Abstract
Importance
Pre-operative hyperglycemia is associated with post-operative adverse outcomes in diabetic and non-diabetic patients. Current pre-operative screening includes random plasma glucose, yet plasma glycated hemoglobin (HbA1c) is a better measure of long-term glycemic control. It is not clear whether pre-operative HbA1c can identify non-diabetic patients at risk of post-operative complications.
Objective
The systematic review summarizes the evidence pertaining to the association of suboptimal pre-operative HbA1c on post-operative outcomes in adult surgical patients with no history of diabetes mellitus.
Evidence review
A detailed search strategy was developed by a librarian to identify all the relevant studies to date from the major online databases.
Findings
Six observational studies met all the eligibility criteria and were included in the review. Four studies reported a significant association between pre-operative HbA1c levels and post-operative complications in non-diabetic patients. Two studies reported increased post-operative infection rates, and two reported no difference. Of four studies assessing the length of stay, three did not observe any association with HbA1c level and only one study observed a significant impact. Only one study found higher mortality rates in patients with suboptimal HbA1c.
Conclusions and relevance
Based on the limited available evidence, suboptimal pre-operative HbA1c levels in patients with no prior history of diabetes predict post-operative complications and represent a potentially modifiable risk factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pre-operative hyperglycemia and insulin resistance are well-established risk factors for post-operative complications in both diabetic and non-diabetic surgical patients [1,2,3,4]. Insulin resistance and post-operative hyperglycemia are accentuated in diabetic patients [5] as well as non-diabetic patients with some degree of pre-operative insulin resistance and/or dysglycemia [6,7,8]. The prevalence of undiagnosed/provisional diabetes and pre-diabetes is unexpectedly high in surgical population, varying from 23% to over 60% [9,10,11,12,13,14,15]. Perioperative hyperglycemia has been linked to infectious complications and even death in non-diabetic patients [16, 17]. As such, a reliable test to screen for dysglycemia and insulin resistance pre-operatively is needed.
However, routine pre-operative screening does not include a reliable test for diagnosing insulin resistance or dysglycemia. Some practice guidelines recommend random blood sugar (RBS) levels as a pre-operative screen for hyperglycemia [18, 19]. Although RBS may identify patients with established uncontrolled diabetes, it is highly dependent on the prandial state of the patient and may not identify patients with provisional diabetes or pre-diabetic patients with some levels of insulin resistance who are prone to become hyperglycemic during and after surgery [10]. Even a fasting blood glucose test might not be able to identify all patients with dysglycemia before surgery [9].
With these limitations in random blood sugar testing, there is increasing interest in the use of plasma level of glycosylated hemoglobin (HbA1c) to diagnose both dysglycemia and insulin resistance in surgical patients [10]. HbA1c is a form of hemoglobin made by non-enzymatic glycation of hemoglobin in exposure to plasma glucose [20]. HbA1c is an indicator of long-term (3–4 months) glycemic control [21] and is an excellent measure for diagnosing both diabetes and pre-diabetes. It does not require the patient to be fasted, is not affected by acute changes in blood glucose levels and is completely independent of the patient’s prandial status [22]. Thus, it could be used as part of routine screening in the pre-operative visit and provides a potentially modifiable risk factor. According to American and Canadian Diabetes Associations guidelines, HbA1c ≥6.5% is diagnostic for diabetes and 5.7% ≤ HbA1c ≤ 6.4% is considered pre-diabetes [22, 23].
While HbA1c level has been studied as indicator of poorer surgical outcomes in diabetic patients [24, 25], whether suboptimal HbA1c levels are also associated with higher post-operative hyperglycemia and complications in non-diabetic is unclear [26, 27]. The introduction of HbA1c screening requires resources and should be supported by evidence. In this systematic review, we summarize the evidence regarding the correlation of suboptimized HbA1c levels with post-operative complications in non-diabetic adult surgical patients.
Methods
Protocol and registration
This systematic review was conducted based on the PRISMA statement guidelines [28]. The review protocol was registered and published on PROSPERO (ID# CRD42015016400) [29].
