Abstract
Background
Previous studies investigating the impact of tumor location on colorectal cancer prognosis only compared two groups by location, e.g., ‘right-sided colon vs. left-sided colon,’ ‘colon vs. rectum,’ and ‘right-sided (right-sided colon) vs. left-sided (left-sided colon and rectum).’ This nationwide multicenter retrospective study aimed to clarify the prognostic impact of tumor location in patients with stage III colorectal cancer by classifying tumors into three groups: right-sided colon, left-sided colon, and rectum.
Methods
Subjects were 9194 patients with stage III colorectal cancer who underwent curative surgery from 1997 to 2012. Relapse-free survival (RFS) after primary surgery and overall survival (OS) after recurrence were examined.
Results
Rectal cancer (n = 2922) was associated with worse RFS compared to right-sided colon cancer (n = 2362) (hazard ratio (HR) 0.65; 95% CI 0.59–0.72; p < 0.001) and left-sided colon cancer (n = 3910) (HR 0.72; 95% CI 0.66–0.78; p < 0.001) after adjusting for key clinical factors (i.e., sex, age, histological type, CEA, adjuvant therapy, T category, and N category). Among patients with recurrence (n = 2823), rectal cancer was associated with better OS compared to right-sided colon cancer (HR 1.23; 95% CI 1.08–1.40; p = 0.002) and worse OS compared to left-sided colon cancer (HR 0.88; 95% CI 0.79–0.99; p = 0.029). Twenty percent of right-sided colon cancer recurrences exhibited peritoneal dissemination, 42% of left-sided colon cancer recurrences were liver metastases, and 33% of rectal cancer recurrences were local recurrences.
Conclusions
The three tumor locations (right-sided colon, left-sided colon, rectum) had different prognostic implications for recurrence after curative resection and overall mortality, suggesting that tumor location serves as a prognostic biomarker in stage III colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In colorectal cancer, the prognostic value of primary tumor sidedness has been a topic of considerable interest. For unresectable colorectal cancer, pooled analyses of several randomized trials that assessed the prognostic influence of primary tumor location in patients treated with molecular targeted agents revealed that right-sided (right-sided colon) tumors were significantly associated with worse overall survival (OS) compared to left-sided (left-sided colon and rectum) tumors [1, 2]. We recently reported that, regardless of use of targeted agents or performance of palliative primary tumor resection, unresectable stage IV right-sided colorectal tumors were associated with shorter OS compared to left-sided tumors [3, 4]. These results support the view that primary tumor sidedness has prognostic value in unresectable stage IV colorectal cancer [5].
The prognostic value of primary tumor sidedness has also attracted attention in nonmetastatic colon cancer. Some have reported that, among patients with stage III colon cancer, those with right-sided colon tumors had worse OS compared to those with left-sided colon tumors [6,7,8], whereas others reported no differences in OS between right-sided vs. left-sided colon tumors [9, 10]. Tumor location has also been shown to affect prognosis after recurrence in nonmetastatic colon cancer, with right colon cancer being associated with significantly shorter cancer-specific survival (CSS) after recurrence compared to left colon cancer in patients with stage II-III colon cancer [11] and those with stage III colon cancer [12].
According to global surveillance of trends in cancer survival 2000–14 (CONCORD-3), the prognosis of colon cancer was better than that of rectal cancer in most countries [13]. In the non-metastatic setting, not only prognosis but also treatment strategies differed between colon cancer and rectal cancer; while surgery is performed without preoperative therapy as the standard treatment for colon cancer globally [14], total mesorectal excision with preoperative chemoradiotherapy or chemotherapy is the current standard for locally advanced rectal cancer in many Western countries [15]. However, in Japan, the standard treatment for rectal cancer is surgery with total mesorectal excision plus lateral lymph node dissection, with no preoperative therapy [16].
Notably, previous studies investigating the impact of tumor location on colorectal cancer prognosis have all compared two groups by location, e.g., ‘right-sided (right-sided colon) vs. left-sided (left-sided colon and rectum),’ ‘right-sided colon vs. left-sided colon,’ and ‘colon vs. rectum.’ The unique treatment strategy for rectal cancer in Japan, which allows for comparison between rectal cancer and colon cancer without any influence of preoperative therapy, prompted us to investigate the prognostic value of primary tumor location by classifying tumors into three groups: right-sided colon, left sided colon, and rectum. Accordingly, the present nationwide multicenter retrospective study aimed to investigate the prognostic impact of the three tumor locations on recurrence and subsequent survival in patients with stage III colorectal cancer. Recurrence patterns in each group and survival after recurrence according to recurrence site were also evaluated.
