Abstract
Background
Transanal endoscopic microsurgery (TEM) after radiochemotherapy (RCT) has been reported in selected cases of locally advanced rectal cancer as an alternative to traditional radical resection with total mesorectal excision with a curative intent or as diagnostic tool to confirm a pathological complete response of the primary tumor. No study has evaluated functional outcome after TEM in preoperatively irradiated patients.
Methods
This study was designed to evaluate short-term morbidity (according to Clavien’s classifications) and establish (by a questionnaire) continence and evacuative function after RCT and TEM, at 1 year from surgery, analyzing the impact of RCT on postoperative outcomes. Patients with locally advanced rectal cancer treated by RCT and TEM (group 1) or with early T1 or adenomas treated only by TEM (group 2) entered this cohort comparative study.
Results
Twenty-two patients entered the study as group 1 and 25 as group 2. No postoperative mortality occurred. The morbidity rate was 36.4 % in group 1 vs. 16 % in group 2 (p = 0.114). The rate of suture dehiscence was 22.7 % in group 1 vs. 4 % in group 2 (p = 0.068). No grade III complications, reoperation, or hospital readmission within 30 days was recorded in either group. One year after surgery, continence and evacuative scores in group 1 were 1.05 ± 1.25 and 24.72 ± 2.79, respectively, which were similar to group 2 (p = 0.081 and 0.288, respectively).
Conclusions
TEM after RCT in selected rectal cancer patients has an acceptable morbidity and functional results at 1 year from surgery. Preoperative irradiation could increase postoperative short-term morbidity, but it does not seem to influence evacuative or sphincter function after 1 year from surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Large, randomized, controlled trials have established radiotherapy (RT) or radiochemotherapy (RCT) followed by total mesorectal excision (TME) as the “gold standard” for the treatment of locally advanced rectal cancer [1, 2]. Neoadjuvant RCT plays an important role in local disease control and tumor regression by significantly reducing tumor size (downsizing), the depth of tumor penetration, and the risk of nodal metastases (downstaging) [1].
TME, since its introduction in 1982, has significantly reduced the rate of local recurrences and improved overall survival compared to surgery without TME [3, 4]. However, it is associated with significant postoperative short-term morbidity (range 20–35 %) and mortality (range 1–4 %) [5, 6]. Moreover, patients who undergo TME frequently experience evacuative dysfunction, commonly known as “anterior resection syndrome,” including gas and fecal incontinence, urgency, a sensation of incomplete rectal emptying, inability to defer defecation, and clustering of bowel movements [7]. Randomized, clinical trials have shown a significant increase of these disorders after neoadjuvant treatment, and it is not clear how important radiation treatment is in these symptoms [8, 9].
For these reasons and because of the increasing incidence of pathological complete response after RCT [10–13], local excision after RCT, especially using transanal endoscopic microsurgery (TEM), has been recently reported in the literature in selected cases as a valid alternative treatment to TME [14, 15]. Our group reported the use of TEM as an essential diagnostic step in clinical complete response to offer a more conservative treatment rather than radical resection to patients with pathological complete response of the primary tumor [16]. The selection of patients is essential in both cases.
The main advantage after local excision by TEM is to preserve the anatomical and functional integrity of the rectum to avoid the functional disorders that are frequent after radical surgery. Authors who have analyzed functional outcome after TEM have reported no change in evacuative function or sphincter parameters between the preoperative and postoperative periods [17–19]. However, in these series, no patients were preoperatively treated with RCT, and to the best of our knowledge, no studies have evaluated functional outcome in patients submitted to TEM after preoperative RCT. Moreover, recent studies evaluated postoperative outcome of patients submitted to TEM after neoadjuvant RT or RCT and reported a significantly higher rate of postoperative morbidity, especially suture dehiscence, compared with patients who underwent TEM without preoperative pelvic irradiation [20, 21].
The purpose of the current study was to evaluate and analyze postoperative mortality, short-term (within 30 days) morbidity, and evacuation and continence function at 1 year from surgery in patients submitted to TEM after RCT, quantifying the influence of preoperative irradiation on postoperative outcomes.