Search strategy
A systematic search of bibliographic databases following PICO framework [30] was conducted by a librarian (AA-Z) to retrieve all publications that evaluated pre-operative measurement of HbA1c as predictive of any kind of post-operative complication. A search strategy was developed for Medline via OvidSP and peer-reviewed by two other hospital librarians. The search was then adapted and run in other databases, including Medline via OvidSP, on October 20, 2014, Embase via OvidSP (1947–2014 October 17), Biosis via OvidSP (1969–2014 week 46), all databases comprising the Cochrane Library via Wiley, CINAHL via Ebsco, Scopus, Web of Science and Medline via PubMed (for records “as supplied by publisher”), all with no date restrictions. A second hospital librarian also reviewed the adapted search strategies.
The search strategy was designed to retrieve (1) HbA1c and possible variations, (2) pre-operative care or the pre-operative period, (3) peri- or per-operative care or the peri- or per-operative period, or post-operative care or the post-operative period, (4) surgery, (5) post-operative complications and (6) risk. All concepts were searched using MeSH or other controlled vocabulary (e.g., Emtree) where available, in combination with text words. The concepts were combined: 1 and 2, or 1 and (3 or 4) and 5. Both these searches were combined, filtered by the final concept of risk (6) and limited to adult humans. Search strategy details are available in “Appendix.” References from the searches were imported into an EndNote library. Duplicates were removed after all database results were imported.
Eligibility criteria
The eligibility of the identified observational and cohort studies was evaluated according to the inclusion and exclusion criteria listed in Table 1.
Outcome measures
The main outcomes of interest were 30-day all-cause post-operative morbidity (complications) and mortality. The other studied outcomes were post-operative infection and inflammatory response as well as any procedure-specific complications, length of hospital stay, re-operation and re-admission.
Study selection and data extraction
Two reviewers (NK and PN) independently assessed the eligibility of bibliographic records. Studies selected after the first screening were retrieved and independently evaluated by two reviewers, and conflicts were resolved. After screening, a citation search was performed using Scopus and Web of Science, retrieving articles cited in the selected studies, as well as articles which have cited the selected studies. These articles were subsequently screened.
The Ovid Medline and PubMed (non-Medline) searches were updated on December 20, 2016. The six included studies, all available in Medline, were also checked January 4, 2017, for corrections, errata, retractions or updates. None had been amended.
Risk of bias and quality of evidence assessment
All the included studies were critically appraised by two separate reviewers (NK and PN) according to the quality in prognostic studies (QUIPS) tool [31]. This tool is specifically designed to evaluate risk of bias in prognostic cohort-type studies across six main domains: (1) study participants, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding and (6) statistical analysis and reporting. Studies could be rated to have either low, moderate or serious bias across different domains [31]. Then we rated the overall evidence by employing Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [32]; while GRADE was originally designed to judge the evidence derived from interventional studies, it can also be used to judge the evidence in systematic reviews of prognostic factor studies [33]. Based on this framework, the level of evidence is judged for each outcome of interest separately; it can be downgraded considering the studies’ limitations, inconsistency, indirectness, imprecision and publication bias and upgraded considering large effect and exposure–gradient responses [33].
Data analysis
Based on the very diverse populations in the reviewed studies, we were not able to combine their results to conduct a meta-analysis; therefore, the results were narratively reviewed.
Results
Study selection
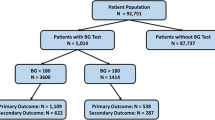
A total of 4153 studies were retrieved using our search strategy; after removing the duplicates (n = 1620), we screened the abstracts based on the eligibility criteria. Fifty-eight full texts were retrieved for extensive review. Six studies met all the eligibility criteria and remained in the systematic review (Fig. 1).
Characteristics of the included studies
The included studies had either low or moderate risk of bias across different domains according to the QUIPS tool [31] (Table 2). Three out of six studies were prospective observational studies, while the other three were retrospective. Overall, a total of 14,363 non-diabetic patients undergoing various types of surgeries were included in the studies; 34% of these patients (n = 4898) had suboptimized HbA1c prior to surgery as defined most commonly as HbA1c ≥6% (Table 3). Three of the six studies [26, 27, 34] did not define an upper limit for HbA1c as an exclusion, defining non-diabetic patients as those without diet/pharmacologically controlled diabetes [34] and/or having fasted glucose levels below 7 mmol/L [26, 27]. The results from the included studies will be summarized in the following categories: 30-day post-operative complication/morbidity, post-operative infection, 30-day mortality, length of stay, re-operation and re-admission. The overall strength of the existing evidence for each outcome is summarized in Table 5 using the GRADE framework.