Methods
Study population
The Japanese Study Group for Follow-Up of Colorectal Cancer (JFUP-CRC, see “Acknowledgements”) collected clinicopathological data from 33,625 patients with pathologically diagnosed stage I-III colorectal cancer who underwent curative surgery between January 1997 and December 2012 across 24 Japanese referral hospitals. In the present study, all patients with stage III colorectal cancer were included in the analysis, excluding those with the following: multiple primary tumors, multiple colorectal cancers, preoperative adjuvant therapy, histology other than adenocarcinoma, insufficient clinical and pathological information, unknown follow-up information, and unknown stage information. The study protocol was approved by the Central Institutional Review Board (Tokyo Medical and Dental University) and the Ethics Review Board of each institution, including the Institutional Review Board of the National Cancer Center Hospital (IRB code: 2018-397).
Follow-up
Patients underwent physical examinations, tumor marker assessment, computed tomography (CT), and colonoscopy to detect postoperative recurrence, as described previously [11, 16]. Typically, serum carcinoembryonic antigen (CEA) and CA19-9 measurements were performed every 3 months for the first 3 years, then every 6 months for 2 years; CT was performed every 6 months for 5 years; and colonoscopy was performed in the 1–3 years. Follow-up data were recorded until an event occurred, or until the study cutoff date of January 2019. Patient data were extracted from the prospectively generated database at each facility and analyzed retrospectively.
Data selection
Patients were divided into three groups according to tumor location: right-sided colon, left-sided colon, and rectum. Right-sided colon was defined as the cecum, ascending colon, and transverse colon. Left-sided colon was defined as the descending colon, sigmoid, and rectosigmoid junction. The following parameters were retrospectively assessed using medical records: treatment year, sex, ECOG performance status, histological type of the tumor, residual tumor (R) classification according to TNM classification (R0: No residual tumor, R1: Microscopic residual tumor, R2: Macroscopic residual tumor), [17] pathological stage according to the TNM classification (8th edition), [17] preoperative CEA levels, and the presence of adjuvant chemotherapy (adjuvant therapy was classified as either a single-agent therapy of fluoropyrimidines or doublets therapy). The site of recurrence was also included for patients with recurrence.
Statistical analysis
Pearson’s Chi-square test was used for categorical variables to examine various factors in the three groups. Relapse-free survival (RFS) after primary surgery was examined in the entire study population, and OS after recurrence was assessed only in those with recurrence. Survival curves were generated using the Kaplan–Meier method to estimate RFS and OS with the log-rank test. RFS was defined as the interval between the date of primary surgery and the date of recurrence or death from all causes. OS was defined as the interval between the date of primary surgery and the date of death from all causes. Patients alive at the end of the follow-up period were censored. Multivariate Cox proportional hazards regression models were subsequently fitted to evaluate factors independently associated with recurrence and death. Since colorectal cancer, once it recurs, metastasizes to multiple sites, each recurrence site was considered to constitute a competing risk. Thus, a competing risk analysis was performed to analyze the initial recurrence site as the cumulative recurrence rate [18]. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using JMP statistical software version 14 (SAS Institute Inc., Cary, NC) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study cohort characteristics
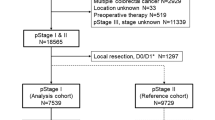
The final study population comprised 9194 patients with colorectal cancer, including 2362 (25.7%) patients with right-sided colon cancer, 3910 (42.5%) with left-sided colon cancer, and 2922 (31.8%) with rectal cancer (Fig. 1). Table 1 summarizes the clinicopathological characteristics of the study cohort by group. The rate of lateral lymph node dissection for stage III rectal cancer was 39.0% (1140/2922), of which 386 patients (33.9%) had lateral lymph node metastases, which accounted for 13.2% of patients with rectal cancer (386/2922). Significant differences were observed in sex, age, histological type, preoperative CEA levels, pT category, pN category, and the presence of adjuvant therapy between the three groups. Briefly, patients with right-sided colon cancer were more likely to be female, significantly older, less likely to have differentiated adenocarcinoma, and more likely to have a higher pathological T category compared to those with left-sided colon cancer and those with rectal cancer (all p < 0.01). Of the 3467 patients for whom adjuvant chemotherapy regimens were known, a higher proportion of those with rectal cancer underwent doublet therapy relative to the proportions of those with right-sided colon cancer and left-sided colon cancers (6.3% versus 4.0%, 3.1%, respectively; p < 0.0001). After the primary surgery, 2823 patients (30.7%) relapsed, and 1813 patients died of colorectal cancer recurrence during the study period. Rates of recurrence after surgery also differed between the three groups (right-sided: 26.0%, left-sided: 27.0, rectum: 39.5%).
