Abstract
Purpose
Mucinous appendiceal tumours were described already 180 years ago, but reliable data on the incidence of these tumours are sparse. The clinical importance of these tumours is increasing since they are now identified as the most common site of origin for pseudomyxoma peritonei (PMP), which is currently recognised as a treatable condition.
Methods
Data on the incidence of mucinous appendiceal tumours were retrieved from the Eindhoven Cancer Registry, which collects data on all patients with newly diagnosed cancer in a large part of the southern Netherlands that comprises about 2.3 million inhabitants. From 1980 to 2010, all cases of primary adenocarcinomas of the appendix were included.
Results
From 1980 to 2010, a mucinous adenocarcinoma was diagnosed in 78 patients being 48 % of all cases of appendiceal adenocarcinoma diagnosed during this period (n = 164). The incidence increased during the study period from 0.6 to 1.9 per 1,000,000 person-years for women and from 0.4 to 1.0 per 1,000,000 person-years for men.
Conclusion
The reported incidence of mucinous adenocarcinomas of the appendix shows an increasing trend. This is probably mainly explained by the increased awareness of this tumour and its relation with PMP, and better registration of this specific diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Already in 1842, Karl Rokitansky was the first to recognise and describe an appendiceal mucocele. The first case of pseudomyxoma peritonei (PMP) was described in literature 42 years later by a gynaecologist named Werth [1]. Next, Frankel was the first to link PMP with the presence of appendiceal cysts in 1901, while Naesland observed an apparently causative relation between the presence of an appendiceal mucocele with an ovarian cystadenoma already at the beginning of the twentieth century [2]. However, for many years, discussion concerning the origin of PMP remained. Initially, most clinicians even thought PMP to originate from the ovaries. This is not surprising given the ovarian involvement in 85 % of women with PMP [1]. Only recently, histopathological research has demonstrated that at least 82 % of PMP cases are of appendiceal origin and that a primary ovarian origin accounts for only 3–10 % of cases [1, 3].
Cells aligning mucinous appendiceal tumours, either benign or malignant, produce mucin. This process eventually results in rupture of the appendiceal cyst or tumour. Then, mucin-producing cells spread throughout the abdominal cavity and continue to produce mucin, resulting in a state commonly referred to as “pseudomyxoma peritonei” or PMP. Because of the vast amounts of free intraperitoneal mucus that is produced, the alternative and more descriptive term is “jelly belly”.
With the introduction of an effective treatment combining cytoreductive surgery with heated intraperitoneal chemotherapy (HIPEC) [4], there is a growing interest in PMP. However, although mucinous appendiceal tumours have been described already 180 years ago, still little is currently known concerning their incidence and prognosis. In order to provide reliable population based data on the incidence of mucinous malignancies of the appendix, a population-based study was performed.
Methods
The Eindhoven Cancer Registry (ECR) collects data on all patients with newly diagnosed cancer in a large part of the southern Netherlands, which comprises about 2.3 million inhabitants. Data represent approximately 14 % of the Dutch population. This population-based registry is notified by 6 pathology departments, 10 community hospitals at 17 locations, and 2 radiotherapy institutions. Information on patient and tumour characteristics is routinely extracted from the medical records by specially trained administrators of the cancer registry. Registration takes place 6–18 months after diagnosis. By means of an independent case ascertainment method, the completeness of the registration is estimated to exceed 95 %. Vital status of all patients diagnosed was assessed on 1 January 2012 through merging with the Municipal Administrative Databases, where all deceased and emigrated persons in the Netherlands are registered.
From 1980 to 2010, all primary adenocarcinomas of the appendix that were diagnosed in the Eindhoven Cancer Registry area were included and analysed for incidence, patient characteristics and survival. In the mucinous group, mucinous cystadenocarcinoma, mucinous adenocarcinoma, and other mucin producing adenocarcinomas were included. The nonmucinous group contained all other types of appendiceal adenocarcinoma, including intestinal type and signet cell adenocarcinoma. Other types of appendiceal neoplasms, e.g., carcinoids, were excluded. Incidence rates according to sex and period of diagnosis were calculated per 1,000,000 person-years. The incidence rates were age standardised to the European standard population (European Standardised Rates). Only the patients who were living inside the region of the ECR at time of diagnosis were included for this analysis (n = 141).
Results
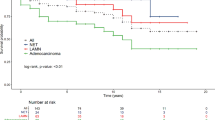
From 1980 to 2010, 164 patients were diagnosed with an appendiceal adenocarcinoma. A mucinous adenocarcinoma was diagnosed in 78 patients (48 %) and a nonmucinous adenocarcinoma in 86 patients (52 %; Table 1). Mean age of diagnosis was 66 years for mucinous adenocarcinoma and 64 years for the nonmucinous group (Table 2). Patients with a mucinous adenocarcinoma demonstrated a female predominance of 63 % compared to 57 % in the nonmucinous tumours (Table 2). Furthermore, the number of patients diagnosed with an adenocarcinoma is increasing from 1990 onwards (Table 3). Chi-square analysis on these data revealed no statistically significant difference. As shown in Fig. 1, the age-standardised incidence rates increased during the study period from 0.6 to 1.9 per 1,000,000 person-years for women and from 0.4 to 1.0 per 1,000,000 person-years for men. Data on survival demonstrated a 5-year survival of 50 % for patients diagnosed with a mucinous adenocarcinoma, compared to 40 % for patients with a nonmucinous adenocarcinoma (Fig. 2).
