Abstract
Introduction
This study explores the association between perioperative hypoglycaemia and surgical outcomes in subjects with diabetes, undergoing colorectal surgery.
Methods
A retrospective review of 149 subjects with Type 2 Diabetes Mellitus (DM) who underwent colorectal surgery between 2010 and 2015 was performed. Perioperative glucose levels, glycated haemoglobin (HbA1c) measurements within 3 months of surgery and surgical complications based on Clavien-Dindo classification were analysed.
Results
The mean age was 67 years (67 ± 11.2). Perioperative hypoglycaemia was found in 7.4% of subjects. The mean HbA1c of subjects with Clavien 2 and above surgical complications were higher than patients with Clavien 1 or no complications, Hba1c 7.6% (7.6 ± 2.5%) and 7.0% (7.0 ± 1.1%, p = 0.008), respectively. Similar findings in subjects with Clavien 3 and above complications, HbA1c of 8.2% (8.2 ± 3.9%) as compared to those with Clavien 2 and below complications, 7.2% (7.2 ± 1.5%, p = 0.001). Adjusted multivariate analysis showed that hypoglycaemia was significantly associated with Clavien 2 and above surgical complications, OR of 19.0 (CI 2.23–162, p = 0.007). Preoperative hypoglycaemia was associated with Clavien 2 and above surgical complications, OR 10.7 (CI 1.22–94.1, p = 0.032). Suboptimal glycaemic control (Hba1c >8.0%) was significantly associated with Clavien 2 and above complications, OR 2.48 (CI 1.04–5.91, p = 0.04), but not with Clavien 3 and above complications, OR 1.50 (CI 0.450–4.98, p = 0.511).
Conclusion
Perioperative hypoglycaemia is associated with adverse surgical outcomes in diabetic patients undergoing colorectal surgery. Prevention of hypoglycaemia may improve surgical outcomes. HbA1c is an independent predictor for adverse surgical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Singapore, like many of its Asian counterparts has been facing an epidemic of diabetes mellitus (DM) [1]. As the burden of diabetes extends to adverse surgical outcomes, it is imperative to optimize glycaemic control to improve surgical outcomes. While perioperative hyperglycaemia in general surgery subjects is associated with an almost twofold higher risk of infection, in-hospital mortality and operative deaths [2], it is equally important to explore the effects of hypoglycaemia in general surgery subjects as overly cautious management of perioperative hyperglycaemia may result in perioperative hypoglycaemia. Hypoglycaemia, when severe, can lead to cardiac arrhythmias, myocardial injury and neurological consequences including coma and death.
A previous systematic review and meta-analysis failed to show any consistent benefits of intensive insulin therapy targeted to strict glycaemic control in terms of short-term and long-term mortality or length of stay but instead increased the risk of severe hypoglycaemia. Despite the limitations of differing blood glucose targets, differing methods of blood glucose sampling (capillary versus arterial blood sampling) and differing hospital settings (intensive care versus non-intensive care, medical versus surgical disciplines), the authors caution against using intensive insulin therapy targeted to strict glycaemic targets due to the potential for serious harm from severe hypoglycaemia [3].
Perioperative hypoglycaemia was found to be an independent risk factor for death [4–6] and was also found to be related to complications in cardiac surgery [7]. In a retrospective study of subjects with sepsis [8], hypoglycaemia was found to be associated with increased risk of hospitalization and 1-year mortality, as well as intensive care unit (ICU)-acquired complications. It remains uncertain whether hypoglycaemia is a causative factor for poorer outcomes or simply a marker of more severe disease, and existing evidence remains conflicting. To date, there is limited evidence on the impact of perioperative hypoglycaemia on surgical outcomes. Therefore, this study aims to explore the association between perioperative hypoglycaemia and surgical outcomes in subjects with diabetes, undergoing colorectal surgery.
Methodology
A retrospective study was performed between 2010 and 2015 on a cohort of subjects who had undergone colorectal surgery at our institution, which is a general and acute care hospital. We included subjects with Type 2 DM, who underwent colorectal surgery. Perioperative point-of-care blood glucose levels and glycated haemoglobin (HbA1c) measurements within 3 months of surgery were collected.
The primary outcomes measured were surgical complication rates, based on the Clavien-Dindo classification system [9]. We hypothesized that perioperative hypoglycaemia would translate to poorer outcomes. Perioperative hypoglycaemia was defined by a glucose level of <4.0 mmol, 24 h before or after surgery [10]. Severe hypoglycaemia was defined as glucose level <3 mmol/l. Suboptimal glycaemic control was defined as HbA1c level of >8.0%. This was compared against in-hospital complication rates (Clavien-Dindo Grade 2 and above).