Study cohort selection process. The Japanese Study Group for Follow-Up of Colorectal Cancer collected clinicopathological data of a total of 33,625 patients with pathologically-diagnosed stage I-III colorectal cancer who underwent curative surgery between January 1997 and December 2012 across 24 Japanese referral hospitals. A total of 9194 patients with colorectal cancer were included in the final study population
Relapse-free survival in right-sided colon, left-sided colon, and rectal cancers
The 3-year and 5-year RFS rates of patients with stage III colorectal cancer (the entire cohort) were 70.2% and 66.0%, respectively (data not shown). Figure 2a shows RFS curves for patients with stage III colorectal cancer, stratified by tumor location. The 3-year and 5-year RFS rates of patients with rectal cancer were 63.1% and 58.4%, respectively, while those of patients with left-sided and right-sided colon cancers were 73.6% and 69.3%, and 73.4% and 70.0%, respectively (p < 0.001).
a Relapse-free survival curves for patients with stage III colorectal cancer, stratified by tumor location. Of 9194 patients, 2362 (25.7%) patients had right-sided colon cancer, 3910 (42.5%) had left-sided colon cancer, and 2922 (31.8%) had rectal cancer. b Relapse-free survival curves for patients with stage III colorectal cancer who were treated with adjuvant therapy (n = 5730). c Relapse-free survival curves for patients with stage III colorectal cancer who were treated without adjuvant therapy (n = 2804)
Relapse-free survival in Stage III colorectal cancer with or without adjuvant therapy
Since the present study examined stage III colorectal cancer patients who were treated with and without adjuvant therapy (n = 5730 and n = 2804, respectively), RFS curves were plotted separately according to the presence of adjuvant therapy (Fig. 2b, c, stratified by tumor location). Regardless of the presence or absence of adjuvant therapy, RFS rates were similar between patients with right-sided colon cancer and those with left-sided colon cancer. Regimens of adjuvant therapy were as follows; intravenous fluoropyrimidines: 14.6%, oral fluoropyrimidines: 81.1% (tegafur-uracil (UFT): 60.7%, capecitabine: 16.9%, S-1: 3.5%), 5- fluoropyrimidines plus oxaliplatin: 4.0%, fluoropyrimidines plus irinotecan: 0.3%.
Factors affecting relapse-free survival in Stage III colorectal cancer
Univariate analysis revealed that sex (male), age (≥ 75 years), histological type (others), CEA (> 5), adjuvant chemotherapy (without), T category (T4), and N category (N2) were significantly associated with worse RFS (all p < 0.0001) (Table 2). Multivariate analyses using Cox proportional hazards regression models revealed that, after adjusting for sex, age, histological type, CEA, adjuvant therapy, T category, and N category, rectal cancer was associated with significantly worse RFS compared to right-sided colon cancer (HR 0.65, 95% CI 0.59–0.72; p < 0.001) and left-sided colon cancer (HR 0.72, 95% CI 0.66–0.78; p < 0.001) (Table 2).
Factors affecting relapse-free survival (in consideration of surgical factor and host status)
Of the data pertaining to the entire cohort (collected 1997–2012), those for residual tumor (R) status and performance status were both available for the last four years of patients (2009–2012). Thus, with 1854 patients for whom data were available for both of these variables, we analyzed factors affecting RFS including these two factors as covariates (Supplementary Table 1). Multivariate analyses revealed that R status was an independent prognostic factor [HR (R1, R2/R0), 2.34, 95% CI 1.58–3.47; p < 0.0001], while performance status was not (p = 0.838). Moreover, although these two factors were included as covariates in the multivariate analyses, tumor location was an independent prognostic factor for relapse-free survival; specifically, rectal cancer was associated with significantly worse RFS compared to right-sided colon cancer (HR 0.54, 95% CI 0.44–0.68; p < 0.001) and left-sided colon cancer (HR 0.62, 95% CI 0.51–0.75; p < 0.001).