Post-operative complications/morbidity
Four out of six studies reported 30-day post-operative complications and overall morbidity [26, 27, 34, 35] (Table 4). All had low risk of bias. Two of them were prospective observational studies on colorectal surgery [34] and vascular surgery patients [26]; the other two were both retrospective observational studies on collected data from large prospectively maintained databases of cardiac surgery [27] and laparoscopic gastric bypass surgery patients [35]. All four studies showed some degrees of association between suboptimized levels of HbA1c and post-operative overall complication rate or specific procedure-related complications. In the colorectal surgery study, after adjusting for different confounders, the authors observed a threefold increase risk of overall post-operative complications. This was mainly due to pneumonia, urinary tract infection, pleural effusion and post-operative ileus (45 vs 25%, adjusted OR = 2.9, 95% CI 1.1–7.9, p = 0.037) [34].
O’Sullivan et al. [26] observed that the non-diabetic vascular surgery patients with suboptimal HbA1c had a significantly higher all-cause 30-day morbidity compared to the normal HbA1c group (56.5 vs 15.7%, 95% CI 2.8–17.2, RR = 7, p < 0.001); when categorized based on the procedures, more specifically, they observed significant differences in non-diabetic patients with suboptimal HbA1c undergoing aortic procedures (50 vs 6.7%; p = 0.009) and peripheral arterial procedures (77.8 vs 20%; p = 0.003). Among the cardiac surgery patients, the risk of acute kidney injury was higher in patients with elevated HbA1c levels even after adjusting for other known renal risk factors (adjusted OR = 1.148, 95% CI 1.003–1.313, p = 0.04) [27]. Among the non-diabetic gastric bypass patients, Stenberg et al. [35] observed that suboptimal HbA1c was associated with having any post-operative complication (OR 1.16, 95% CI 1.00–1.33, p = 0.043) and a severe (≥3b Clavien–Dindo [36]) post-operative complication (OR = 1.3, 95% CI 1.05–1.61, p = 0.012), which remained after adjusting for confounding factors (adjusted OR = 1.26, 95% CI 1.01–1.59, p = 0.042). More specifically, higher HbA1c was associated with increased risk of pulmonary complications (OR = 1.92, 95% CI 1.17–3.14, p = 0.009) and small bowel obstruction/prolonged ileus (OR = 1.55, 95% CI 1.09–2.22, p = 0.016) [35].
Post-operative infection
Three studies with low risk of bias reported the rate of post-operative infection among cardiac, colorectal and vascular surgery patients [26, 27, 34], and one study reported the risk of leakage and abscesses among the non-diabetic gastric bypass patients [35]. Three out of four studies did not see any association between rate of post-operative infection and the pre-operative level of HbA1c, while only one found a significant association in their study populations (Table 4). While the 30-day post-operative infection rate was increased in the higher pre-operative HbA1c subgroup, this did not reach statistical significance among the non-diabetic colorectal surgery patient (29% vs 17%, adjusted OR 2.3, 95% CI 0.8–5.2, p = 0.129) [34]. Likewise, among the non-diabetic patients undergoing laparoscopic gastric bypass, while higher HbA1c was associated with increased risk of anastomotic leakage/abscess, this did not reach statistical significance (OR = 1.33, 95% CI 0.98–1.82, p = 0.071) [35]. In addition, in the study on the cardiac surgery patients, no association was observed between post-operative infection and elevated HbA1c levels (p = 0.48) [27]. Only, O’Sullivan et al. [26] documented an overall higher incidence of overall post-operative infection (21.1 vs 5.9%, p = 0.037), specifically surgical wound infection (9.9 vs 0%, p < 0.05) among non-diabetic patients with suboptimized HbA1c levels undergoing vascular surgery.
Mortality
Two of the included studies with low risk of bias reported 30-day mortality as their primary outcome [26, 27] (Table 4). One in vascular surgery patients did not find any significant difference in all-cause 30-day mortality between the non-diabetic patients with higher than normal HbA1c and with normal HbA1c (6% < HbA1c < 7% vs HbA1c ≤ 6%) [26]. In a second study in cardiac surgery patients, HbA1c >6% in non-diabetic patients was independently associated with increased risk of 30-day mortality, i.e., 53% increase in the risk of early post-operative mortality per percent increase in HbA1c level (OR 1.53; 95% CI +1.24 to 1.91, p = 0.0005); similar results were found even after excluding the borderline diabetic patients (with fasting blood glucose >7 mmol/l; p = 0.05) [27].