Baseline characteristics such as age, comorbidities, medications and creatinine levels were collected. Estimated glomerular filtration rate (eGFR) was calculated via CKD-EPI as it has increased precision in multi-ethnic Asian population [11].
Data analysis was conducted using IBM SPSS Statistics Version 21. Results are presented as mean ± SD values or median [interquartile range (IQR)]. Univariate analyses for categorical variables were performed using χ2 test and Fisher’s exact test. Continuous data were analysed using Student’s t test and analysis of variance method, while multivariate analyses were performed using multinomial and binary logistic regression methods. Adjusted analyses were performed after adjusting for confounding factors. A p value of less than 0.05 was considered to be statistically significant.
Results
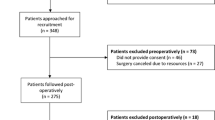
A total of 149 subjects with Type 2 DM who underwent colorectal surgery were analysed. The mean age of subjects was 67 years (67 ± 11.2). Eighty-three (56%) of the subjects were males (Table 1). Perioperatively, 144 (96.7%) subjects were on glucose monitoring. HbA1c was measured within 3 months of surgery for 130 (87.2%) subjects.
Eleven out of the 144 (7.4%) subjects had hypoglycaemia in the perioperative period. Nine of these 11 subjects had preoperative hypoglycaemia while the remaining 2 had post-operative hypoglycaemia. The glucose range of subjects with hypoglycaemic episodes ranged from 2.7 to 3.9 mmol/l. Only one out of the 11 subjects had severe hypoglycaemia with a glucose level of 2.7 mmol (<3.0 mmol), the remainder of the subjects had glucose levels between 3.0 to 3.9 mmol. The mean value of Hba1c of all subjects was 7.3% (7.3 ± 1.95%).
Ninety out of 149 (60%) of the subjects had surgical complications. Twenty (13.4%) of the subjects had Clavien 1 complications, 70 (47.0%) of the subjects had Clavien 2 and above surgical complications and 19 (12.8%) of the subjects had Clavien 3 and above surgical complications. There was no Clavien 5 surgical complication of inpatient mortality.
The mean HbA1c of subjects with Clavien 2 and above surgical complications were higher than patients without surgical complications or Clavien 1 complications. Subjects with Clavien 2 and above complications had Hba1c of 7.6% (7.6 ± 2.5%) as compared to 7.0% (7.0 ± 1.1%, p = 0.008) in those without surgical complications or Clavien 1 complications, while subjects with Clavien 3 and above complications had a mean HbA1c of 8.2% (8.2 ± 3.9%) as compared to those with Clavien 2 and below complications with a mean HbA1c of 7.2% (7.2 ± 1.5%, p = 0.001) (Table 2). Suboptimal glycaemic control, defined as HbA1c >8.0% was found to be significantly associated with Clavien 2 and above complications, OR 2.57 (CI 1.11–5.94, p = 0.024), but not with Clavein 3 and above complications, OR 1.71 (CI 0.537-5.454, p = 0.359) (Table 3).
Adjusted multivariate analysis showed that hypoglycaemia is significantly associated with Clavien 2 and above surgical complications, OR of 18.9 (CI 2.23–162, p = 0.007). Preoperative hypoglycaemia was significantly associated with Clavien 2 and above surgical complications, OR 10.7 (CI 1.22–94.1, p = 0.032). Subjects with post-operative hypoglycaemia were insufficient for statistical analysis. Hypoglycaemia was not significantly associated with Clavien 3 and above surgical complications, OR 4.38 (CI 0.896–21.4, p = 0.068) (Table 4).
Most of the subjects, 116 out of 149 (77.9%) subjects, had serum creatinine and eGFR measured before surgery. The majority, 84 out of the 116 (72.4%) subjects, had eGFR >60 ml/min/1.73 m2. Twenty-one out of the 116 (18.1%) subjects, had eGFR between 45 and 60 ml/min/1.73 m2 or Stage 3A Chronic Kidney Disease (CKD). The remaining 11 of the 116 (9.5%) subjects had eGFR <45 ml/min/1.73 m2. Diabetic subjects who had Stage 3B CKD (eGFR <45 ml/min/1.73 m2) was associated with perioperative hypoglycaemia, OR 5.05 (CI 1.09–23.3, p = 0.025) (Table 5).
There were no significant associations between the use of insulin (p = 0.746) or oral antidiabetic agents (p = 0.218) with hypoglycaemia. Age, gender and comorbidities such as hypertension, dyslipidaemia and previous cerebrovascular events/ischaemic heart disease were also not associated with hypoglycaemia.
