Abstract
Purpose
This study aimed to investigate the outcome for stage II and III rectal cancer patients compared to stage II and III colonic cancer patients with regard to 5-year cause-specific survival (CSS), overall survival, and local and combined recurrence rates over time.
Methods
This prospective cohort study identified 3,355 consecutive patients with adenocarcinoma of the colon or rectum and treated in our colorectal unit between 1981 and 2011, for investigation. The study was restricted to International Union Against Cancer (UICC) stages II and III. Postoperative mortality and histological incomplete resection were excluded, which left 995 patients with colonic cancer and 726 patients with rectal cancer for further analysis.
Results
Five-year CSS rates improved for colonic cancer from 65.0 % for patients treated between 1981 and 1986 to 88.1 % for patients treated between 2007 and 2011. For rectal cancer patients, the respective 5-year CSS rates improved from 53.4 % in the first observation period to 89.8 % in the second one. The local recurrence rate for rectal cancer dropped from 34.2 % in the years 1981–1986 to 2.1 % in the years 2007–2011. In the last decade of observation, prognosis for rectal cancer was equal to that for colon cancer (CSS 88.6 vs. 86.7 %, p = 0.409).
Conclusion
Survival of patients with colon and rectal cancer has continued to improve over the last three decades. After major changes in treatment strategy including introduction of total mesorectal excision and neoadjuvant (radio)chemotherapy, prognosis for stage II and III rectal cancer is at least as good as for stage II and III colonic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis for colon cancer has long been held superior to that of rectal cancer due to better accessibility during surgery, shorter operating time, fewer complications, and consequently a much lower local recurrence rate [1–5]. Since the 1980s, however, the prognosis for rectal cancer has caught up after the introduction of total mesorectal excision (TME) [3–9], specimen-directed pathologic work-up [10, 11], MRI-guided decision making [12], and preoperative (neoadjuvant) radio(chemo)therapy [13]. For colon cancer, an embryologic concept similar to TME called complete mesocolic excision (CME) was developed after 2000 but has gained wide acceptance only slowly [14], while adjuvant chemotherapy has become standard in many countries for International Union Against Cancer (UICC) stage III tumors [15]. This study was undertaken to compare outcomes for rectal and colon cancers over the last three decades in which major therapeutic improvements were introduced for both tumor entities using a prospectively held database.
Patients and methods
All patients with malignancies of the colon and rectum treated in our Department of Colorectal Surgery have been prospectively documented in a database since 1981. Follow-up was organized in a coloproctologic clinic where patients were seen at least annually for 5 years and every 2 years thereafter for 10 years in total. During the follow-up visits, all relevant data were collected in a meticulous search for local or distant recurrence. For patients who did not show up, a form was sent out to the family doctor inquiring about the life status and evidence of recurrence.
Management of colorectal cancer including operative technique changed considerably over time. Concerning rectal cancer surgery, blunt dissection of the rectum was carried out in the 1980s as in many other centers worldwide. After 1990, a more radical approach was pursued with stripping off the aorta and the iliac vessels of all tissue. TME was introduced in our department in 1996; 1 year later, we started to participate in the CAO/ARO/AIO-94 Study on preoperative vs. postoperative RCT in rectal cancer [13]. From that time on, MRI was regularly used for clinical staging and planning of surgery. After publication of the CAO/ARO/AIO-94 Study in 2004, neoadjuvant treatment became the standard for UICC stage II and III tumors. Adjuvant chemotherapy was regularly considered for all UICC stage III colon cancer after publication of the first German guideline for colorectal cancer in 1997. The principles of CME were introduced from 2006 on. Figure 1 shows a timeline when changes in treatment strategy were implemented in our clinic (Fig. 1).