Materials and methods
From 2000 to 2010, all patients affected by extraperitoneal rectal cancer were enrolled in a pretreatment workup, including digital examination, colonoscopy with biopsy, endorectal ultrasound, abdominal computed tomography scan, pelvic magnetic resonance imaging, positron emission tomography (PET) scan, and chest x-ray. Patients with early (cT1-2 N0 M0) extraperitoneal (up to 12 cm from the anal margin) rectal cancer were submitted directly to surgery with a TME or with a local excision by TEM (in selected cT1 cases). If pretreatment workup staged the cancer as nonmetastatic locally advanced (T3-4 N0 M0/any T N + M0), the patients were treated with neoadjuvant long-term RCT. Radiation therapy consisted of 50.4 Gy of external-beam radiation therapy delivered by a 3-field approach with daily doses of 1.8 Gy on weekdays to the pelvis. During the study, different protocols of chemotherapy were adopted: cisplatin and 5-fluorouracil (5-FU PVI protocol) [22], raltitrexed and oxaliplatin (TOMOX protocol) [23], or oxaliplatin and capecitabine (CAPOX [24] and XELOX protocols [25]).
Six weeks after the end of RCT, the patients underwent restaging examinations (endoscopy, endorectal ultrasound, pelvic magnetic resonance, PET scan) to estimate the tumor response to RCT. The clinical response to RCT was assessed according to the World Health Organization score [26] using a reference index (IND) defined as the product of the quarter of the circumference of the rectal wall involved (categorized 1–4) multiplied by the craniocaudal length of the tumor in mm [27]. This index was calculated at the time of initial diagnosis (IND-pre) and 6 weeks after radiation (IND-post). Patients with a complete (absence of residual disease) or partial (IND-post < 50 % IND-pre) response were considered responders to RCT; all others (no change or progression) were considered nonresponders. We defined a clinical complete response, at restaging, as there was a negative pelvic magnetic resonance and PET scan and, at endoscopy, the absence of macroscopic intraluminal tumor residue or the presence of only a small scar at the site of the tumor.
Standard surgical treatment was radical resection with TME, anterior resection, or abdominoperineal resection (APR), performed 8 weeks after the completion of RCT. TEM was performed in selected cases: patients unfit for or refusing radical surgery with TME or patients in whom a clinical complete response to RCT was obtained, to assess the pathological complete response of the primary tumor. All patients preoperatively signed an informed consent. TEM was performed under general anesthesia, using Richard Wolf’s (Knittlingen, Germany) TEM equipment, according to the standard technique described by Buess et al. [28]. The patients were positioned in the lithotomy, prone or lateral position, depending on the location of the lesion. Only one surgeon (C.C.) performed TEM. In all patients, a full-thickness excision was performed and the wound was closed with one or more running sutures (Biosyn 3/0) secured with silver clips. All patients had a urinary catheter in place at the time of surgery, which was removed 24 h after operation.
All patients were given antibiotics with gram-negative, aerobic and anaerobic coverage, e.v., ½ hour before and for 2 days after surgery and orally for the next 4 days. Narcotics were prescribed on demand and included ketorolac 10 mg or paracetamol 1,000 mg. The specimens were staged according to TNM system [29], and tumor response after neoadjuvant RCT was evaluated according to Mandard’s tumor regression grade [30]. When specimen examination confirmed a complete (ypT0 and TRG1) or nearly complete (ypT1 and TRG2) pathological response with margins free of tumors, no adjunctive radical surgery was proposed to the patient. This group of patients were enrolled in an intensive follow-up program, including digital examination, assessment of blood carcinoembryonic antigen, endoscopy, pelvic magnetic resonance, and PET/computed tomography scan. In all other cases (pT >1 or pT1 TRG >2), immediate (within 1 month) radical surgery with TME was suggested. Short-term (within 30 days) postoperative morbidity and mortality were recorded, and complications were graded according to the classification proposed by Clavien and colleagues [31].
