Abstract
Purpose
The standard treatment of rectal adenocarcinoma is total mesorectal excision (TME), in many cases requires a temporary or permanent stoma. TME is associated with high morbidity and genitourinary alterations. Transanal endoscopic microsurgery (TEM) allows access to tumors up to 20 cm from the anal verge, achieves minimal postoperative morbidity and mortality rates, and does not require an ostomy. The treatment of T2, N0, and M0 cancers remains controversial. Preoperative chemoradiotherapy (CRT) in association with TEM reduces local recurrence and increases survival. The TAU-TEM study aims to demonstrate the non-inferiority of the oncological outcomes and the improvement in morbidity and quality of life achieved with TEM compared with TME.
Methods
Prospective, multicenter, randomized controlled non-inferiority trial includes patients with rectal adenocarcinoma less than 10 cm from the anal verge and up to 4 cm in size, staged as T2 or T3-superficial N0-M0. Patients will be randomized to two areas: CRT plus TEM or radical surgery (TME). Postoperative morbidity and mortality will be recorded and patients will complete the quality of life questionnaires before the start of treatment, after CRT in the CRT/TEM arm, and 6 months after surgery in both arms. The estimated sample size for the study is 173 patients. Patients will attend follow-up controls for local and systemic relapse.
Conclusions
This study aims to demonstrate the preservation of the rectum after preoperative CRT and TEM in rectal cancer stages T2–3s, N0, M0 and to determine the ability of this strategy to avoid the need for radical surgery (TME).
Trial registration
ClinicalTrials.gov identifier: NCT01308190. Número de registro del Comité de Etica e Investigación Clínica (CEIC) del Hospital universitario Parc Taulí: TAU-TEM-2009-01.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Local surgery for superficial T2–3, N0, M0 rectal cancer is limited by node involvement, which reaches rates of between 12 and 28% [1]. Recurrence after local excision is higher than 20%, a figure considered unacceptable. Recent meta-analyses of local surgery followed by adjuvant therapy in these tumors have not reported any great improvements, with rates between 11 and 19% [2]. Therefore, the treatment of choice for T2–3s, N0, M0 rectal cancer in the middle and lower third is total mesorectal excision (TME), which achieves local recurrence rates of between 2 and 11% and systemic recurrence between 2 and 13%; however, the morbidity and mortality rates associated with TME are high, at 30–40 and 2%, respectively [3]. TME patients usually require temporary or permanent ostomies and the genitourinary and sexual dysfunction alterations reach non-negligible rates of between 20 and 30% [4, 5]. These circumstances directly compromise the quality of life of these patients.
To a large extent, transanal endoscopic microsurgery (TEM) is able to correct these problems. First described by Buess [6], TEM is an endoscopic procedure that preserves the sphincter apparatus and allows access to rectal tumors located up to 20 cm from the anal verge. Postoperative morbidity rates are below 10% and mortality is close to 0%, and no genitourinary alterations or sexual dysfunctions have been reported [7, 8].
Attempting to preserve the rectum, the combination of preoperative chemoradiotherapy (CRT) and local excision has been proposed as a less aggressive alternative to TME, since it appears to obtain similar results [9, 10]. The main objective of neoadjuvant treatment is to achieve the highest percentage of pathological complete response (pCR) and thus to obtain the best oncological results. The pCR values published in the literature for locally advanced rectal cancer range from 15 to 27% [11]. In T2 and T3, they vary widely, between 11.7 and 73% [12]; a recent study by the group leading the present study found a rate of 37.5% [13].
In order to address the issues of local and systemic disease and quality of life, since 2010, our group has led a randomized, controlled, prospective, multicenter clinical trial of T2–T3 (superficial) N0, M0 rectal cancer, comparing the combination of neoadjuvant treatment (CRT) plus TEM with standard TME.
Hypothesis and objectives
Hypothesis
In patients with rectal adenocarcinoma (ADK) located less than 10 cm from the anal verge and with a size of 4 cm or less, staged as T2-N0-M0 and superficial T3-N0-M0 by endorectal ultrasound (ERUS) and magnetic resonance imaging (MRI), who undergo preoperative CRT and subsequent local surgery (TEM), the efficacy results will be similar to those obtained radical surgery (TME) in terms of local recurrence and tolerance; in addition, quality of life will be improved.