From the database, all patients with histologic proven adenocarcinoma of the colon and rectum (excluding carcinoma of the appendix) were identified for this study. We excluded patients classified as UICC stage I and IV and investigated only locally advanced cancers (i.e., UICC stages II and III). With the introduction of neoadjuvant chemoradiotherapy for rectal cancer, it was no longer possible to determine the initial tumor stage by pathological examination because of intended tumor shrinking and lymph node sterilization by therapy. For these tumors, only clinical staging reflected the extent of the tumor at the start of therapy. Clinical staging has well-known limitations, especially with regard to accuracy of lymph node metastases. To circumvent this problem, we analyzed stages II and III together. Thus, we selected all patients with pathologic stages II and III when no preoperative therapy was given. Patients who had had preoperative treatment were selected by using clinical stages II and III. In a further subanalysis, stages II and III were considered separately. Furthermore, we considered all cancers only for patients who achieved a histologic complete resection (R0, irrespective of width of circumferential margin) and excluded postoperative mortality [16].
For survival analysis, we defined the date of diagnosis as the starting point. For cause-specific survival (CSS) estimations, we censored all deaths without tumor. This comprised all patients who died of other causes (including other malignancies). In overall survival, all deaths irrespective of the cause were counted as events. Local recurrences as well as combined recurrence (comprising distant and local recurrence) were also calculated as cumulative rates. The start of inclusion for recurrences was the date of operation. Survival analyses and recurrence rate analyses were performed according to the product limit method of Kaplan-Meier. For comparisons between the groups, we used the log rank test. Patients were divided in time intervals of 5 years. For reasons of clarity, we chose only two time intervals (1981–2001 vs. 2002–2011) for the depiction of the Kaplan-Meier curves.
Data collection was done with a d-base software throughout the time. For statistical analysis, we used SPSS vs. 21 (IBM Corp. Armonk, New York, USA). The follow-up period for this study ended on September 30, 2014.
Results
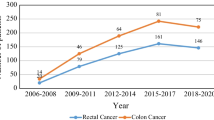
From 1981 through 2011, we treated a consecutive series of 3,355 patients with histologically proven adenocarcinoma of the colon or rectum in our department. We excluded 716 patients with distant metastases (stage IV) and another 35 patients with unknown M-category. Furthermore, we excluded 598 patients with stage I disease. Of the remaining patients with stage II and III tumors, 147 patients were excluded for in-hospital mortality and another 138 patients because of an R1 or an R2 status. This left 995 patients with colon carcinoma and 726 patients with rectal carcinoma for analysis. Only 2.4 % (22 of 929 alive at last follow-up) of patients were lost to follow-up at 5 years and 5.1 % (47 of 929) at 10 years. Median follow-up for patients treated from 2007 to 2011 was 56.4 months, and for all other time periods, it exceeded 5 years. Complete (R0) resection rate for colon carcinoma increased from 89.7 % (1981–1986) to 96.0 % (2007–2011), whereas R0 resection rate for rectal carcinoma increased from 84.2 % (1981–1986) to 95.5 % (2007–2011). Selected demographic and treatment data are given in Table 1. Patients with colon cancer were a median 3 years older than patients with rectal carcinoma, but age did not change significantly over time within the groups. Emergency operations with removal of the primary tumor were performed in 15.5 % of colon carcinoma cases. This rate varied over the time period but did not show any clear trend. Primary tumor resection of rectal carcinoma as an emergency was avoided whenever possible, resulting in a rate of 1.2 % for the entire time period. Of the patients with rectal carcinomas, 38.9 % had been treated with some kind of neoadjuvant therapy. As expected, neoadjuvant treatment was given more often during the later study period, reflecting the change of policy in Germany. In the last time interval (2007–2011), 76.5 % of patients with clinical stages II and III received preoperative radio(chemo)therapy. In colon carcinoma, neoadjuvant treatment was only given in eight (0.8 %) locally advanced cases. However, in this group of patients, the change in treatment strategy concerning adjuvant therapy started earlier in the 1990s. From this time on, adjuvant 5FU-based chemotherapy was administered in 55.0 % of patients with pathologic UICC stage III, with oxaliplatin added in an increasing proportion of cases over time.
For the whole study period, there was an increase in the 5-year CSS rate for colon cancer patients from 65.0 % in the first study period (1981–1986) to 88.1 % in the last study period (2007–2011) (Table 2 and Fig. 2). A 5-year CSS rate of more than 80 % was reached already in the period from 1992 to 1996 with only a slight increase in the following periods.