At follow-up visit 1 year after surgery, the study population was asked to answer a pool of questions to evaluate the anorectal function and quantify evacuative and sphincter disorders. Seven questions were intended to define the evacuative function and considered the following parameters: number of bowel movements/day; sensation of incomplete evacuation, defined as the need, after leaving the toilet, to return within a few minutes for second or multiple evacuations; the necessity to return to the bathroom less than 15 min after evacuation; the ability to evacuate completely in less than 15 min; the ability to defer evacuation more than 15 min; the use of laxatives and/or enemas; and the use of medications for retarding transit. Five questions were used to define continence status: incontinence to gas, liquid, or solid stools; need to wear a pad; and modification of lifestyle.
The answers were subsequently evaluated to establish the frequency of each symptom, defining an evacuation and continence score according to those proposed by Gervaz et al. [32] and Jorge and Wexner [33]. The former evaluates the frequency of each of the seven evacuative parameters on a scale varying from never to always. Each degree of the scale corresponds to a score, varying from 4, the most desirable option, to 0. This allowed us to calculate an evacuation score varying from 0 to 28, with the highest value corresponding to the best function [32]. The Jorge and Wexner continence score evaluates the frequency of each of the five continence parameters that we considered in our questionnaire by the same grading scale as described above. In this index, each degree of the scale corresponds to a score, varying from 0, the most desirable option, to 4 [33]. The calculated continence score can vary from 0 to 20 points, with the lowest value corresponding to the best function. To quantify the incidence of each functional disturbance in the study population, we considered only those patients who complained of the symptom at least once a week to be affected (often/usually or always in the grading scale). Strict adherence to validated questionnaires guaranteed maximally from questioner’s personal interpretation. Apart from the scores, evacuation also was studied by asking questions regarding the patient’s ability to distinguish flatus from stools and pain at defecation.
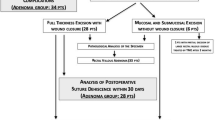
The inclusion criteria for this study were preoperative radiation therapy dose of almost 50.4 Gy, local full-thickness excision of the tumor residue or scar by TEM, no other surgical rectal procedure performed after TEM, no adjunctive postoperative radiation dose, no history of inflammatory bowel disease, no rectal comorbidities, and the absence of local or distant relapses at the time of the questionnaire. Patients operated on by TEM after RCT observing these inclusion criteria entered in the study as group 1.
During the same period (2000–2010), patients with clinically early rectal cancer (pTis, pT1sm1) or with villous adenomas were managed only by TEM, performed by the same surgeon (C.C.). This group of patients were analyzed about short-term postoperative morbidity and investigated, at 1 year from surgery, about long-term functional outcome with the same methods of group 1 patients. The inclusion criteria were local full-thickness excision of the tumor by TEM, no other surgical rectal procedure performed after TEM, no preoperative or postoperative radiation dose, and no history of inflammatory bowel disease. Patients operated only by TEM observing these inclusion criteria entered in the study as group 2. The short-term postoperative morbidity and long-term functional results of group 1 were compared with the results recorded in group 2.
Statistical analysis was performed using the χ2 and Fisher’s exact probability tests for categorical variables and Student’s t test for continuous variables. p < 0.05 was considered statistically significant. This study was approved by the institutional review board.