Objectives
The primary objective is to compare local recurrence at 2 years in patients treated with preoperative CRT and TEM and in patients undergoing conventional radical TME surgery.
The secondary objectives are to compare the 3-year survival results in patients treated with preoperative CRT and TEM or with conventional radical TME surgery and to evaluate postoperative morbidity and mortality and quality of life in patients undergoing one or other treatment, both before and after the procedure. The following measures will be taken:
-
Study of postoperative morbidity and mortality in the groups assessed, using the Dindo-Clavien classification [14] and the Comprehensive Complication Index (CCI) [15].
-
Comparison of specific elements with a bearing on quality of life; bladder, urinary, and sexual dysfunction, and the need for an ostomy in the two groups.
-
Assessment of quality of life and morbidity associated with preoperative CRT in the local surgery group.
-
Study of the clinical and histological response of patients undergoing preoperative CRT and subsequent local surgery (TEM).
Methods/design
Study design
Prospective, multicenter, randomized controlled non-inferiority trial of the treatment of T2–T3 (superficial) N0, M0 cancer with preoperative CRT and TEM versus TME.
Recruitment and scope of the study
To achieve the predetermined sample size and to increase the external validity of the study, a multicenter design will be used.
Spanish hospitals with specialized colorectal surgery, radiodiagnosis, oncology, and radiotherapy units will be enlisted. The hospitals included are Hospital Universitario Parc Tauli de Sabadell, Hospital Clínic de Barcelona, Hospital de Sant Pau de Barcelona, Hospital de Bellvitge, Hospital de la Vall d’Hebron de Barcelona, Hospital General Universitario de Valencia, Hospital La Paz de Madrid, Hospital de Getafe de Madrid, Hospital Marqué de Valdecillas, Hospital Torrecardenas de Almería, Hospital del Mar, Hospital Fundación Jiménez Díaz, Hospital Reina Sofía de Córdoba, Hospital de Sagunto, Hospital Cabueñes de Gijón, Hospital Universitario de Donostia, Hospital General Universitario de Elche, and Hospital Universitario Joan XXIII de Tarragona.
Patients: definition of the study population
All patients diagnosed with a rectal tumor will undergo a complete colonoscopy with multifocal biopsy to indicate the tumor’s distance from the anal verge and its size. Endorectal ultrasound (ERUS), pelvic magnetic resonance imaging (MRI), and abdominal computed tomography (CT) will be performed. Tumor markers CEA and CA 19.9 will be determined preoperatively. Preoperative staging with ERUS will apply the criteria described by Hildebrandt [16], and staging with pelvic MRI the criteria described by Brown [17, 18]. T3 tumors will be subdivided into two groups: superficial, when tumor invasion of the mesorectum is less than 4 mm, and deep when invasion is 4 mm or more [19].
The maximum diameter of the tumor will be measured in order to apply the RECIST (Response Evaluation Criteria In Solid Tumors) [20] criteria and thus assess the response of patients to neoadjuvant treatment.
Inclusion criteria
Inclusion criteria are as follows: rectal ADK located within 10 cm of the anal verge, measured by rigid rectoscopy at the time of ERUS; preoperative staging of T2 N0 by ERUS and pelvic MRI; in the case of disparity, the more advanced staging will be considered as definitive diagnosis; superficial T3, N0 by MRI, when the tumor invasion of the mesorectum is less than 4 mm; tumors with a maximum diameter of 4 cm or less as measured by MRI; ASA Index III or less; the absence of distant metastases on abdominal CT and chest X-ray (if not conclusive, chest CT); pathological variables in the TME specimen (distal margin more than 2 cm, circumferential margin more than 1 mm, the presence of more than 12 nodes in the specimen); TEM pathology specimen with full thickness excision and minimal distance of 1 mm between the tumor and the lateral and deep resection margin.
Exclusion criteria
Exclusion criteria are as follows: preoperative staging by ERUS or pelvic MRI of T1, deep T3–T4 or N1–2; the presence of distant metastases; synchronicity with other colorectal ADKs; radiotherapy; failure to sign informed consent.