Five-year cause-specific survival. Five-year cause-specific survival in patients with rectum carcinoma vs. patients with colon carcinoma. Survival rate is given as a percentage. The date of surgery was used for forming groups. Only patients with UICC stages II and III are included. Death without tumor is censored
The 5-year CSS rate for rectal carcinoma patients improved from 53.4 % (1981–1986) to 89.8 % (2007–2011) (Table 2 and Fig. 2). The most pronounced improvement was observed in the time periods 1997–2001 and 2002–2006, when the 5-year CSS rate rose from 74.4 to 87.9 %. In 2002–2006, the 5-year CSS rate was similar to that of colonic cancer, and both rates remained on a plateau in the following period. This plateau phase from 2002 to 2011 was compared for the two different tumor sites in contrast to the earlier phase from 1981 to 2001 (Figs. 3 and 4). Whereas we could detect a significant difference for the earlier phase (77.9 (74.4–81.4) % for colon cancer vs. 64.5 (59.6–69.4) % for rectal cancer (p < 0.001)), the 5-year CSS rate was almost identical in the later phase (86.7 (83.0–90.4) % for colon cancer vs. 88.6 (84.9–92.3) % for rectal cancer (p = 0.409)). Trends for CSS were similar for stages II and III separately, albeit more inhomogeneous over time (Fig. 5).
Cause-specific survival 1981–2001. Cause-specific survival in patients with rectum carcinoma vs. patients with colon carcinoma. Survival rate is given as a percentage. Five-year cause-specific survival (CSS) rate for rectum (n = 394) 64.5 % (CI 59.6–69.4 %); CSS rate for colon (n = 611) 77.9 % (CI 74.4–81.4 %), p < 0.001. Only patients with UICC stages II and III are included. Death without tumor is censored. CI confidence interval
Cause-specific survival 2002–2011. Cause-specific survival in patients with rectum carcinoma vs. patients with colon carcinoma. Survival rate is given in percent. Five-year cause-specific survival (CSS) rate for rectum (n = 332) 88.6 % (CI 83.0–90.4 %); CSS rate for colon (n = 384) 86.7 % (CI 83.0–90.4 %) p = 0.409. Only patients with UICC stages II and III are included. Death without tumor is censored. CI confidence interval
Overall survival in the period from 1981 to 2001 for colon carcinoma patients was calculated with 61.9 (58.0–65.8) % and for rectum carcinoma patients with 54.3 (49.4–59.2) %. This difference reached a significant level with p = 0.01. For the second period under observation from 2002 to 2011, overall survival for colon carcinoma was 72.5 (67.8–77.2) % and 78.5 (73.8–83.2) % (p = 0.179) for rectal carcinoma, respectively.
After emergency operation for colon cancer, 5-year CCS dropped significantly by 12.4 % for emergency resections if all patients over the entire study period were included. In rectal cancer, the number of emergency resections was too small for a meaningful analysis. If we excluded all emergency resections, prognosis for colon cancer increased by approximately 2 % in each time interval, but the relation in prognosis to rectal cancer did not change (data not shown).
Similar trends were seen when we analyzed the 5-year local and combined recurrence rates for colon and rectal cancer patients. In the early years of observation, the cumulative isolated local recurrence rate for patients with rectal cancer was 34.2 %. The combined recurrence, including distant metastases, was 51 %. In the same time period, the patients with colon cancer had a recurrence rate of 13.9 % for isolated local recurrence and 38.7 % for local and distant recurrence combined (Table 2). Over the observed time periods, this changed significantly for the patients with rectal cancer, to an isolated local recurrence rate of 2.1 % in the latest period observed and an overall recurrence of 20.1 %, which approached the combined recurrence rate for colon cancer with 19.0 %. Figures 6 and 7 illustrate the trend for isolated local and combined recurrence rates over time.