Length of stay
Four of the included studies compare length of stay in non-diabetic surgical patient population stratified by pre-operative HbA1c levels (Table 4). None of the three prospective observational studies in colorectal [34], cardiac [37] and vascular [26] surgery patients found any association between levels of pre-op HbA1c and length of stay [26, 27, 34, 37]. Only the retrospective study on spine surgery patients observed that LOS and total cost (hospital and physician) were significantly higher in non-diabetics with HbA1c >6.1 compared to non-diabetics with normal HbA1c (<6.1%) [14].
Re-operation and re-admission
None of the included studies included re-operation or re-admission rates as outcomes.
Discussion
The purpose of this systematic review was to summarize the available evidence to better understand whether pre-operative level of HbA1c in the non-diabetic adult surgical patients is an indicator of increased risk of post-operative adverse outcomes. This is a critical question to address prior to evaluating the candidacy of HbA1c as a potential screening test pre-operatively. In synthesizing the available data on 14,363 patients without previously diagnosed diabetes undergoing various types of surgeries, we found that high pre-operative HbA1c (generally defined as >6%) was associated with higher risk of overall post-operative complications after colorectal, bariatric, vascular and cardiac surgery.
We specifically addressed the prognostic value of pre-operative HbA1c screening in patients without previously diagnosed diabetes. A previous systematic review reported an association of high pre-operative glucose and HbA1c levels with increased post-operative complications [38]; however, this review also included diabetic patients. The HbA1c levels of a diabetic patient who is receiving pharmacological treatment might be as low as the levels of a non-diabetic individual; however, similar HbA1c levels do not necessarily reflect the same metabolic status. The increased risk of post-operative complications in studies with mixed population could be attributed to diabetes status and not to HbA1c level alone [38], and the review concluded that neither blood glucose testing nor HbA1c screening was recommended for non-diabetic patients, except for procedures where there is a high prevalence of undiagnosed diabetes, e.g., vascular and orthopedics surgery.
In the studies included in the present review, higher levels of HbA1c were seen in patients with higher BMI and older age and were regarded as confounding factors adjusted for in the statistical analysis [27, 34, 35]. These are well-defined risk factors for the development of perioperative complications [39,40,41,42]. A state of increased insulin resistance, as identified by higher HbA1c levels, could accentuate the surgery induced metabolic stress in this population and further complicate or slow their recovery after surgery. Therefore, it might be particularly beneficial to target these patients for HbA1c screening prior to surgery.
Pre-operative HbA1c screening can help identify patients with undiagnosed diabetes as well as pre-diabetic patients and can differentiate patients with stress hyperglycemia [10, 12]. Undiagnosed diabetes has been suggested to represent a much higher risk factor for post-operative complications than known diabetic state [43, 44]. In USA alone, the overall prevalence of pre-diabetes in 2011–2012 was estimated to be 38%; the same data also indicated that among the diabetic population in USA, up to 36% were undiagnosed [45]. Both diabetes and pre-diabetes may result in some degrees of insulin resistance [23] which may warrant pre-operative physiological conditioning and/or pharmacological interventions as well as closer post-operative glucose monitoring and control [46,47,48]. Higher pre-operative HbA1c levels are associated with post-operative hyperglycemia and possible need for insulin infusion among non-diabetic patients [6, 27, 34, 49]. As insulin resistance is a central feature of the metabolic response to surgery, identification of interventions that preserve insulin sensitivity is a key strategy to improve outcomes [50]. Regardless of diabetes status, a 20% increase in insulin resistance was associated with a more than twofold increase in the risk of serious complications after cardiac surgery [4].
The four studies that investigated post-operative complication/morbidity were all consistent in observing strong associations between higher levels of HbA1c (>6%) and elevated rate of post-operative complications. Based on GRADE approach (Table 5), the strength of evidence regarding this finding is at high level because for prognostic studies, retrospective and prospective cohorts could be the best approach to investigate the association between the prognostic factor and outcomes [33]. Furthermore, the large effects observed in at least two of the included studies and the gradient response of HbA1c levels in relation to post-operative outcomes contribute to upgrading the evidence [33].