Overall survival after recurrence stratified by tumor location
We next investigated OS after recurrence by limiting the analysis to patients with recurrence. After primary surgery, a total of 2823 patients (30.7%) had recurrence, including 613 (21.7%) patients with right-sided colon cancer, 1057 (37.4%) with left-sided colon cancer, and 1153 (40.9%) with rectal cancer. Of these, 2746 patients were analyzed for OS after recurrence, excluding 77 with unknown recurrence date. Figure 3a shows OS curves after recurrence in patients with recurrence, stratified by tumor location. The 3-year and 5-year OS rates were 48.4% and 31.6%, respectively, in patients with rectal cancer (n = 1114); 50.7% and 36.6%, respectively, in those with left-sided colon cancer (n = 1040); and 36.8% and 23.3%, respectively, in those with right-sided colon cancer (n = 592) (p < 0.001). These results demonstrate that, among patients with recurrence, those with right-sided colon cancer had the worst OS after recurrence even though RFS after curative surgery was better compared to those with left-sided colon cancer and those with rectal cancer.
a OS curves after recurrence in 2746 patients with recurrent colorectal cancer, stratified by tumor location; 592 patients with right-sided colon cancer, 1040 patients with left-sided colon cancer, and 1114 patients with rectal cancer were analyzed. b OS curves after recurrence in 2746 patients with recurrent colorectal cancer, stratified by recurrence site. Sites of recurrence were as follows: lung only (n = 712), liver only (n = 871), peritoneal only (n = 259), locoregional only (n = 652), and others/multiple sites (n = 252)
Factors affecting overall survival after recurrence in Stage III colorectal cancer
Table 3 shows the results of univariate and multivariate analyses of factors affecting OS in patients with recurrent stage III colorectal cancer. After adjusting for sex, age, histological type, CEA, adjuvant therapy, T category, and N category, right-sided colon cancer was associated with significantly worse OS compared to rectal cancer (HR 1.23, 95% CI 1.08–1.40; p = 0.002), whereas left-sided colon cancer was associated with significantly better OS compared to rectal cancer (HR 0.88, 95% CI 0.79–0.99; p = 0.029).
Overall survival after recurrence stratified by recurrence site
Sites of recurrence differed among 2746 patients with recurrence: lung only (n = 712), liver only (n = 871), peritoneal only (n = 259), locoregional only (n = 652), and others/multiple sites (n = 252). Analyses of OS after recurrence stratified by recurrence site revealed that 3-year and 5-year OS rates were 59.4% and 41.9%, respectively, in patients with lung only recurrence; 51.4% and 37.4%, respectively, in those with liver only recurrence; 42.4% and 25.8%, respectively, in those with locoregional only recurrence; 25.0% and 12.6%, respectively, in those with peritoneal only recurrence; and 28.7% and 18.0%, respectively, in those with other/multiple-site recurrence.
Competing risk analysis by recurrence site
Figures 4a–c show competing risk analysis of recurrence sites for right-sided colon, left-sided colon, and rectal cancers, respectively. Proportions of recurrence occurring over the cumulative three years in liver only, lung only, peritoneal only, locoregional only, and others/multiple sites were 36.0%, 18.6%, 20.1%, 13.6%, and 4.2%, respectively, among those with right-sided colon cancer; 42.1%, 24.2%, 10.1%, 14.9%, and 3.7%, respectively, among those with left-sided colon cancer; and 24.0%, 29.2%, 2.5%, 33.4%, and 3.9%, respectively, among those with rectal cancer. Recurrence in the liver/lung accounted for 66.3% of all recurrences originating from left-sided colon cancer, or 54% of all recurrences originating from right-sided colon/rectal cancers (p < 0.001). Local recurrence accounted for 33% of all recurrences originating from rectal cancer, or less than 15% of all recurrences originating from right-sided and left-sided colon cancers (p < 0.001). Peritoneal metastases accounted for roughly 20% of all recurrences originating from right-sided colon cancer, or 10% and 3% of recurrences originating from left-sided colon cancer and rectal cancer, respectively (p < 0.001). No significant differences were observed in recurrences for others/multiple sites (p = 0.591).