Patients who meet these exclusion criteria will be assigned to the conventional cancer protocol.
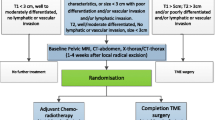
All patients who meet the inclusion criteria will complete two quality of life questionnaires validated by the EORTC (European Organization for Research and Treatment of Cancer) [21]. A general questionnaire, the EORTC QLQ-C30 (version 2) [22], and the specific module for colorectal cancer, EORTC QLQ-CR38 [23], and also the Karnofsky Scale to measure general QoL [24]. Figure 1 shows the outline of the study protocol.
Trial flow chart. FCS fibrocolonoscopy, ERUS endorectal ultrasound, MRI magnetic resonance, CT CT scan, ADK adenocarcinoma, ASA American Society of Anesthesiologists, CRT chemoradiation, TEM transanal endoscopic microsurgery, TME total mesorectal excision. EORTC QLQ C-30/CR38, Karnofsky: quality of life questionnaires
Informed consent and legal considerations
Patients who meet the inclusion criteria and provide signed informed consent will enter the randomization process.
The study protocol, patient information, and informed consent documents have been approved by the Clinical Research Ethics Committees of all participating testing centers, in accordance with Spanish Royal Decree 1090/2015. The Ethics Committee of the Parc Taulí University Hospital is the reference (ID: TAU-TEM-2009-01). The trial has been recorded in the ClinicalTrials.gov database (ID: NCT01308190) and in the EU Clinical Trials Registry database (EudraCT number: 2009-014310-94). Prior to randomization, all patients must give signed informed consent to participate in the clinical trial.
This test is carried out in accordance with the 7th revision of the Declaration of Helsinki [25], the SPIRIT 2013 Standard Protocol Articles for Clinical Trials [26], and the Spanish laws and regulations for biomedical research, with the authorization of the Spanish Agency of Medicines and Medical Products.
Description of randomization
Patients will be randomly assigned to one or other group: CRT/TEM or TME (1:1) using a computer-generated list of random numbers centralized by the Contract Research Company (CRO) “Effice Servicios para la Investigación SL.”
Procedure
All patients will undergo mechanical preparation of the colon prior to surgery. The standard antibiotic and thromboembolic prophylaxis protocol will be applied. Patients will complete the EORTC QLQ-C30, EORTC QLQ-CR38, and Karnofsky quality of life questionnaires either at the time of admission or previously at the outpatient clinic.
CRT/TEM group
ᅟ
Neoadjuvant treatment: preoperative CRT
Neoadjuvant treatment with chemotherapy will be performed concomitantly with radiotherapy and will consist of the administration of capecitabine 825 mg/m2 every 12 h orally on radiotherapy days. Radiotherapy is given in daily fractions of 1.8 Gy 5 days a week according to the standard regimen [27, 28]. The total dose will be 45 Gy plus a boost of 5.4 Gy in the tumor area.
Seven weeks after the end of the CRT, the clinical response will be evaluated by means of a new MRI. Standard protocols for monitoring morbidity and mortality secondary to CRT will be followed.
Surgical technique: 8th week after neoadjuvant treatment
Local surgery applying transanal endoscopic surgery includes transanal endoscopic microsurgery (TEM) [6], transanal endoscòpic operation (TEO) [29], or transanal minimally invasive surgery (TAMIS) [30].
The patient will be operated under general or spinal anesthesia. Surgery will comprise excision with lesion margins superior to 15–20 mm. Full thickness resection will be followed by washing with physiological saline plus povidone iodine 1%. Suturing of the wall defect will also be followed by washing with saline plus povidone iodine 1%.
Radical surgery group (TME)
Surgical technique: Surgery will be carried out within 1 month of the first outpatient visit. The classical TME procedure described by Heald [31] will be performed, with the choice of one of the following techniques depending on the tumor location and on the criteria applied at the particular service: TME-abdomino-perineal resection (APR), TME-lower anterior resection (LAR), TME-LAR and TME-APR laparoscopy, TME-LAR laparoscopy, TME-LAR and loop ileostomy-laparoscopy, transanal TME and loop ileostomy.