Post-operative infections are among the most resource-consuming and costly complications after surgery, contributing to longer hospital stays, re-admissions and emergency visits [51, 52]. Perioperative hyperglycemia is a risk factor for post-operative infections [2, 15, 53]. Post-operative hyperglycemia was associated with superficial site infections, sepsis and even death in non-diabetic patients undergoing colorectal surgery [16]. Hence, an efficient screening tool for pre-operative hyperglycemia such as plasma HbA1c could play an important role in preventing post-operative infections by identifying non-diabetic patients who may benefit from monitoring and treatment of hyperglycemia. Yet the data from the few studies reporting post-operative infections were conflicting, with an increase rate of infection in the non-diabetic vascular surgery patients with high pre-operative HbA1c [26], a trend toward increased infections and risk of anastomotic leakage/abscess in patients with suboptimal HbA1c undergoing colorectal surgery [34] and gastric bypass surgery [35], but no increased risk in cardiac surgery patients [27].
Recently, pre-operative HbA1c screening has been included in the 2014 draft of best practices for perioperative glucose control from Strong for Surgery which issues guidelines for perioperative care in Washington State. Although it is unclear whether optimization of patients with poorly controlled diabetes improves outcomes, this guideline recommends screening all diabetic patients and patients at risk of diabetes or pre-diabetes (i.e., age ≥ 40 or BMI ≥ 30) by HbA1c or fasting blood glucose prior to surgery [54]. Other major guidelines also recommend testing for HbA1c in all diabetic patients [55, 56]; however, they have not recommended HbA1c screening as the initial test to diagnose dysglycemia in the pre-operative visit for people without diabetes [55, 56]. These guidelines instead recommend random blood glucose testing [18, 19, 55, 56]. However, this test is highly dependent on prandial state of patients and high rates of false negatives in diagnosing pre-diabetes and diabetes makes it inappropriate as an efficient screening tool for pre-operative dysglycemia [10]; more importantly, it results in missing the patients with dysglycemia who could benefit from better perioperative monitoring and control of blood glucose.
What is attractive about HbA1c screening is the potential to intervene and improve outcomes. In a risk-predictive model study on cardiac surgery patients, reduction of HbA1c from 8 to 5.5% predicted reduction in LOS by almost half a day; this suggest HbA1c as modifiable risk factor [57]. Upon diagnosis of pre-operative dysglycemia and/or insulin resistance by suboptimal HbA1c levels, various pharmacologic or physiological interventions could be reinforced in order to modify the risks associated with these conditions. These interventions could include but are not limited to pre-operative diet modification, exercise and administration of insulin sensitizers. Perioperative glucose control by insulin administration decreased renal complications in non-diabetic cardiac surgery patients [58] and overall mortality and morbidity in surgical patients at intensive care unit [59]. Perioperative glucose control has been also recommended for prevention of surgical site infections [60].
This review has several limitations. The restricted number of eligible studies included in this review and the diverse study populations limit the strength of the conclusions and recommendations for some of the outcomes. Therefore, there is need for more studies focusing on the association of suboptimal HbA1c with these post-operative outcomes in non-diabetic patients and whether this represents a therapeutic target in order to reduce hyperglycemia and complications in this at-risk population. In some of the included studies, considering a higher than 6.5% upper limit for categorizing the patients with suboptimal HbA1c might have resulted in including some undiagnosed diabetic patients in the non-diabetic category. Therefore, for future studies giving more attention to this matter is warranted. Furthermore, plasma HbA1c is a laboratory test which is subject to significant laboratory-to-laboratory variation and variation over time. This may impact the utility of a single HbA1c value especially in the pre-operative period. Thus, it might be a good practice to couple this measure with another measure such as random or fasting blood sugar level to screen for dysglycemia in non-diabetic patients with risk factors such as advanced age or obesity. In addition, it should be noted that short term interventions cannot modify HbA1c levels and this limits the value of HbA1c as modifiable risk factor in pre-operative settings.