Discussion
This study analyzed long-term outcomes of stage III colorectal cancer to investigate the prognostic impact of tumor location by classifying tumors into three groups: right-sided colon, left-sided colon, and rectum. After adjusting for key clinical factors (i.e., sex, age, histological type, CEA, adjuvant therapy, T category, and N category), rectal cancer was associated with worse RFS compared to right-sided colon cancer (HR 0.65; p < 0.001) and left-sided colon cancer (HR 0.72; p < 0.001), while right-sided and left-sided colon cancers had almost identical RFS. These findings were also confirmed in subsequent analyses performed in two cohorts: those who were treated with adjuvant therapy and those who were treated without adjuvant therapy. Another significant finding of the present study was that, when analyses were limited to patients with recurrence, OS after recurrence was significantly longer among those with left-sided colon cancer, followed by those with rectal cancer and those with right-sided colon cancer. These results were partly consistent with a previous study reporting a significantly shorter 5-year CSS after recurrence in patients with right colon cancer compared to those with left colon cancer [11, 12]. Taken together, different tumor locations (i.e., right-sided colon, left-sided colon, rectum) have different prognostic implications for recurrence after curative resection and overall mortality of patients with stage III colorectal cancer, suggesting that primary tumor location might serve as a prognostic biomarker in stage III colorectal cancer. Our current study proposes that right-sided colon cancer, left-sided colon cancer, and rectal cancer should be considered distinct entities in stage III colorectal cancer and emphasizes implications of this distinction for clinical practice, which is novel insight in this field.
One reason that may explain why primary tumor location could be a prognostic factor may lie in the embryological origin of normal tissue. Briefly, the right-sided colon is derived from the embryonic mid-gut, while the left-sided colon and rectum are derived from the embryonic hind-gut. Consistent with this difference in embryological origin, the genetic carcinogenic pathways also differ [19]. Similarly, since the right-sided colon is vascularized by the superior mesenteric artery, the left-sided colon by the inferior mesenteric artery, and the rectum by the inferior mesenteric artery and middle rectal artery, sites and patterns of lymph node metastases differ by tumor location. In fact, lateral pelvic lymph node metastases occur only from tumors located in the rectum. These differences could result in diverse clinical, pathological, and biological features. Another potential explanation we have identified in this report is the difference of recurrence site. As shown in Fig. 1, 66% of recurrences originating from left-sided colon cancer were liver/lung metastases, 33% of those originating from rectal cancer were local recurrences, and roughly 20% of those originating from right-sided colon cancer were peritoneal disseminations. Consistent with previous reports [20, 21], OS rates after recurrence differed by recurrence site, with lung/liver metastases showing the most favorable OS after recurrence, followed by local recurrence and peritoneal dissemination, regardless of primary tumor location (Fig. 3b). Taken together, these results indicate that longer OS after recurrence in patients with primary tumors of the left-sided colon, rectum, and right-sided colon, in decreasing order, might be explained by the difference in distribution of recurrence sites.
Another possible explanation may lie in clinicopathological characteristics of patients that differ by tumor location. As shown in Table 1, in stage III patients, pathological T category was more advanced in those with right-sided colon cancer compared to those with left-sided colon cancer and rectal cancer. This could partly be explained by clinical issues in the diagnosis of right-sided colon cancer, which can be asymptomatic because of the more distal location from the anus and the passage of softer bowel content through the lesions [11]. On the other hand, pathological N category was more advanced in patients with rectal cancer compared to those with right-sided and left-sided colon cancers, possibly due to the higher proportion of metastases to lateral lymph nodes in rectal cancer patients (13.2%) compared to colon cancer patients (0% for both right-sided and left-sided) (data not shown). As rectal cancer with lateral lymph node metastases is considered locally advanced [22], it is not surprising that a high proportion of patients with rectal cancer were classified as pathological N2. Thus, multivariate analyses were performed to adjust for these different characteristics, which still revealed that the three tumor locations (right-sided colon, left-sided colon, rectum) of stage III colorectal cancer had different prognostic implications for recurrence after curative resection and overall mortality.