Pathology study
Two classifications are used to study the degree of histological regression in rectal tumors treated with chemoradiotherapy: the Bouzourene [32] and the Dworak classifications [33].
The presence of a correct histological response after chemoradiotherapy is determined by the pathology study of the tumor excised after TEM, in order to establish TRG1, TRG 2, or TRG 3 in Bouzourene’s classification, or TRG 4, TRG 3, or TRG 2 in Dworak’s classification.
The pathology study of the local surgery specimen will determine the size of the lesion (maximum and minimum diameter in mm), the degree of ADK differentiation, the degree of response to CRT, according to Bouzourene’s or Dworak’s tumor regression grade, T stage, presence of venous, lymphatic, or perineural infiltration, and margins of the TEM specimen (mm).
The pathology study of the TME specimen will determine the size of the lesion (maximum and minimum diameter in mm); the degree of ADK differentiation; the presence of venous, lymphatic, or perineural infiltration; and margins of the TME specimen, distal, and circumferential in millimeters. N stage (nodes found, nodes affected).
Definition of clinical and pathological responses to preoperative chemoradiotherapy
Definition of the possible clinical responses of the tumor 7 weeks after preoperative CRT, prior to local surgery (TEM). According to the RECIST [20] criteria by MRI: Complete response: Complete disappearance of tumor. Partial response: Reduction of maximum diameter of the lesion by more 30%. Disease stabilization: Reduction of diameter by less than 30% or increase in size of less than 20%. Disease progression: increase in maximum lesion diameter above 20%.
By MRI: Onset or growth of non-target lesions (lymphadenopathies), Reduction in T stage.
Histological response to CRT according to the degree of regression: TRG1, TRG 2, or TRG 3 in Bouzourene’s classification. TRG4, TRG3, or TRG2 in Dworak’s classification.
Patients in the CRT-TEM group with complete or partial clinical response or pathological response will enter the comparative per protocol analysis with conventional surgery (TME).
Criteria for abandoning protocol
Patients who after neoadjuvant treatment present the following: absence of clinical response: disease stabilization, disease progression, and absence of histological response. Degree of response to CRT of TRG 4 or TRG 5 in Bouzourene’s classification or TRG 1 or TRG 2 in Dworak’s classification.
Patients who present the following features in the pathology study of the tumor after TEM: characteristics of poor prognosis (lack of differentiation, venous, lymphatic, or perineural infiltration), a stage superior to superficial T3, N0 (ypT3), surgical margin affected.
Patients with criteria for abandoning the study will be rescued with conventional radical surgery within 4–6 weeks. These patients will be analyzed independently on an intention-to-treat basis.
Follow-up (Fig. 1)
EORTC QLQ-C30, EORTC QLQ-CR38, and Karnofsky quality of life questionnaires will be administered to all patients in both groups 6 months after surgery.
CRT/TEM group
During the first 2 years, rectosigmoidoscopy will be performed every 4 months along with a multifocal scar biopsy (if the biopsy is positive for adenocarcinoma, ERUS, MRI, and measurements of tumor markers CEA and CA 19.9 will be performed every 4 months. Abdominal-pelvic CT and chest X-ray every 6 months). Full colonoscopy annually: if normal, the follow-up protocol in place at each center will be applied.
TME group
During the 2-year follow-up: measurement of tumor markers (CEA and CA 19.9) every 4 months. Abdominal-pelvic CT and chest X-ray every 6 months. Full colonoscopy annually: if normal, the follow-up protocol in place at each center will be applied.
Subsequently, the two groups will follow the protocolized follow-up at each center.
Study variables
Primary outcome
Local recurrence defined as the presence of ADK in the biopsy on the residual scar, anastomosis, or the defect area of the excised tumor.
Secondary outcomes
Survival, disease-related mortality, and quality of life were determined using the questionnaires described.
Other variables to be recorded
Demographic: age, sex, hospital of origin.