In summary, HbA1c is a practical and informative test for screening non-diabetic patients prior to surgery for dysglycemia, pre-diabetes and undiagnosed diabetes who are at risk of developing post-operative hyperglycemia. The association of suboptimal HbA1c levels >6% with post-operative hyperglycemia and complications highlights its value for risk stratification prior to surgery. Furthermore, suboptimal HbA1c levels may identify patients who may benefit from more intensive monitoring and treatment of perioperative hyperglycemia. However, whether suboptimal pre-operative HbA1c represents a modifiable risk factor requires further study.
Conclusion
The current evidence suggests that elevated pre-operative HbA1c levels among patients without prior diagnosis of diabetes might be associated with an increased risk of post-operative complications. Future studies are essential to assess this possible association and to further explore HbA1c as a modifiable pre-operative risk factor.
References
Kiran RP, Turina M, Hammel J, Fazio V (2013) The clinical significance of an elevated postoperative glucose value in nondiabetic patients after colorectal surgery: Evidence for the need for tight glucose control? Ann Surg 258(4):599–604 (discussion 604–595)
Jackson RS, Amdur RL, White JC, Macsata RA (2012) Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg 214(1):68–80
Kotagal M, Symons RG, Hirsch IB et al (2015) Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 261(1):97–103
Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T (2010) The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 95(9):4338–4344
Feve B, Bastard JP (2009) The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nature reviews. Endocrinology 5(6):305–311
Perna M, Romagnuolo J, Morgan K, Byrne TK, Baker M (2012) Preoperative hemoglobin A1c and postoperative glucose control in outcomes after gastric bypass for obesity. Surg Obes Relat Dis 8(6):685–690
Donatelli F, Corbella D, Di Nicola M et al (2011) Preoperative insulin resistance and the impact of feeding on postoperative protein balance: a stable isotope study. J Clin Endocrinol Metab 96(11):E1789–E1797
Bagry HS, Raghavendran S, Carli F (2008) Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology 108(3):506–523
Tekumit H, Cenal AR, Polat A, Uzun K, Tataroglu C, Akinci E (2010) Diagnostic value of hemoglobin A1c and fasting plasma glucose levels in coronary artery bypass grafting patients with undiagnosed diabetes mellitus. Ann Thorac Surg 89(5):1482–1487
Koumpan Y, VanDenKerkhof E, van Vlymen J (2014) An observational cohort study to assess glycosylated hemoglobin screening for elective surgical patients. Can J Anaesth 61(5):407–416
Engoren M, Habib RH, Zacharias A et al (2008) The prevalence of elevated hemoglobin A1c in patients undergoing coronary artery bypass surgery. J Cardiothorac Surg 3:63
Hackman KL, Snell GI, Bach LA (2013) An unexpectedly high prevalence of undiagnosed diabetes in patients awaiting lung transplantation. J Heart Lung Transplant 32(1):86–91
McGinn JT Jr, Shariff MA, Bhat TM et al (2011) Prevalence of dysglycemia among coronary artery bypass surgery patients with no previous diabetic history. J Cardiothorac Surg 6:104
Walid MS, Newman BF, Yelverton JC, Nutter JP, Ajjan M, Robinson JS Jr (2010) Prevalence of previously unknown elevation of glycosylated hemoglobin in spine surgery patients and impact on length of stay and total cost. J Hosp Med 5(1):E10–E14
Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS Jr (2001) The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol 22(10):607–612
Mohan S, Kaoutzanis C, Welch KB et al (2015) Postoperative hyperglycemia and adverse outcomes in patients undergoing colorectal surgery: results from the Michigan surgical quality collaborative database. Int J Colorectal Dis 30(11):1515–1523
Wang R, Panizales MT, Hudson MS, Rogers SO, Schnipper JL (2014) Preoperative glucose as a screening tool in patients without diabetes. J Surg Res 186(1):371–378
Umpierrez GE, Hellman R, Korytkowski MT et al (2012) Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 97(1):16–38
Feely MA, Collins CS, Daniels PR, Kebede EB, Jatoi A, Mauck KF (2013) Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician 87(6):414–425
Letourneau J, Bui H, Schricker T, Hatzakorzian R (2013) HbA1c: a prognostic biomarker in the surgical and critically ill patient population. J Cardiothorac Vasc Anesth 27(4):760–764
Saudek CD, Derr RL, Kalyani RR (2006) Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 295(14):1688–1697
American Diabetes Association (2015) Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes 33(2):97–111
Canadian Diabetes Association Clinical Practice Guidelines Expert, Goldenberg R, Punthakee Z (2013) Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes 37(Suppl 1):S8–11
Rollins KE, Varadhan KK, Dhatariya K, Lobo DN (2016) Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin Nutr 35(2):308–316
Takahashi S, Suzuki A, Toyoda H et al (2013) Characteristics of diabetes associated with poor improvements in clinical outcomes after lumbar spine surgery. Spine 38(6):516–522