Results
From Jan 2000 to Dec 2010, a total of 178 patients with locally advanced extraperitoneal rectal cancer were treated with preoperative long-term RCT, were restaged 6 weeks after the end of RCT, and, after 6–8 weeks, underwent surgery. One hundred twenty-five had an anterior resection, 24 had an abdominoperineal resection, 3 had palliative stoma, and 1 patient had a Hartmann resection. Twenty-five patients underwent TEM after neoadjuvant RCT: 22 had a clinical complete response, 2 refused radical surgery with TME after partial clinical tumor response, and 1 was unfit for radical resection. Pathological complete response (ypT0; TRG1) was found in 17 patients (the ratio between pathological complete response and clinical complete response was 77.27 %), and pathological major response (ypT1-TRG2) was found in 3. In the remaining five cases, pathological examination showed one ypT2-TRG2, one ypT2-TRG4, and three ypT3- TRG2, all with clear margins. Two patients with (ypT3-TRG2) agreed to radical resection and, consequently, were excluded from the study: one (ypT3-TRG 2) patient, who previously refused surgery with TME, was submitted to postoperative RT and was excluded from the study; two patients (1 ypT2-TRG2 and 1 ypT2-TRG4) refused radical surgery with TME and were not submitted to postoperative RT. Thus, 22 patients (15 males; median age 63 (range 41–78) years) entered the study as group 1. Neither intraoperative nor short-term (within 30 days) postoperative mortality occurred. Short-term complications occurred in eight patients (36.4 %). Five patients (22.7 %) had a dehiscence of the suture line, all of whom were conservatively treated (2 required prolonged antibiotic therapy, and 3, reporting only rectal pain, required only occasional oral analgesic drugs). One patient had persistent rectal pain, without evidence of dehiscence of the suture line, requiring chronic use of oral analgesic drugs. One patient developed pneumonia, which was treated with antibiotics, and one case had an unspecific fever that required antibiotics. According to Clavien’s classification, grade I complications occurred in four patients (18.2 %), and grade II complications occurred in four patients (18.2 %); no grade III complications occurred. Particularly, dehiscence of the suture line caused grade II postoperative complications in two cases, and no patients who experienced a wound dehiscence required a reoperation or a hospital readmission. None of the eight patients with complications required surgical reintervention. The median hospital stay was 4 days (range 3–12 days), and no hospital readmission within 30 days was recorded.
During the same period, 27 patients (15 male) were operated on by TEM for adenomas (14 patients) or early adenocarcinomas (13 patients: 3 in situ adenocarcinomas, 9 pT1 sm1 adenocarcinomas, and 1 pT2 adenocarcinoma). One patient with low rectal pT1 sm1 adenocarcinoma, after 1 month, was operated on by anterior resection for a synchronous sigmoid adenocarcinoma; the pT2 patient was unfit for radical surgery and was submitted to postoperative radiation therapy. So, 25 patients entered the study as group 2. They had undergone no other rectal surgical procedures or any type of adjuvant treatment. No intraoperative or short-term (within 30 days) postoperative mortality occurred. Short-term postoperative complications occurred in four patients (16 %): one patient had a dehiscence of the suture line, which was conservatively treated with antibiotics; two patients reported occasional rectal pain, which was treated with oral analgesic drugs; and one patient had a urinary tract infection, which was treated with antibiotics. According to Clavien’s classification, grade I complications occurred in two patients (8 %), and grade II complications occurred in two patients (8 %). The median hospital stay was 4 (range 2–14) days, and no hospital readmission within 30 days was recorded.
Table 1 shows a comparative analysis of the patient characteristics and short-term postoperative outcomes recorded in group 1 and group 2. Tumor and defect size were significantly greater in group 2 (respectively, 40 mm vs. 12.5 mm, p < 0.001; and 57.5 mm vs. 32.5 mm, p < 0.001). The rate of grade II postoperative complications was slightly but not significantly higher in group 1. The rate of suture line dehiscence was higher in group 1, but this difference also was not statistically significant (22.7 vs. 4 %; p = 0.068).
Patients of both groups were interviewed about their functional status during the follow-up evaluation 12 months (median value; range 11–14 months) after surgery (Table 2). In group 1, the mean evacuation score was 24.72 ± 2.79, and the only evacuation disorder reported was urgency, in one case (4.5 %). The mean continence score was 1.05 ± 1.25, and incontinence to flatus was reported in two cases (9.1 %); no patients reported soiling, incontinence to solid stools, or the necessity of wearing a pad. In group 2, the mean evacuation score was 25.6 ± 2.24 (not significantly different from group 1; p = 0.288), and the only evacuation disorder reported was urgency, in three cases (12 %; not significantly different from group 1; p = 0.354). The mean continence score was 0.84 ± 1.43 (not significantly different from group 1; p = 0.081), and no patients reported incontinence to flatus (not significantly different from group 1; p = 0.213), soiling, incontinence to solid stools, or the necessity of wearing a pad. One year after surgery, no patients reported difficulty in distinguishing gas from liquid stools or painful evacuation.