Preoperative: prior to neoadjuvant treatment: lesion size (colonoscopy, ERUS, and MRI), lesion height, tumor location (anterior, posterior, right or left lateral), degrees of the circumference occupying the rectum, ERUS staging, MRI staging (tumor size in mm), the presence of distant metastases by abdominal CT, preoperative CEA, and CA 19.9, pathology biopsy.
After preoperative neoadjuvant treatment: acute and chronic adverse effects of radiotherapy, acute, and chronic adverse effects of chemotherapy. Assessment of the clinical response of chemoradiotherapy by MRI: tumor size (mm). Measurement of the maximum diameter of the tumor.
Surgical: surgical technique, surgical time, estimated blood loss, perioperative complications, conversion after TEM, single TEM specimen, and defect suture.
Pathology: lesion size, degree of differentiation, degree of response to CRT, T stage, N stage (in TME), the presence of venous, lymphatic, or perineural infiltration. Margins in TEM specimen, margins in TME specimen.
Postoperative (30 days post-surgery): nosocomial, surgical, and non-surgical postoperative complications; complications according to the Dindo-Clavien classification and the CCI (Comprehensive Complication Index); hospital stay.
Follow-up: date of local recurrence, date of distant recurrence, mortality due to rectal cancer, date of death, and mortality due to other pathologies.
Statistical analysis
Predetermination of sample size
Assuming a one-sided 5% significance level, a power of 80%, and non-recurrence rate of 95% in the control group (TME) and a non-inferiority limit of 10% for the experimental group (TEM), 78 patients will be needed for each group. Bearing in mind the estimated losses during follow-up, the total of 173 patients will be recruited.
Analysis of patients by intention to treat, per protocol, and patients lost
Intention-to-treat analyses (ITT) of all randomized patients will be performed.
Per protocol analyses will be performed of all patients in the TME group and of all patients undergoing surgery, and in the CRT-TEM group, all those receiving at least one dose of chemotherapy.
Statistical analysis of the lost patients will be performed.
Monitoring
Monitoring will be carried out by CRO Effice Servicios para la Investigación S.L. and a data manager. The variables will be recorded on an Access database which the data manager will manage via the various hospitals.
Statistical analysis
To evaluate the main objective of the study, i.e., the non-inferiority (10%) of the TEM experimental treatment in terms of non-relapse, a one-sided 95% confidence interval of the difference in the percentage of non-relapse between the two groups [34, 35] will be used in both the intention-to-treat and the per protocol samples.
The quantitative variables will be described as means and standard deviations or as medians, interquartile range and range, as appropriate. Categorical variables will be described in absolute numbers and percentages.
The statistical analysis of the quantitative variables, with independent groups, will be performed with the parametric Student’s t test, provided that its conditions for application are met. Otherwise, the non-parametric Mann-Whitney U test will be used.
Statistical analysis for categorical variables will use the Pearson Χ 2 test or the Fisher exact test.
Specifically, the above methods will be used to compare the two groups in terms of baseline characteristics in order to assess whether the randomization has been effective.
Statistical analysis of the survival analysis of relapse and mortality will be performed using the Kaplan-Meier estimation method and the log-rank test.
A p value below 0.05 will be considered statistically significant.
Discussion
TME is the standard treatment for rectal ADK staged T2, N0, M0, or above. However, it is associated with high morbidity and a reduced quality of life due to the resection of the rectum and the need for an ostomy. This situation has prompted the search for other less aggressive therapeutic strategies, such as a neoadjuvant approach in combination with local surgery.
Several methods are used for the local excision of rectal tumors. With the classic endoanal surgery, it is difficult to ensure full thickness excision and free margins, and this approach is limited by the height of the lesion with respect to the anal verge. Transanal endoscopic microsurgery (TEM) overcomes these limitations [6, 8]. Since the introduction of TEM, other forms of transanal endoscopic surgery have been described such as TEO (transanal endoscopic operations) [29] and TAMIS [30] (transanal minimally invasive surgery), with the intention of simplifying the technique and the equipment required.
The controversial study published by Habr-Gama [10]assessed the effect of CRT in advanced stage distal rectal cancer, reporting complete clinical or pathological response in up to 30% of patients. Neoadjuvant CRT has reduced local recurrence rates and has increased survival in patients with stage pT3–4 pN0 or any pT pN1–2 rectal cancer [36, 37]..