Five-year isolated local recurrence rates. Isolated cumulative local recurrence rates after 5 years of follow-up in patients with rectum carcinoma vs. patients with colon carcinoma. The recurrence rate is given as a percentage and was calculated as a cumulative rate. The start of inclusion was the date of operation. Only patients with UICC stages II and III are included. Postoperative mortality and histologic incomplete resection are excluded
Five-year combined recurrence rate (local and distant). Combined (local and distant) recurrence rates after 5 years of follow-up in patients with rectum carcinoma vs. patients with colon carcinoma. The recurrence rate is given as a percentage and was calculated as a cumulative rate. The start of inclusion was the date of operation. Only patients with UICC stages II and III are included. Postoperative mortality and histologic incomplete resection are excluded
Discussion
Our study based on a prospective clinical database shows that the outcomes for rectal and colon cancer have continued to improve over the last three decades. Until recently, the survival rates for colon cancer have always been better than those for rectal cancer [2, 3]. Andreoni et al. calculated 5-year survival rates for colon cancer at 100 % (UICC I), 91 % (UICC II), and 76 % (UICC III), whereas rectal cancer patients showed 5-year survival rates of 93 % (I), 83 % (II), and 68 % (III) [3]. Similarly, Rutter et al. stated that patients diagnosed with rectal cancer had a poorer survival than colon cancer patients across all stages except stage IV in an analysis of 233,965 patients from the Surveillance, Epidemiology and End Results (SEER) program, collected from 1975 through 2003 [5]. Our study provides proof that in recent years, the prognosis for rectal cancer has at least caught up with the prognosis for colon cancer, due to profound changes in treatment strategies. A major step towards improved results for rectal cancer was the introduction of TME by Richard Heald in the early 1980s [9]. This specimen-oriented surgery following embryologic planes was able to reduce local recurrence rates from more than 20 % to well below 10 %. This could be demonstrated by nationwide changes of surgical technique by means of comprehensive workshops in Sweden, Norway, and the Netherlands [17–19]. The improvement in local recurrence rates was mirrored by an improvement of overall and cause-specific survival in a range of 10–13 % [6–8].
Parallel to the changes in surgical approach, in-depth pathology work-up of the specimens revealed the importance of an uninvolved circumferential resection margin (CRM) and the quality of the specimen itself [10, 20–23]. Both factors proved to be predictive for outcome and showed a high correlation with each other [22]. Nagtegaal et al. were able to show in a case series of 180 specimens an increased risk for local and distant recurrence if the circumferential resection margin was involved. This led to a combined local and distant recurrence rate of 36.1 vs. 20.3 % in the group with an uninvolved circumferential resection margin [21]. Furthermore, MRI was able to predict the relation of the tumor to the future CRM, i.e., the mesorectal fascia, with high accuracy, allowing the surgeon to adapt the extension of surgery to the individual requirement of the tumor [12, 24, 25]. The MERCURY Study Group was able to show a diagnostic accuracy of 94 % in a series of 408 patients for preoperative magnetic resonance imaging to predict CRM involvement by the tumor [26]. Iannicelli et al. were able to confirm this data by calculating a predictive CRM accuracy of 94.5 % for high-resolution staging MRI in rectal cancer [27].
Another important development in rectal cancer treatment was the introduction of radiotherapy or chemoradiotherapy, preferably administered as preoperative or neoadjuvant therapy [13, 18, 19]. (Chemo)radiotherapy is able to reduce local recurrence rates by approximately 50 %, even in series in which TME was the surgical standard [18]. However, no improvement of overall survival could be demonstrated in combination with TME [18]. Nowadays, all evidence about rectal cancer treatment is bundled in multidisciplinary team (MDT) discussions to individualize therapy for each patient [24, 28, 29].
For colon cancer, awareness of surgical principles based on embryologic preconditions was raised by Hohenberger in 2009 [14]. The cornerstone of CME is the removal of the entire lymphatic package of the involved colonic segment within embryologic planes and a central tie of the artery feeding this colonic segment. Furthermore, in transverse colon cancers and cancers of both flexures, special consideration must be given to gastroepiploic and inferior pancreatic lymph nodes [30]. Even in lymph node-positive cases, this kind of surgery leads to excellent results. Weber et al. presented data with improving 5-year cause-specific survival rates from 82.1 to 90.1 % as well as a reduction of local recurrence rate from 6.5 to 3.2 % [30, 31]. In the 1980s and 1990s, many studies were undertaken to demonstrate the benefit of adjuvant chemotherapy [32]. The current standard for stage III colon cancer is a regime that combines 5FU, leucovorin, and oxaliplatin [32].