Discussion
Patients with DM are known to have a higher risk of surgical complications than non-DM patients such as surgical site infections, stroke and heart disease [12, 13]. One key aspect of the perioperative management in these patients is glycaemic control. Multiple factors predispose DM patients to labile blood glucose levels perioperatively such as the combination of operative procedures, anaesthesia and post-operative factors such as sepsis, disrupted meal schedules, altered nutritional intake, hyperalimentation and emesis. Hypoglycaemia, even for short periods of time, increases the risk of arrhythmias, other cardiac events and transient cognitive deficits. In anaesthetized patients, hypoglycaemia and subsequent neuroglycopenia can be difficult to detect resulting in delayed diagnosis.
The prevalence of hypoglycaemia varies depending on glucose thresholds used in defining hypoglycaemia. The prevalence of hypoglycaemia defined as blood glucose levels <4 mmol/l in our study is 7.4%, This is lower as compared to previous studies found in hospitalized patients (7–22.4%) [14, 15]. In our institution, since January 2015, an inpatient diabetes care team, comprising a consultant endocrinologist and advanced specialty trainees in endocrinology, monitor and detect blood glucose ranges that are out of target (pre-defined as <4 and >14 mmol/l) from the blood glucose data management system. Depending on the clinical context, the team will offer suggestions on perioperative diabetes management with the aim of reducing hypo- and hyperglycaemia occurrences.
Glycaemic targets for perioperative patients undergoing colorectal or major surgery remain uncertain. This is due to the lack of consensus of glycaemic targets and the fact that strict glycaemic control has been shown to have limited benefits. It may however be wise to consider a higher glycaemic target range which results in less hypoglycaemia but no significant differences in morbidity compared with a stricter target. This has been shown in a study involving patients undergoing cardiac surgery [16].
In our study, perioperative hypoglycaemia was associated with Clavien 2 surgical complications. The management of Clavien 2 surgical complications require pharmacological treatment with drugs other than anti-emetics, antipyretics, analgesia, diuretics and physiotherapy [Fig1]. Examples of management of Clavien 2 surgical complications include requirements for blood transfusions and total parenteral nutrition (TPN). A possible mechanism for an increased risk of this complication is that patients with perioperative hypoglycaemia may have a more severe, prolonged state of malnutrition and gastrointestinal impairment. They may also lack the appropriate neurohormonal responses during periods of acute stress, requiring post-operative interventions such as inotropic support and blood products to maintain an optimal physiological state.
Our study found that patients with impaired renal function at Stage 3B CKD and above are at risk of hypoglycaemic episodes. Use of insulin and oral antidiabetic agents in patients with eGFR <45 ml/min/1.73 m2 was associated with increased risk of hypoglycaemia but not in patients with higher eGFR. A proposed mechanism for the increased risk of hypoglycaemia in people with eGFR less than 15–20 ml/min/1.73 m2 is the prolonged duration of action of insulin due to reduced renal clearance of insulin and oral antidiabetic agents and its active metabolites. [17]. Poor renal function itself is an independent predictor of hypoglycaemia and possible adverse surgical outcomes. Thus, glycaemic targets should be less strict in this vulnerable patient group.
Detection of hypoglycaemia should rely on frequent, careful glucose determination in patients with diabetes undergoing surgery. Surgical outcomes can be optimized by striking a balance between hyperglycaemia and hypoglycaemia. There may be a role for intra-operative glucose testing especially for long and complex procedures. This is important to avoid hypoglycaemia during anaesthesia and in the post-operative phase as hypoglycaemic symptoms are difficult to detect in the anaesthetized patient. Prioritizing patients with DM in the operative list and as a morning case may assist in limiting disruptions to meals and minimizing the risk of hypoglycaemia.
Besides avoiding hypoglycaemic episodes perioperatively, our study demonstrated that good glycaemic control within 3 months of surgery, translates to better surgical outcomes. Preoperative HbA1c values should be determined in patients undergoing colorectal surgery, especially in elective surgical patients with diabetes. This may influence the timing of surgery and allow optimization of patients prior to major surgery. Studies have shown that delaying elective major surgery due to suboptimal glycaemic control is predicted to decrease mortality, serious morbidity and influence length of hospital stay [18, 19].
Further studies should be done to explore other factors contributing to perioperative hypoglycaemia. These factors include duration of anorexia, patterns of glucose control including aggressive lowering of blood glucose and timing at which first meal was served post-operatively. Better inpatient diabetes protocols for patients with diabetes who are to be kept fasted and/or started on transitional types of feeds are needed to enhance the quality of inpatient DM care.