Discussion
In selected groups of extraperitoneal rectal cancer patients, local excision by TEM after RCT is increasingly common as an alternative to traditional radical resection with TME. The only prospective, randomized study comparing these modalities was published by Lezoche in 2008, who compared the oncologic results obtained after TEM (35 patients) and after laparoscopic TME resection (35 patients) for T2N0 rectal cancer after neoadjuvant RCT. At a follow-up of 84 months, they found no significant differences between the two approaches, and the probability of survival for rectal cancer was the same (94 %) after TEM as after TME [14]. In our previous study, in response to the reported increasing rate (8–35 %) of pathological complete response after RCT [10–13], we proposed TEM as an essential diagnostic step to identify patients in whom clinical complete response corresponds to a true pathological complete response of the primary tumor and a radical resection can be avoided [16]. Full-thickness excision of the rectal wall disc previously containing a tumor allows the surgeon to assess the grade of pathologic response (ypT) of the primary tumor with high accuracy. The major criticism of this approach is the impossibility of radically removing the mesorectum and, consequently, obtaining direct pathological information regarding mesorectal lymph-node status [34]. Nevertheless, a direct correlation between ypT status and mesorectal lymph node involvement has been observed and the rate of positive nodes is very low when a complete response (ypT0) is found [10, 13, 16, 34].
Short-term postoperative complications after TEM occur at a rate of approximately 4 % and include suture line dehiscence, bleeding, abscess formation, transient incontinence, and stenosis [19, 35–37]. Studies of TEM after RCT are scant but have reported short-term postoperative complication rates from 11 to 61 % [14, 20, 21, 38]. In 2009, an American prospective study from Thomas Jefferson University analysed the postoperative outcome of 43 patients operated on by TEM after RT, comparing these results with a group of 19 patients treated by TEM alone. In the irradiated group, the overall rates of morbidity and dehiscence of the suture line were significantly higher than among nonirradiated cases (respectively, 33 vs. 5.3 and 25.6 vs. 0 %) [20]. Along the same lines, in 2011, a Brazilian prospective study analysed 23 irradiated patients and 13 nonirradiated patients treated by TEM. Patients undergoing neoadjuvant RCT were more likely to develop grade II/III complications (56 vs. 23 %) and wound dehiscence (70 vs. 23 %) with a consequently higher readmission rate within 30 days (43 vs. 7 %) [21]. In our prospective study, overall morbidity in the irradiated group was 36.4 %, but it was only 18.2 % if only grade II complications were considered; this morbidity rate was slightly but not significantly higher than that in nonirradiated cases. We found a tendency toward a higher rate of suture dehiscence in previously irradiated patients, but also in this case the difference was not statistically significant. The absence of statistical significance is probably due to the small size of the sample. The high rate of postoperative short-term complications and, in particular, suture dehiscence could be a consequence of the detrimental effects of RCT on the tissue, e.g., free radical formation, DNA damage and vascular injury, with a consequently higher risk of suture line dehiscence and infection [39]. Moreover, when TEM is performed on irradiated tissue, both wound edges used for the suture were previously irradiated, which carries a higher hypothetical risk of wound healing [20]. However, in our series of irradiated patients, the occurrence of postoperative complications did not give rise to a significantly prolonged hospital stay or to any hospital readmissions.