Our effort was to demonstrate the impact of the change of treatment strategy on survival rates of patients with rectal cancer. For a reasonable comparison, we excluded patients in stage I and stage IV. The prognosis in stage I is so excellent that a detection of improvement over time seemed unlikely. In stage IV, prognosis is generally poor and depends on the rate of patients able to undergo metastatectomy. It was assumed that this would unduly bias the outcomes of surgery directed towards the primary tumor. For analysis, we excluded postoperative mortality, because improvement in that respect rather reflected achievements in perioperative care and not in oncologic therapy [16]. We found a substantial increase of CSS from 65.0 to 88.1 % for stage II and III colon cancer within the study period. For stage II/III rectal cancer, the increase in CSS was even more pronounced (53.4 vs. 89.8 %). The figures for the last two time intervals under investigation (2002–2011) compare favorably with the literature (Table 3). Most importantly, in our study population, the survival rates for both tumor entities seem to be almost equal for the last decade. Some authors still describe a difference of about 10 % worse survival for rectal cancer [3]. Birgisson et al. [19] and Nedrebo [17] showed on a register level that relative survival for rectal cancer had caught up with that for colon cancer (compare Table 3). However, it is difficult to estimate the net impact of treatment strategy in their studies because data was not stratified for tumor stage. Thus, the effects of increased awareness, screening, and consecutively a higher number of patients with earlier tumor stages may have biased the data. In our study, only stages II and III were selected to avoid this kind of bias. A similar approach as ours was used by Renouf et al., who selected stage II and III colon and rectal carcinoma for comparison as well [4]. Again, their study was based on register data. They were able to show an improvement in cause-specific survival for rectal cancer patients from 53.0 % in 1989/1990 to 65.7 % in 2001/2002 after implementing TME surgery in the affiliated surgery units. The improvement in disease-specific survival for colon cancer patients was only marginal from 63.2 to 68.7 % in the respective time periods. The differences in survival rates compared to our study might be explained by different extend of TME usage (42.8 % in the 2001/2002 time period vs. almost 100 % from 1996 on in our series) as well as the introduction of CME for colon cancer surgery in our unit. The rates of chemotherapy as well as radiotherapy were comparable to our study. Adjuvant therapies did not differ very much from our policy regarding type of chemotherapy, patient selection, or radiation protocols. In general, follow-up is a complex challenge for registries [33]. We had the opportunity to follow-up almost every single patient and were able to perform follow-up examinations in our department, allowing us to document the entire course of the disease.
Our study has some limitations that need to be addressed. First, restriction of the study to stage II and stage III patients may have led to some bias, especially for rectal cancer when clinical staging was used. Especially with regard to the lymph node evaluation, this might contain some inaccuracy. However, to ensure a high quality of staging, we have been using MRI as the standard diagnostic measure since introduction of neoadjuvant treatment in our center. The main problem of clinical staging is lymph node evaluation, with rates of accuracy ranging from 63 to 82 % in MRI scan as compared to accuracy for the T-category (81–94 %) [27, 34, 35]. To compensate for this uncertainty, we first analyzed stages II and III together. The separate analysis of the two stages showed similar trends with an improvement in prognosis for all four groups over time and insignificant differences in the later time periods. However, trends were more inhomogeneous and reflect the problem of stage migration provoked by neoadjuvant therapy. Nevertheless, we are convinced that the combination of stages II and III defines the group of patients that benefited most from modern treatment strategies and gives the most informative results. Second, we provided cause-specific survival rates and overall survival rates, being well aware of the problems inherent in that. Whereas accuracy of cause-specific survival depends heavily on the completeness of follow-up, overall survival is biased by age and general health of the population under investigation. However, for colorectal cancer, cause-specific survival is almost identical to relative survival and provides a high degree of accuracy in a population with close follow-up [36].