Conclusion
Perioperative hypoglycaemia has been shown to be associated with adverse surgical outcomes in DM patients undergoing colorectal surgery. Glucose monitoring and optimal glucose balance is imperative as derangements can have dire consequences to surgical outcomes. Poor glycaemic control as reflected by most recent HbA1c is an independent predictor for adverse surgical outcomes. Improved glucose control prior to surgery may serve to improve overall surgical outcomes. In addition, glycaemic targets have to be individualized for different groups of patients. Glycaemic targets should be less stringent in patients with renal impairment, in particular eGFR <45 ml/min/1.73 m2, in view of the higher risk of hypoglycaemic episodes. Further exploration into factors contributing to hypoglycaemia should be carried out.
References
Hu FB (2011) Globalization of diabetes. Diabetes Care 34(6):1249–1257. doi:10.2337/dc11-0442
Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D (2013) Importance of perioperative glycemic control in general surgery: a report from the surgical care and outcomes assessment program. Ann Surg 257:8–14. doi:10.1097/SLA.0b013e31827b6bbc
Kansagara D, Fu R, Freeman M, Wolf F, Helfand M (2011) Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med 154(4):268–282. doi:10.7326/0003-4819-154-4-201102150-00008
Preiser JC, Devos P, Ruiz-Santana S, et al. (2009) A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009 Oct;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2
Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C (2009) The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care 2009;13(3):R91. doi: 10.1186/cc7921.
Van den Berghe G, Wilmer A, Hermans G et al (2006) Intensive insulin therapy in the medical ICU. New Eng J Med 354(5):449–461
Engoren M, Schwann TA, Habib RH (2014) Hyperglycemia, hypoglycemia, and glycemic complexity are associated with worse outcomes after surgery. J Crit Care 29(4):611–617. doi:10.1016/j.jcrc.2014.03.014
Park S, Kim D, Suh GY et al (2012) Mild hypoglycemia is independently associated with increased risk of mortality in patients with sepsis: a 3-year retrospective observational study. Crit Care 16(5):R189. doi:10.1186/cc11674
Dindo D, Demartines N, Clavien P (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
Clayton D, Woo V, Yale J (2003) Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: hypoglycemia. Can J Diabetes 37:S69–S71. doi:10.1016/j.jcjd.2013.01.022
Teo BW, Koh YY, Toh QC et al (2014) Performance of the CKD-EPI creatinine-cystatin C glomerular filtration rate estimation equations in a multiethnic Asian population. Singap Med J 55(12):656–659. doi:10.11622/smedj.2014181
Ata A, Valerian BT, Lee EC, Bestle SL, Elmendorf SL, Stain SC (2010) The effect of diabetes mellitus on surgical site infections after colorectal and noncolorectal general surgical operations. Am Surg 76(7):697–702
McAnulty GR, Robertshaw HJ, Hall GM (2000) Anaesthetic management of patients with diabetes mellitus. Br J Anaesth 85(1):80–90. doi:10.1093/bja/85.1.80
Health and Social Care Information Centre (2013) National diabetes inpatient audit 2012. Leeds, West Yorkshire, England
Hypoglycemia and Clinical Outcomes in Patients with Diabetes Hospitalized in General Ward. Diabetes Care. 2009; 32(7): 1153–1157.
Mulla I, Schmidt K, Cashy J et al (2014) Comparison of glycemic and surgical outcomes after change in glycemic targets in cardiac surgery patients. Diabetes Care 37(11):2960–2965. doi:10.2337/dc14-1199
Alsahli M, Gerich JE, Navarro-González JF (2015) Hypoglycemia in patients with diabetes and renal disease. J Clin Med 4(5):948–964. doi:10.3390/jcm4050948
Aldam P, Levy N, Hall GM (2014) Perioperative management of diabetic patients: new controversies. Br J Anaesth. doi:10.1093/bja/aeu259
Halkos ME, Lattouf OM, Puskas JD et al (2008) Elevated preoperative hemoglobin A1c level is associated with reduced long-term survival after coronary artery bypass surgery. Ann Thorac Surg 86(5):1431–1437. doi:10.1016/j.athoracsur.2008.06.078
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
For this type of study, formal consent is not required.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Goh, S.N.S., Yeoh, E. & Tan, K.Y. Impact of perioperative hypoglycaemia in subjects with diabetes undergoing colorectal surgery. Int J Colorectal Dis 32, 209–214 (2017). https://doi.org/10.1007/s00384-016-2680-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2680-9