone changes using in vivo micro-CT in ovariectomized, zoledronic acid-treated, and sham-operated rats. Osteoporos Int 21:1371–1382
Hwang JS, Chin LS, Chen JF, Yang TS, Chen PQ, Tsai KS, Leung PC (2011) The effects of intravenous zoledronic acid in Chinese women with postmenopausal osteoporosis. J Bone Miner Metab 29:328–333
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, Pivotal Fracture Trial HORIZON (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Acknowledgments
This study was supported by a grant from the National Science Funds for Distinguished Young Scholars (no. 30825040) and Program for New Century Excellent Talents in University (NCET-10-0597).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y.F., Zhou, C.C., Li, J.H. et al. The effects of combined human parathyroid hormone (1-34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int 23, 1463–1474 (2012). https://doi.org/10.1007/s00198-011-1751-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1751-6