Two studies have evaluated long-term functional outcome after TEM, reporting very good results [17, 19]. In 2004, Cataldo and colleagues [17] reported the results of a prospective, comparative study based on interviews of 41 patients (using the Faecal Incontinence Severity Index [FISI] and the Faecal Incontinence Quality of Life [FIQL] score) 6 weeks after TEM. The number of bowel movements per 24 h, urgency, FISI and FIQL were unchanged when preoperative and postoperative data were compared. A recent large (93 patients) Italian study published the long-term (60 months) functional results and quality of life results after TEM, based on clinical scores (Wexner score, FIQL score, EORTC QLQ-C30, EORTC QLQ-CR38, EuroQoL EQ-5D, and EQ-VAS) and manometry. Three months after TEM, postoperative continence (evaluated with Wexner’s score) became worse than that preoperatively (but without statistical significance), improved at 12 months, and returned to the preoperative status at 60 months. Urgency occurred in 65 % at 3 months, 30 % at 12 months, and 5 % at 60 months after TEM. The same trend was noted in QoL scores and in postoperative manometry values, which were significantly lower than those at baseline at 3 months but returned to preoperative values at 12 months (no significant difference between results at 12 and 60 months) [19]. One explanation for these results could be that the effects of prolonged insertion of the 40-mm-diameter operating proctoscope during the procedure or that the necessity of performing a large full-thickness excision impairs (although not permanently) evacuative function, and evacuative and sphincter functions usually return to physiological status within 12 months [40, 41].
However, no published studies have evaluated long-term functional outcome after TEM in patients previously treated with RCT. Therefore, ours is the first such study. In our study, long-term functional results recorded 1 year after TEM in previously irradiated patients were excellent, with near-optimal continence and evacuative scores that were not significantly different from those of nonirradiated patients and with a low rate of weekly or daily occurring functional disorders. The absence of statistically significant differences between irradiated and nonirradiated patients could represent the marginal impact of neoadjuvant treatments in determining long-term evacuative and sphincter disorders when the rectum is spared. Moreover, if preoperative RCT could increase the short-term postoperative complication rate, these events do not seem to affect functional outcome after 1 year from TEM, with similar low rate of functional disorders between irradiated and not irradiated patients.
It is our opinion that the type of surgery plays a major role in determining functional disorders after integrated treatment for rectal cancer. It seems obvious to assume that evacuative and continence functions after TEM are better than after anterior resection with TME. However, only one study has investigated this aspect, and it only concerned patients not previously treated with RCT. In that study, 31 patients operated on by TEM (for T1 rectal cancers) were compared with 31 patients treated with radical surgery and TME (for more advanced rectal cancers). The evaluation, performed after a median interval of 28 (range 5–91) months using four cancer-specific questionnaires (EuroQoL EQ-5D, EQ-VAS, EORTC QLQ-C30, and QLQ-CR38) showed that TEM patients had fewer defecation problems than TME patients [18]. In the setting of irradiated patients, in 2007, we published the functional results of 100 patients preoperatively irradiated (long-term RCT) treated by anterior resection with TME and evaluated the same length of time after surgery and with the same questionnaire reported in the current study [7]. Although these series are not comparable because of the dissimilar numbers of cases analyzed, it is clearly evident from Table 3 that patients who underwent TME had more functional disorders than patients treated by TEM.
The main limitation of this study was the small number of cases analyzed due to the strict selection criteria for performing TEM after neoadjuvant RCT. We did not compare functional status before neoadjuvant treatment or TEM with postprocedure status. We believe that because our study population was affected by locally advanced rectal cancers, located in the middle or lower rectum, pretreatment evaluation of the evacuation and continence functions could have been impaired by the presence of the rectal mass itself. Moreover, we did not perform a manometric evaluation, because we wanted to evaluate what patients felt independently of objective measurements.
Conclusions
In highly select locally advanced extraperitoneal rectal cancer patients previously treated with RCT, TEM is increasingly reported as a therapeutic option or as a valid diagnostic tool to assess whether a pathological complete response of a primary tumor has been obtained. Our study shows, contrary to a recent report [21], that the short-term postoperative morbidity rate in this group of patients is acceptable, especially considering that no grade III complications were reported and no reoperations or readmissions were needed, even if a relatively high rate of suture dehiscence is reported. Another important aspect, unique to this study, is the rate of evacuative and continence disorders in patients treated with preoperative long-term radiochemotherapy and TEM. Our results indicate that, in both group treated by TEM, a very low rate of functional disorders after 1 year from surgery was reported, probably due to rectum preservation, without differences between not irradiated and irradiated patients. In particular, for irradiated patients, the not statistically significant higher rate of suture dehiscence and higher rate of postoperative complications do not seem to affect functional outcome at 1 year from surgery.