In our study, we include superficial T3 N0, M0 ADKs. Merkel et al. reported 5-year survival rates of 54% in patients with deep T3 tumors and 85% in those with superficial T3 tumors, regardless of lymph node involvement [38]. The results are thus similar to those obtained for T2, N0, M0 cancers.
The vast majority of pCRs published in the literature have been achieved with long cycles of CRT, similar to the one described in our study. Short CRT cycles do not obtain the same results, and postoperative morbidity rates are higher [39]. In an attempt to improve the results for pCR, some authors have changed the CRT regimens. Combining capecitabine in standard doses and oxaliplatin with RT, García Aguilar et al. achieved a pCR rate of 48% [40]; however, 44% of patients had adverse effects of grade ≥ 3, which required a reduction in the capecitabine dose. At the reduced dose, a pCR rate of 36% was obtained and the incidence of adverse events grade ≥ 3 fell to 30%. In a recent publication reviewing, the results of that study, with a mean follow-up of 56 months, 6% of the 79 patients had distant metastases and 4% local recurrence [41].
Complete clinical response (cCR) does not always coincide with pCR. In a previous study by our group, 12 out of 24 patients (50%) presented cCR, while nine (37.5%) presented pCR. Therefore, three out of 24 patients considered to have cCR would have relapsed if we had applied the “wait and see” policy. For this reason, we consider this strategy to be dangerous and advocate excision of the lesion in order to confirm cPR [13].
No previous studies have assessed the impact of complications measured by the Clavien-Dindo classification and the Comprehensive Complication Index. The studies performed to date have considered that the morbidity associated with TEM in combination with neoadjuvancy is unacceptable [42].
Another important strength of the present study is the measurement of quality of life in the two groups of patients. As noted above, radical abdominal surgery controls the disease but at the price of sexual alterations, urinary dysfunctions, and the need for a temporary or definitive ostomy [43]. To date, quality of life in these two groups of patients has not been compared.
Similar studies which have been published or are in the final stages include “Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial” [44], by the Memorial Sloan Kettering Cancer Center, but the main objective of that study was to assess consolidation and induction chemotherapy in an evaluation protocol that allows non-surgical observational treatment. In Europe, the UK-TREK study at the University of Birmingham and the already finalized GRECCAR 2 in France [45] used different designs but had the same objective with regard to neoadjuvant treatment and local surgery, from the point of view of control of local and systemic disease. But none of these studies have compared t quality of life in patients with superficial T2–3, N0, M0 rectal cancer treated with neoadjuvant CRT plus local excision or with radical surgery.
References
Sun G, Tang Y, X L, Meng J, Liang G (2014) Analysis of 116 cases of rectal cancer treated by transanal local excision. World J Surg Oncol 12:202
Borstlap WAA, Coeymans TJ, Tanis PJ, Marijnen CAM, Cunningham C, Bemelman WA, Tuynman JB (2016) Meta-analysis of oncological outcomes after local excision of pT1–2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg 103:1105–1116
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240:260–268
Kneist W, Junginger T (2004) Residual urine volume after total mesorectal excision: an indicator of pelvic autonomic nerve preservation? Results of a case-control study. Color Dis 6:432–437
Shah EF, Huddy SPJ (2001) A prospective study of genito-urinary dysfunction after surgery for colorectal cancer. Color Dis 3(122):5
Buess G, Hutterer F, Theiss J et al (1984) A system for a transanal endoscopic rectum operation. Chirurg 55:677–680
Lee W, Lee D, Choi S, Chun H (2003) Transanal endoscopio microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 17:1283–1287
Serra Aracil X, Bombardó Junca J, Mora López L, Alcántara Moral M, Ayguavives Garnica I, Navarro Soto S (2006) La microcirugía endoscopica transanal (TEM). situacion actual y expectativas de futuro. Cir Esp 80:123–132
Serra Aracil X, Bombardó Juncá J, Mora López L et al (2009) Lugar de la cirugía local en el adenocarcinoma de recto T2N0M0. Cir Esp 85:103–109
Habr-Gama A, Perez RO, Nadalin W et al (2004) Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 240:711–717
Maas M, Nelemans PJ, Valentini V et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844
Coco C, Rizzo G, Mattana C et al (2013) Transanal endoscopic microsurgery after neoadjuvant radiochemotherapy for locally advanced extraperitoneal rectal cancer: Short-term morbidity and functional outcome. Surg Endosc 27:2860–2867