Third, this study shows an improvement of prognosis in general but is not sufficient to determine the impact of single treatment measures on patient survival. Before the introduction of TME in rectal cancer surgery, 30–50 % of recurrences were only local [37]. Eradication of local recurrence obviously resulted in higher survival rates. This could either be accomplished by improved surgery or by radiotherapy that compensated for suboptimal surgery. Series with quality-controlled TME surgery failed to show a survival benefit from radiotherapy for rectal cancer patients when given to an unselected patient population [18].
Comparing our observation periods from 2002 to 2006 with the period from 2007 to 2011, we almost see the same 5-year cause-specific survival for patients with rectal cancer. In the latter period, 76.5 % of the patients underwent some kind of neoadjuvant treatment compared to 54.1 % in the period before without observing major benefits in survival rates. This observation suggests that a more selective approach to neoadjuvant therapy based on high-quality MRI and high-quality TME is necessary. Recent studies have shown that this strategy results in short-term outcomes that are not inferior to unselected preoperative RCT in all UICC stage II and III patients [24, 38]. For colon cancer, it is even more difficult to outline the impact of improved surgery, because local recurrence is rare and often difficult to measure. Surrogate parameters like number of retrieved lymph nodes depend not only on surgical quality but also on the dedication and resources of pathological investigation. In our population, we found a decrease in combined local and distant recurrence from 25 to 19 % in the last three periods, although the rate of patients who received adjuvant chemotherapy plateaued at approximately 25 %. This may reflect the benefit of the introduction of CME in our department.
Conclusion
Our study provides evidence that 5-year cause-specific and overall survival rates for rectal cancer are as good as those for colon cancer. The implementation of modern treatment strategies has led to a better prognosis for both tumor entities. Room for improvement was greater for rectal carcinoma, and measures taken to avoid local recurrence, especially the application of proper surgical techniques, have leveled the commonly reported differences in outcome. This emphasizes the importance of well-organized multidisciplinary teams with well-trained surgeons for the adequate management of colorectal cancer.
References
Berrino F, Capocaccia R, Estève J, Gatta G, Hakulinen T, Micheli A, Sant M, Verdecchia A (1999) Survival of cancer patients in Europe: the EUROCARE-2 Study. IARC Scientific Publication 151
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, Bielska-Lasota M, Engholm G, Nennecke A, Siesling S, Berrino F, Capocaccia R, the EUROCARE-5 Working Group (2013) Cancer survival in Europe 1997–2007 by country and age: results of the EUROCARE-5-a population-based study. Lancet Oncol. 1470-2045(13)70546-1. doi:10.1016/S1470-2045(13)70546-1
Andreoni B, Chiappa A, Bertani E, Bellomi M, Orecchia R, Zampino MG, Fazio N, Venturino M, Orsi F, Sonzogni A, Pace U, Monfardini L (2007) Surgical outcomes for colon and rectal cancer over a decade: results from a consecutive monocentric experience in 902 unselected patients. World J Surg Oncol 5:73–81
Renouf DJ, Woods R, Speers C, Hay J, Phang PT, Fitzgerald C, Kennecke H (2013) Improvements in 5-year outcomes of stage II/III rectal cancer relative to colon cancer. Am J Clin Oncol 36:558–564
Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D (2013) Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst 105:1806–1813
Martling AL, Holm T, Rutqvist LE et al (2000) Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 356:93–96
Wibe A, Moller B, Norstein J et al (2002) A national strategic change in treatment policy for rectal cancer—implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 45:857–866
Kapiteijn E, Puffer H, van de Velde CJ (2002) Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 89:1142–1149
Heald RJ, Ryall RDH (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1479–82
Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2:996–999
Tilney HS, Rasheed S, Northover JM, Tekkis P (2009) The influence of circumferential resection margins on long-term outcomes following rectal cancer surgery. Dis Colon Rectum 52(10):1723–1729
The MERCURY study Group (2007) Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 243(1):132–139