References
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Eng J Med 351:1731–1740
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg 69:613–616
Kapiteijn E, Putter H, van de Velde CJ, Cooperative investigators of the Dutch ColoRectal Cancer Group (2002) Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 89:1142–1149
Ridgway PF, Darzi AW (2003) The role of total mesorectal excision in the management of rectal cancer. Cancer Control 10:205–211
Pinsk I, Phang PT (2007) Total mesorectal excision and management of rectal cancer. Expert Rev Anticancer Ther 7:1395–1403
Coco C, Valentini V, Manno A, Rizzo G, Gambacorta MA, Mattana C et al (2007) Functional results after radiochemotherapy and total mesorectal excision for rectal cancer. Int J Colorectal Dis 22:903–910
Dahlberg M, Glimelius B, Graf W, Påhlman L (1998) Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum 41:543–549
Bujko K, Nowacki MP, Oledzki J, Sopyło R, Skoczylas J, Chwaliński M (2001) Sphincter preservation after short-term preoperative radiotherapy for low rectal cancer–presentation of own data and a literature review. Acta Oncol 40:593–601
Pucciarelli S, Urso E, DeSalvo GL, Aschele C, Friso ML, Rugge M et al (2006) 5-fluorouracil and weekly oxaliplatin combined with radiotherapy for locally advanced rectal cancer: surgical complications and long-term results. Arch Med Res 37:860–865
Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R et al (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72:99–107
Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W et al (2010) Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09–01). Ann Surg 252:998–1004
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844
Lezoche G, Baldarelli M, Guerrieri M, Paganini AM, De Sanctis A, Bartolacci S et al (2008) A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc 22:352–358
Callender GG, Das P, Rodriguez-Bigas MA, Skibber JM, Crane CH, Krishnan S (2010) Local excision after preoperative chemoradiation results in an equivalent outcome to total mesorectal excision in selected patients with T3 rectal cancer. Ann Surg Oncol 17:441–447
Coco C, Manno A, Mattana C, Verbo A, Rizzo G, Valentini V et al (2007) The role of local excision in rectal cancer after complete response to neoadjuvant treatment. Surg Oncol 16(Suppl 1):S101–S104
Cataldo PA, O’Brien S, Osler T (2005) Transanal endoscopic microsurgery: a prospective evaluation of functional results. Dis Colon Rectum 48:1366–1371
Doornebosch PG, Tollenaar RA, Gosselink MP, Stassen LP, Dijkhuis CM, Schouten WR et al (2007) Quality of life after transanal endoscopic microsurgery and total mesorectal excision in early rectal cancer. Colorectal Dis 9:553–558
Allaix ME, Rebecchi F, Giaccone C, Mistrangelo M, Morino M (2011) Long-term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg 98:1635–1643
Marks JH, Valsdottir EB, DeNittis A, Yarandi SS, Newman DA, Nweze I et al (2009) Transanal endoscopic microsurgery for the treatment of rectal cancer: comparison of wound complication rates with and without neoadjuvant radiation therapy. Surg Endosc 23:1081–1087
Perez RO, Habr-Gama A, São Julião GP, Proscurshim I, Scanavini Neto A, Gama-Rodrigues J (2011) Transanal endoscopic microsurgery for residual rectal cancer after neoadjuvant chemoradiation therapy is associated with significant immediate pain and hospital readmission rates. Dis Colon Rectum 54:545–551
Coco C, Valentini V, Manno A, Mattana C, Verbo A, Cellini N et al (2006) Long-term results after neoadjuvant radiochemotherapy for locally advanced resectable extraperitoneal rectal cancer. Dis Colon Rectum 49:311–318