O’Sullivan CJ, Hynes N, Mahendran B et al (2006) Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 32(2):188–197
Hudson CC, Welsby IJ, Phillips-Bute B et al (2010) Glycosylated hemoglobin levels and outcome in non-diabetic cardiac surgery patients. Can J Anaesth 57(6):565–572
Group P, Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Karimian N, Niculiseanu P, Amar-Zifkin A, Carli F, Feldman L (2014) Value of pre-operative HbA1C screening in identifying the pre-diabetic patients at higher risk of developing post-operative complications. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015016400
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16
Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158(4):280–286
Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64(4):380–382
Huguet A, Hayden JA, Stinson J et al (2013) Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev 2:71
Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J (2009) Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg 96(11):1358–1364
Stenberg E, Szabo E, Naslund I (2014) Is glycosylated hemoglobin A1 c associated with increased risk for severe early postoperative complications in nondiabetics after laparoscopic gastric bypass? Surg Obes Relat Dis 10:801–805
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Medhi M, Marshall MC Jr, Burke HB et al (2001) HbA1c predicts length of stay in patients admitted for coronary artery bypass surgery. Heart Dis 3(2):77–79
Bock M, Johansson T, Fritsch G et al (2014) The impact of preoperative testing for blood glucose concentration and haemoglobin A1c on mortality, changes in management and complications in noncardiac elective surgery: a systematic review. Eur J Anaesthesiol 32:152–159
Doyle SL, Lysaght J, Reynolds JV (2010) Obesity and post-operative complications in patients undergoing non-bariatric surgery. Obes Rev 11(12):875–886
Mullen JT, Moorman DW, Davenport DL (2009) The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 250(1):166–172
Wigfield CH, Lindsey JD, Munoz A, Chopra PS, Edwards NM, Love RB (2006) Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI > or =40. Eur J Cardiothorac Surg 29(4):434–440
Polanczyk CA, Marcantonio E, Goldman L et al (2001) Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 134(8):637–643
Kohl BA, Schwartz S (2010) How to manage perioperative endocrine insufficiency. Anesthesiol Clin 28(1):139–155
Sebranek JJ, Lugli AK, Coursin DB (2013) Glycaemic control in the perioperative period. Br J Anaesth 111(Suppl 1):i18–i34
Menke A, Casagrande S, Geiss L, Cowie CC (2015) Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 314(10):1021–1029
Bergman M (2012) Treatments of prediabetes. Louvain Med 131(3):104–113
Dhatariya K, Levy N, Kilvert A et al (2012) NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 29(4):420–433
Canadian Diabetes Association Clinical Practice Guidelines Expert C, Ransom T, Goldenberg R, Mikalachki A, Prebtani AP, Punthakee Z (2013) Reducing the risk of developing diabetes. Can J Diabetes 37(Suppl 1):S16–S19
Gianchandani RY, Saberi S, Patil P, Prager RL, Pop-Busui R (2015) Prevalence and determinants of glycemic abnormalities in cardiac surgery patients without a history of diabetes: a prospective study. Front Endocrinol 6:125
Carli F (2015) Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: implications of the stress response. Can J Anaesth 62(2):110–119
Urban JA (2006) Cost analysis of surgical site infections. Surgical infections. 7(Suppl 1):S19–S22
Shepard J, Ward W, Milstone A et al (2013) Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA surgery. 148(10):907–914
Tee AH (2015) Is pre-operative haemoglobin A1c level a successful predictor of adverse outcome after cardiac surgery? J Cardiothorac Surg 10(1):A328
Thompson RE, Montgomery P (2014) Perioperative glucose control best practices. 2014. http://www.becertain.org/strong_for_surgery/hospitals/glycemic_control. Accessed July 27, 2015
National Guideline C. National Institute for Health and Care Excellence: Clinical Guidelines (2016) Preoperative tests (Update): routine preoperative tests for elective surgery. National Institute for Health and Care Excellence (UK) Copyright (c) National Institute for Health and Care Excellence 2016, London
Merchant R, Chartrand D, Dain S et al (2015) Guidelines to the practice of anesthesia—revised edition 2015. Can J Anaesth 62(1):54–67
Ad N, Holmes SD, Shuman DJ et al (2015) Potential impact of modifiable clinical variables on length of stay after first-time cardiac surgery. Ann Thorac Surg 100(6):2102–2107 (discussion 2107–2108)
Lecomte P, Van Vlem B, Coddens J et al (2008) Tight perioperative glucose control is associated with a reduction in renal impairment and renal failure in non-diabetic cardiac surgical patients. Crit Care (London, England) 12(6):R154
van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345(19):1359–1367
Anderson DJ, Podgorny K, Berríos-Torres SI et al (2014) Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35(6):605–627
Acknowledgements
The first author is supported by a collaborative fund from MITACS and Medtronic, Canada, for duration of her Ph.D. training.