Serra-Aracil X, Pericay C, Mora-Lopez L, Garcia Pacheco JC, Latorraca JI, Ocaña-Rojas J, Casalots A, Ballesteros E, Navarro-Soto S (2017) Neoadjuvant therapy and transanal endoscopic surgery in T2-T3 superficial, N0, M0 rectal tumors. Local recurrence, complete clinical and pathological response. Cir Esp 95:199–207
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Slankamenac K, Graf R, Barkun J et al (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258:1–7
Hildebrandt U, Feifel G (1985) Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon rectum 28:42–46
Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, Bourne MW, Williams GT (1999) Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 211:215–222
Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 227:371–377
Mercury study grup (2007) Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 243:132–139
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van OosteromAT CMC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Arraras JI, Arias de la Vega F, Vera R, Manterota A, Martinez M, Villafranca E, Salgado E (2006) Quality of life assessment through the EORTC questionnaires of locally advanced rectal cancer patients treated with preoperative chemo-radiotherapy. Clin Transl Oncol 8:423–429
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Sprangers MA, te Velde A, Aaronson NK (1999) The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 35:238–247
Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH (1948) The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1:634–656
Emanuel EJ (2013) Reconsidering the Declaration of Helsinki. Lancet (London, England) 381:1532–1533
Chan A-W, Tetzlaff JM, Altman DG et al (2013) SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 158:200–207
Mohiuddin M, Regine WF, John WJ et al (2000) Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys 46:883–888
Ahmad NR, Marks G, Mohiuddin M et al (1993) High dose preoperative radiation for cancer of the rectum: impact of radiation dose on patterns of failure and survival. Int J Radiat Oncol Biol Phys 27:773–778
Rocha JJ, Feres O (2008) Transanal endoscopic operation: a new proposal. Acta Cir Bras 23:93–104 discussion 104
Atallah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24:2200–2205
Heald RJ, Ryall RDH (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1:1479–1482
Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P (2002) Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 94:1121–1130
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Color Dis 12:19–23
Kaul S, Diamond GA (2006) Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 145:62–69
Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ, CONSORT Group (2006) Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 295:1152–1160
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postopreative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
(1990) NHI consensus conference: adjuvant therapy for patients with colon and rectal cancer. JAMA 264:1444–1450
Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P (2001) The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Color Dis 16:298–304
Arezzo A, Arolfo S, Allaix ME et al (2015) Results of neoadjuvant short-course radiation therapy followed by transanal endoscopic microsurgery for T1-T2 N0 extraperitoneal rectal cancer. Int J Radiat Oncol Biol Phys 92:299–306
Garcia-Aguilar J, Shi Q, Thomas CR Jr et al (2012) A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 19:384–391
Garcia-Aguilar J, Renfro LA, Chow OS et al (2015) Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 16:1537–1546
Perez RO, Habr-Gama A, GP S˜o J˜o, Proscurshim I, Scanavini Neto A, Gama-Rodrigues J (2011) Transanal endoscopic microsurgery for residual rectal cancer after neoadjuvant chemoradiation therapy is associated with significant immediate pain and hospital readmission rates. Dis Colon rectum 54:545–551
Arraras JI, Arias de la vega F, Vera R, Manterota A, Martinez M, Villafranca E, Salgado E (2006) Quality of life assessment through the EORTC questionnaires of locally advanced rectal cancer patients treated with preoperative chemo-radiotherapy. Clin Transl Oncol 8:423–429
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, Guillem JG, Paty PB, Avila K, Garcia-Aguilar J, Rectal Cancer Consortium (2015) Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 15:767
Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A (2017) Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 390:469–479
Acknowledgements
We thank all the members of the multidisciplinary committee for colorectal tumors at our hospital for their support. We are also grateful to Cristina Gomez Vigo for correcting the manuscript and Michael Maudsley for help with the English.