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Color Dis 11(4):354–365
Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR et al (2004) Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 22(10):1797–1806
Hermanek P, Mansmann U, Staimmer DS, Riedl S, Hermanek P (2000) The German experience: the surgeon as a prognostic factor in colon and rectal cancer surgery. Surg Oncol Clin N Am 9(1):33–49, vi
Nedrebo BS, Soreide K, Eriksen MT, Dorum LM, Kvaloy JT, Soreide JA, Korner H (2011) Survival effect of implementing national treatment strategies for curatively resected colonic and rectal cancer. Br J Surg 98:716–723
Van Gijn W, Krijnen P, Lemmens VE, den Dulk M, Putter H, van de Velde CJ (2009) Quality assurance in rectal cancer treatment in the Netherlands: a catch up compared to colon cancer treatment. Eur J Surg Oncol 36:340–344
Birgisson H, Talbäck M, Gunnarsson U, Pahlman L, Glimelius B (2005) Improved survival in cancer of the colon and rectum in Sweden. EJSO 31:845–853
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26(2):303–312
Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH (2002) Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol 20:1729–1734
Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P (1994) Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 344(8924):707–711
Quirke P, Steele R, Monson J, Grieve R et al (2009) Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373(9666):821–828
Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G (2014) Preoperative magnetic resonance imaging assessment of circumferential resection Margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY Study. J Clin Oncol 32(1):34–43
Brown G (2005) Thin section MRI in multidisciplinary pre-operative decision making for patients with rectal cancer. Br J Radiol 78:S117–S127
MERCURY Study Group (2006) Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333:779
Iannicelli E, Di Renzo S, Ferri M et al (2014) Accuracy of high-resolution MRI with lumen distention in rectal cancer staging and circumferential margin involvement prediction. Korean J Radiol 15(1):37–44
Burton S, Brown G, Daniels I, Norman A, Swift I, Abulafi M, Wotherspoon A, Tait D (2006) MRI identified prognostic features of tumors in distal sigmoid, rectosigmoid, and upper rectum: treatment with radiotherapy and chemotherapy. Int J Radiat Oncol Biol 65(2):445–451
Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D, Hospital RM, Network CC (2006) MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 94:351–357
Perrakis A, Weber K, Merkel S, Matzel K, Agaimy A, Gebbert C, Hohenberger W (2014) Lymph node metastasis of carcinomas of transverse colon including flexures. Consideration of the extramesocolic lymph node stations. Int J Color Dis 29:1223–1229
Weber K, Merkel S, Perrakis A, Hohenberger W (2013) Is there a disadvantage to radical lymph node dissection in colon cancer? Int J Color Dis 28:217–226
André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116
Gunnarsson U (2003) Quality assurance in surgical oncology. Colorectal cancer as an example. Eur J Surg Oncol 29:89–94
Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS (1999) Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum 42:770–775
Topova L, Hellmich G, Puffer E, Schubert C, Christen N, Boldt T, Wiedemann B, Witzigmann H, Stelzner S (2011) Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum 54(4):401–411
Stelzner S, Hellmich G, Koch R, Witzigmann H (2009) Exactitude of relative survival compared with cause-specific survival and competing risk estimations based on a clinical database of patients with colorectal carcinoma. Dis Colon Rectum 52(7):1264–1271
Holm T, Johansson H, Cedermark B, Ekelund G, Rutquist LE (1997) Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without radiotherapy. Br J Surg 84:657–663
Ptok H, Ruppert R, Stassburg J, Maurer CA, Oberholzer K, Junginger T, Merkel S, Hermanek P (2013) Pretherapeutic MRI for decision-making regarding selective neoadjuvant radiochemotherapy for rectal carcinoma: interim analysis of a multicentric prospective observational study. J Magn Reson Imaging 37(5):1122–1128
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joern, F., Gunter, H., Thomas, J. et al. Outcome for stage II and III rectal and colon cancer equally good after treatment improvement over three decades. Int J Colorectal Dis 30, 797–806 (2015). https://doi.org/10.1007/s00384-015-2219-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2219-5