Author information
Authors and Affiliations
Corresponding author
Appendix: Ovid Medline Search
Appendix: Ovid Medline Search
-
1.
Hemoglobin A, Glycosylated/
-
2.
Hemoglobins/
-
3.
Limit 2 to year = ”1963–1975”
-
4.
Hemoglobin A/
-
5.
Limit 4 to year = ”1975–1983”
-
6.
((glycat* or glycosylat*) adj2 (hemoglobin* or haemoglobin* or hemo-globin* or haemoglobin*)).tw, kf.
-
7.
(hba1c or “hb a1c” or hbaic or “hb aic”).tw, kf.
-
8.
((ic or 1c or aic or a1c) adj2 (hemoglobin* or haemoglobin* or hemo-globin* or haemoglobin* or hb or hba)).tw, kf.
-
9.
(glycohemoglobin* or glyco-hemoglobin* or glycohaemoglobin* or glycohaemoglobin*).tw, kf.
-
10.
1 or 3 or 5 or 6 or 7 or 8 or 9
-
11.
exp preoperative care/
-
12.
exp preoperative period/
-
13.
(pre-op* or preop*).tw, kf.
-
14.
(presurg* or pre-surg*).tw, kf.
-
15.
((before or prior or previous or undergoing) adj3 (surger* or surgic* or procedure*)).tw, kf.
-
16.
11 or 12 or 13 or 14 or 15
-
17.
exp perioperative care/
-
18.
Perioperative period/
-
19.
(perop* or per-op* or periop* or peri-op*).tw, kf.
-
20.
Postoperative Period/
-
21.
(postoperati* or (post adj2 operati*) or postsurg* or ((post or after or following) adj2 surg*) or posttransplant* or ((post or after or following) adj2 transplant*) or ((postdischarg* or post-discharg*) adj3 surg*)).tw, kf.
-
22.
or/17–21
-
23.
exp general surgery/
-
24.
exp surgical procedures, operative/
-
25.
(surger* or surgic*).tw, kf.
-
26.
su.fs.
-
27.
or/23–26
-
28.
exp postoperative complications/
-
29.
(Co or mo).fs.
-
30.
(morbi* or mortalit* or adverse outcome* or complicat*).tw, kf.
-
31.
28 or 29 or 30
-
32.
10 and 16
-
33.
10 and (22 or 27) and 31
-
34.
32 or 33
-
35.
“Predictive value of tests”/
-
36.
Reference values/
-
37.
Forecasting/
-
38.
Prognosis/
-
39.
exp risk/
-
40.
(risk or risks or prognos* or predict*).tw, kf.
-
41.
or/35–40
-
42.
34 and 41
-
43.
Animals/not (animals/and humans/)
-
44.
42 not 43
-
45.
(exp child/or exp infant/or adolescent/) not exp adult/
-
46.
44 not 45
-
47.
Remove duplicates from 46
Rights and permissions
About this article
Cite this article
Karimian, N., Niculiseanu, P., Amar-Zifkin, A. et al. Association of Elevated Pre-operative Hemoglobin A1c and Post-operative Complications in Non-diabetic Patients: A Systematic Review. World J Surg 42, 61–72 (2018). https://doi.org/10.1007/s00268-017-4106-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4106-4