Gambacorta MA, Valentini V, Coco C, Morganti AG, Smaniotto D, Miccichè F et al (2004) Chemoradiation with raltitrexed and oxaliplatin in preoperative treatment of stage II-III resectable rectal cancer: phase I and II studies. Int J Radiat Oncol Biol Phys 60:139–148
Hospers GA, Punt CJ, Tesselaar ME, Cats A, Havenga K, Leer JW et al (2004) Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I–II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol 14:2773–2779
Glynne-Jones R, Sebag-Montefiore D, Maughan TS, Falk SJ, McDonald AC (2006) A phase I dose escalation study of continuous oral capecitabine in combination with oxaliplatin and pelvic radiation (XELOX-RT) in patients with locally advanced rectal cancer. Ann Surg Oncol 17:50–56
Miller AB (1981) Reporting result of cancer treatment. Cancer 47:207–214
Valentini V, Coco C, Cellini N, Picciocchi A, Fares MC, Rosetto ME et al (2001) Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys 51:371–383
Buess G, Hutterer F, Theiss J, Bobel M, Isselhard W, Pichlmaier H (1984) A system for a transanal endoscopic rectum operation. Chirurg 55:677–680
Sobin LH (2001) TNM classification of malignant tumors, 6th edn. Wiley, Hoboken
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer 73:2680–2686
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Gervaz P, Rotholtz N, Wexner SD, You SY, Saigusa N, Kaplan E et al (2001) Colonic J-pouch function in rectal cancer patients. Impact of adjuvant chemoradiotherapy. Dis Colon Rectum 14:1667–1675
Jorge JMN, Wexner SD (1993) Etiology and management of faecal incontinence. Dis Colon Rectum 36:77–97
Read TE, Andujar JE, Caushaj PF, Johnston DR, Dietz DW, Myerson RJ (2004) Neoadjuvant therapy for rectal cancer: histologic response of the primary tumor predicts nodal status. Dis Colon Rectum 47:825–831
Guerrieri M, Baldarelli M, Morino M, Trompetto M, Da Rold A, Selmi I et al (2006) Transanal endoscopic microsurgery in rectal adenomas: experience of six Italian centers. Dig Liver Dis 38:202–207
Bretagnol F, Merrie A, George B, Warren BF, Mortensen NJ (2007) Local excision of rectal tumors by transanal endoscopic microsurgery. Br J Surg 94:627–633
Suppiah A, Maslekar S, Alabi A, Hartley JE, Monson JR (2008) Transanal endoscopic microsurgery in early rectal cancer: time for a trial? Colorectal Dis 10:314–327
Caricato M, Borzomati D, Ausania F, Tonini G, Rabitti C, Valeri S et al (2006) Complementary use of local excision and transanal endoscopic microsurgery for rectal cancer after neoadjuvant chemoradiation. Surg Endosc 20:1203–1207
Stone HB, Coleman N, Anscher MS, McBride WH (2003) Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol 4:529–536
Kennedy ML, Lubowski DZ, King DW (2002) Transanal endoscopic microsurgery excision: is anorectal function compromised? Dis Colon Rectum 45:601–604
Herman RM, Richter P, Walega P, Popiela T (2001) Anorectal sphincter function and rectal barostat study in patients following transanal endoscopic microsurgery. Int J Colorectal Dis 16:370–376
Disclosures
C. Coco, G. Rizzo, C. Mattana, M.A. Gambacorta, A. Verbo, B. Barbaro, F.M. Vecchio, D.P. Pafundi, M.G. Mastromarino, and V. Valentini have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coco, C., Rizzo, G., Mattana, C. et al. Transanal endoscopic microsurgery after neoadjuvant radiochemotherapy for locally advanced extraperitoneal rectal cancer: short-term morbidity and functional outcome. Surg Endosc 27, 2860–2867 (2013). https://doi.org/10.1007/s00464-013-2842-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-2842-6