TAU-TEM study group
Hospital Parc Taulí: Xavier Serra-Aracil, Carles Pericay, Laura Mora, Sheila Serra, Eugeni Saigi, Emma Dotor, Aleidis Pisa, Ismael Macias, Anna Pallisera, Salvador Navarro. Hospital Clinic I Provincial: Antonio Lacy, Anna Otero. Hospital de Bellvitge: Sebastiano Biondo, Thomas Golda. Hospital de la Santa Creu i Sant Pau: Eduardo Tarragona, Pilar Hernández; Mª Carmen Martínez, Juan Carlos Pernas, Marta Martín, Dolores González, David Paez, Xavier Cussó, C. Balagué. Hospital General Universitari de Valencia: Mª José García Coret, Francisco Villalba Ferrer. Hospital Universitario La Paz: Beatriz Díaz San Andrés, Álvarez Gallego, Higuera, Prieto. Hospital Universitario de Getafe: Jose Luis Ramos, Javier Jiménez Miramó, Javier García Septiem, Francisco Angulo. Hospital Marqués de Valdecilla: Julio Castillo, Joaquín Alonso Martín, Isabel Seco, Carlos Manuel Palazuelo. Hospital Torrecárdenas de Almeria: Ángel Reina, Francisco A. Rubio Gil, Carmen Caro, Rubén Varela, Fco. Manuel Ramos, Ana Fernández, Ricardo Belda, Ramon Solbes, Begoña Medina, Piedad Reche. Hospital Universitari Vall d’Hebrón: Eloy Espín, Francesc Vallribera, Stefania Landolfini, Jaume Capdevila. Hospital del Mar: Marta Pascual, Silvia Salvans, Miguel Pera. Hospital Reina Sofía. Córdoba: César Díaz, Jose Gomez Barbadillo, Amalia Palacios, Carlos Villar Pastor, María Pleguezuelo, Francisco Triviño, José L. Martínez de Dueñas, Auxiliadora Gómez España y Elena Navarro Rodriguez. Hospital de Sagunto: Roberto Lozoya Trujillo, Andrés Frangi, Mª Dolores Ruiz Carmona, Rodolfo Rodríguez Carrillo, Mireia Gil, Vicente Miranda. Hospital de Cabueñes. Gijón: Carlos Álvarez Laso, Paola Lora. Hospital de Donosti: José Mª Enriquez Navascues, Carlos Placer, Dra. Nerea Borda, Adelaida La Casta, JL Elosegui, Yolanda Saralegui, Elena Guimón, JA Múgica. Hospital General Universitario de Elche: Javier Gallego Plazas, Antonio Arroyo. Hospital Universitari Juan XXIII: Aleidis Caro, Monica Millan.
Funding
Funding obtained by the principal investigator (PI): Xavier Serra-Aracil, Olga Torres Grant, Grant from the Ministry of Health and Social Policy, Independent Clinical Research grants Ayudas de Investigación Clínica Independiente, Parc Taulí Foundation grant, and Spanish Coloproctology Foundation grant.
Author information
Authors and Affiliations
Consortia
Contributions
XSA, CP, and LM wrote and edited the paper. TG, AR, SD, and ET will contribute patients to the study and are active in the management of the protocol. All authors have reviewed the research protocol, revising it critically for intellectual content. Each author has participated sufficiently in the work of reviewing and approving the protocol as written. In addition, FV, JMEN, AA, MP, JC, CD, AC, and RL will contribute patients and have reviewed and approved the protocol. JCGP and SS are the clinical research managers of the trial.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study has been approved by the local ethics committees of the participating centers.
Availability of data and materials
Not applicable.
Rights and permissions
About this article
Cite this article
Serra-Aracil, X., Pericay, C., Golda, T. et al. Non-inferiority multicenter prospective randomized controlled study of rectal cancer T2–T3s (superficial) N0, M0 undergoing neoadjuvant treatment and local excision (TEM) vs total mesorectal excision (TME). Int J Colorectal Dis 33, 241–249 (2018). https://doi.org/10.1007/s00384-017-2942-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-017-2942-1