Regarding the association between tumor location and genetic characteristics in stage III colorectal cancer, it is known that a mutation in the BRAF or KRAS codon 12 was enriched in right-sided colon cancer, whereas nonmutated BRAF/KRAS was increased in left-sided colon cancers [23]. In addition, another study found that the incidence of BRAF mutation and microsatellite deficient mismatch repair (dMMR) in tumors gradually decreased as tumor location shifted from the right-sided colon to the rectum [24]. Mutant KRAS tumors showed poorer OS [23] and dMMR tumors showed longer survival after recurrence [25]. Thus, a different prognosis by tumor location can be partially explained by molecular differences in colorectal cancers according to their location in the bowel.
This study has several limitations. First, the study population included patients who were treated more than 20 years ago. During this long period, treatment strategies, including laparoscopic surgery [26] and intensive chemotherapeutic regimens in both adjuvant and metastatic settings, have changed significantly. Although we analyzed and obtained similar RFS after curative resection in the entire cohort as well as the two cohorts stratified by the presence of adjuvant therapy, the obtained results might not be fully reflective of current medical practice. Second, there was a lack of data regarding genetic phenotypes. Prognostic differences by tumor location might be explained partly by genetic differences, since right-sided colon cancers are, for example, known to be characterized by a high frequency of microsatellite instability and BRAF mutations [27, 28]. Third, information on comorbidities and postoperative complications was not available in our database. According to Japan Clinical Oncology Group (JCOG) studies, the complication rate for rectal cancer surgery is approximately 20% (JCOG0212) whereas that for colon cancer surgery is roughly 10% (JCOG0404). One reason given for the poor prognosis for rectal cancer was the higher rates of postoperative complications; however, we surmise that other factors are involved as well. One study evaluated the impact of postoperative complications on survival of colorectal cancer using pooled individual patient’s data from three large phase III randomized trials and found that surgical complications as well as tumor location (colon versus rectum) were independent risk factors for disease-free survival and OS [29]. For this reason, the extent to which these parameters might have influenced our analyses remains unclear. Fourth, information on chemotherapy regimens such as usage of molecular target drugs after recurrence were not available in our database, although these factors are known to influence OS [3]. Fifth, while cases involving tumor perforation at the time of surgery were few in number, information on these cases was not available in our database. Notably, tumor perforation could influence survival, as it is associated with lower disease-free survival [30], and because diastatic perforation proximal to an obstructing tumor is known to associate with higher operative mortality [31]. Sixth, while we adjusted for known confounders, there may have been bias due to unknown confounding factors. Despite these limitations, in the present study, multivariate analyses revealed that rectal cancer had worse RFS compared to right-sided colon cancer, and when the analyses were limited to patients with recurrence, rectal cancer was associated with better OS compared to right-sided colon cancer.
In conclusion, this nationwide multicenter retrospective study demonstrated that the three tumor locations (right-sided colon, left-sided colon, rectum) of stage III colorectal cancer had different prognostic implications for recurrence after curative resection and overall mortality. Differences in OS after recurrence by location might be due to the difference in distribution of recurrence sites, which defines the prognosis, suggesting that primary tumor location might serve as a prognostic biomarker, as well as a surrogate for tumor biology, in stage III colorectal cancer. Thus, as is the case for metastatic colorectal cancer, future randomized trials for stage III colorectal cancer should consider tumor location as a stratification parameter.
Abbreviations
- CI:
-
Confidence interval
- CSS:
-
Cancer-specific survival
- dMMR:
-
Deficient mismatch repair
- HR:
-
Hazard ratio
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
References
Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29.
Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3:194–201.
Shida D, Tanabe T, Boku N, et al. Prognostic value of primary tumor sidedness for unresectable stage IV colorectal cancer: a retrospective study. Ann Surg Oncol. 2019;26:1358–65.
Shida D. ASO author reflections: prognostic impact of primary tumor sidedness for unresectable stage IV colorectal cancer. Ann Surg Oncol. 2019;26:666–7.
Kamath SD, Khorana AA. Does sidedness matter in unresectable colorectal cancer? Ann Surg Oncol. 2019;26:1588–91.
Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs. right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3:211–9.