Discussion
In this population-based study, an increasing trend in the incidence of mucinous appendiceal malignancies is demonstrated, especially in women. In the last decade, the age-standardised incidence of mucinous adenocarcinoma was 1.0 and 1.9 per 1,000,000 person-years for men and women respectively. Chi-square analysis on these data revealed no statistically significant difference, but might be due to the small number of patients in each group.
These incidences are remarkably higher than those reported in a previous population based study by McCusker et al. They performed an analysis with data from the National Cancer Institute’s Surveillance, Epidemiology and End-Results programme and reported an age-adjusted incidence of appendiceal cancer of 0.12 cases per 1,000,000 people per year [5]. An explanation may be that their study included patients between 1973 and 1998. In the present study, the incidence was also remarkably lower in the earliest decade. Indeed, an update of the McCusker study including patients until 2007 as published by Turaga and collegues, also revealed an increased incidence of appendiceal carcinoma, probably attributable to a higher incidence in most recent years [5, 6].
Another way to investigate the incidence of mucinous appendiceal tumours is to study appendectomy specimens. Reported malignancy rates in these specimens typically range from 0.9 to 1.7 % [3, 5–8]. Among appendiceal malignancies, a mucinous adenocarcinoma is usually the predominant histological subtype ranging from 23 to 55 % of malignancies [1, 5–7, 9]. The mean age of diagnosis for mucinous adenocarcinoma is around 60 years and survival (5 years after the diagnosis) is reported to be 58 % [4, 5]. These data are all in accordance to our findings.
The increased incidence of mucinous tumours probably results from improved diagnostic accuracy through modern imaging and pathological techniques. The observation that histological subtype of an appendiceal tumour is directly correlated with the prognosis is another reason why more attention is paid to a more detailed description of subtype of these tumours [5]. Finally, the relationship between appendiceal neoplasms and the development of PMP urges the pathologist to diagnose an appendiceal tumour correctly, especially regarding the mucinous component. PMP is currently getting more attention since a combination of cytoreduction and HIPEC now offers long-term survival or even cure in selected patients [4].
In literature, there is no convincing explanation for the female predominance in this disease. The ovaries are a common metastatic site and in fact an ovarian mass is the initial presentation in many patients but this may only partially explain this phenomenon [1, 10].
The current study presents data concerning malignant mucinous tumours only. It is known that benign neoplasms of the appendix (mucocele and mucinous or cystadenoma) can also cause peritoneal spread of mucin producing tumour cells resulting in PMP. However, the likelihood to develop PMP is much lower in patients with a benign appendiceal mucinous tumour. Patients with appendiceal mucocele are estimated to develop PMP in only 2 % of cases, in contrast to 20 % of patients with a malignant mucinous adenocarcinoma [1]. This is confirmed by a study of Nitecki et al. where PMP occurred in 23 % of all patients with a primary tumour of the appendix but only in those that had a mucinous adenocarcinoma (58 %) [8].
Taken together, the reported incidence of mucinous appendiceal neoplasms shows an increasing trend and the incidence of the last decade as reported in the study is higher than previously published in literature. With current knowledge that the subtype of an appendiceal neoplasm is directly correlated with prognosis and that the mucinous subtype may result in PMP, the awareness of mucinous appendiceal neoplasms—and thus the reported incidence—is likely to increase even further in the near future. It is of increasing clinical significance that appendiceal tumours are diagnosed in more detail and this probably reflects the increased number of mucinous tumours in recent years.
References
Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA (2008) Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 34:196–201
Smeenk RM, Bruin SC, van Velthuysen ML, Verwaal VJ (2008) Pseudomyxoma peritonei. Curr Probl Surg 45:527–75
Trivedi AN, Levine EA, Mishra G (2009) Adenocarcinoma of the appendix is rarely detected by colonoscopy. J Gastrointest Surg 13:668–75
Chua TC, Moran BJ, Sugarbaker PH et al (2012) Early- and Long-Term Outcome Data of Patients With Pseudomyxoma Peritonei From Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J Clin Oncol
McCusker ME, Cote TR, Clegg LX, Sobin LH (2002) Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 94:3307–12
Turaga KK, Pappas SG, Gamblin T (2012) Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol 19:1379–85
Benedix F, Reimer A, Gastinger I et al (2010) Primary appendiceal carcinoma–epidemiology, surgery and survival: results of a German multi-center study. Eur J Surg Oncol 36:763–71
Bucher P, Mathe Z, Demirag A, Morel P (2004) Appendix tumors in the era of laparoscopic appendectomy. Surg Endosc 18:1063–6
Nitecki SS, Wolff BG, Schlinkert R, Sarr MG (1994) The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg 219:51–7
Evers DJ, Verwaal VJ (2011) Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg 98:287–92
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van den Heuvel, M.G.W., Lemmens, V.E.P.P., Verhoeven, R.H.A. et al. The incidence of mucinous appendiceal malignancies: a population-based study. Int J Colorectal Dis 28, 1307–1310 (2013). https://doi.org/10.1007/s00384-013-1714-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1714-9