Ha GW, Kim JH, Lee MR. Oncologic effects of primary tumor-sidedness on patients with stages 1–3 colon cancer: a meta-analysis. Ann Surg Oncol. 2019;26:1366–75.
Yahagi M, Okabayashi K, Hasegawa H, et al. The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg. 2016;20:648–55.
Karim S, Brennan K, Nanji S, et al. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol. 2017;3:1386–92.
Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I-III colon cancer patients. BMC Cancer. 2016;16:554.
Ishihara S, Murono K, Sasaki K, et al. Impact of primary tumor location on postoperative recurrence and subsequent prognosis in nonmetastatic colon cancers: a multicenter retrospective study using a propensity score analysis. Ann Surg. 2018;267:917–21.
Kishiki T, Kuchta K, Matsuoka H, et al. The impact of tumor location on the biological and oncological differences of colon cancer: Multi-institutional propensity score-matched study. Am J Surg. 2019;217:46–52.
Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Benson AB 3rd, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:370–98.
Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:874–901.
Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2019;25:1–42.
UICC. TNM classification of malignant tumours eighth edition. Brierley JD, Gospodarowicz MK, Wittekind C, editors. New York: John Wiley and Sons, Ltd; 2017.
Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–9.
Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–6.
Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17:1709–19.
Shida D, Yoshida T, Tanabe T, et al. Prognostic impact of R0 resection and targeted therapy for colorectal cancer with synchronous peritoneal metastasis. Ann Surg Oncol. 2018;25:1646–53.
Akiyoshi T, Watanabe T, Miyata S, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg. 2012;255:1129–34.
Sinicrope FA, Mahoney MR, Yoon HH, et al. Analysis of molecular markers by anatomic tumor site in stage III colon carcinomas from adjuvant chemotherapy trial NCCTG N0147 (Alliance). Clin Cancer Res. 2015;21:5294–304.
Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54.
Taieb J, Shi Q, Pederson L, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol. 2019;30:1466–71.
Shida D, Ochiai H, Tsukamoto S, et al. Long-term outcomes of laparoscopic versus open D3 dissection for stage II/III colon cancer: Results of propensity score analyses. Eur J Surg Oncol. 2018;44:1025–30.
Pai RK, Jayachandran P, Koong AC, et al. BRAF-mutated, microsatellite-stable adenocarcinoma of the proximal colon: an aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am J Surg Pathol. 2012;36:744–52.
Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–32.
Aoyama T, Oba K, Honda M, et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med. 2017;6:1573–80.
Ho YH, Siu SK, Buttner P, et al. The effect of obstruction and perforation on colorectal cancer disease-free survival. World J Surg. 2010;34:1091–101.
Carraro PG, Segala M, Orlotti C, et al. Outcome of large-bowel perforation in patients with colorectal cancer. Dis Colon Rectum. 1998;41:1421–6.
Acknowledgements
This study is based on data from 24 hospitals, which are members of the Japanese Study Group for Postoperative Follow-up of CRC, as follows: Ichiro Takemasa (Sapporo Medical University); Kenichi Hakamada (Hirosaki University); Hitoshi Kameyama (Niigata University); Yasukimi Takii (Niigata Cancer Center Hospital); Hideki Ueno (National Defense Medical College); Heita Ozawa (Tochigi Cancer Center); Soichiro Ishihara (the University of Tokyo); Keiichi Takahashi (Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital); Yukihide Kanemitsu (National Cancer Center Hospital); Michio Itabashi (Tokyo Women’s Medical University); Tomomichi Kiyomatsu (National Center for Global Health and Medicine); Yusuke Kinugasa (Tokyo Medical and Dental University); Koji Okabayashi (Keio University); Yojiro Hashiguchi (Teikyo University); Tadahiko Masaki (Kyorin University); Masahiko Watanabe (Kitasato University); Akio Shiomi (Shizuoka Cancer Center); Tsunekazu Hanai (Fujita Health University); Koji Komori (Aichi Cancer Center Hospital); Yoshiharu Sakai (Kyoto University); Masayuki Ohue (Osaka International Cancer Institute); Shingo Noura (Osaka Rosai Hospital); Naohiro Tomita (Hyogo College of Medicine); and Yoshito Akagi (Kurume University).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shida, D., Inoue, M., Tanabe, T. et al. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol 55, 958–968 (2020). https://doi.org/10.1007/s00535-020-01706-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01706-7