Abstract
Background
Most investigations on MutY human homolog (MYH)-associated polyposis (MAP) have been conducted in Western countries. Limited data on MAP in Asia are currently available. The present study investigated germline mutations of the MYH gene among patients with 10 to 99 adenomatous colorectal polyps and familial adenomatous polyposis (FAP) without adenomatous polyposis coli (APC) germline mutations in Korea.
Materials and methods
The study population included 46 patients with 10 to 99 adenomatous polyps in the colorectum and 16 FAP patients with no identified APC germline mutations. Subjects were screened for MYH germline mutations, and we additionally screened for MYH mutations in 96 normal control individuals.
Results
Two of 46 (4.3%) patients with multiple polyps displayed heterozygous biallelic germline mutations of the MYH gene. A 39-year-old male patient with biallelic MYH mutations (p.G272E and p.A359V) received total proctocolectomy for rectal cancer and 36 colorectal polyps. A 58-year-old female patient with biallelic MYH mutations (p.Q253X and p.Q440P) received right hemicolectomy for ascending colon cancer and 16 colonic polyps. The frequency of biallelic MYH mutation in 14 of 46 multiple-polyp patients, who had 15 to 99 polyps, was 14.3% (2 of 14). No biallelic MYH mutations were detected in the 32 patients with 10 to 14 colorectal polyps, 16 FAP patients, or 96 normal controls.
Conclusion
We identified biallelic MYH germline mutations in 2 of 14 (14.3%) Korean patients with 15 to 99 colorectal polyps. In this study, there was no Y165C or G382D hot-spot mutation, which had been reported most frequently in previous studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
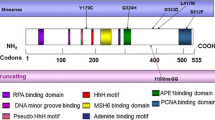
MutY human homolog (MYH)-associated polyposis (MAP) is caused by biallelic germline mutations of the MYH (MIN# 604933) gene located between 1p34.2 and 1p33 [22]. The MYH protein is an adenine-specific DNA glycosylase involved in the base excision repair (BER) system [13]. Human BER system repairs DNA mutations caused by reactive oxygen produced in aerobic metabolism and involves three enzymes, OGG1, MTH, and MYH [16, 18]. The oxidation of guanine in DNA by reactive oxygen generates 8-oxo-7,8-dihydroguanine (8-oxoG), the most stable product of DNA oxidative damage. 8-OxoG is highly mutagenic because it mispairs with adenosine instead of cytosine, leading to the substitution of thymine–adenine pairs for guanine–cytosine pairs (G:C→T:A transversion) [20]. The MYH protein repairs DNA damage by excising adenines from mispairs with 8-oxoguanine that occur during replication of oxidized DNA. Dysfunction of MYH leads to increased G:C→T:A transversion mutations in genes, such as KRAS and adenomatous polyposis coli gene (APC; MIN# 175100), which plays an important role in cellular proliferation of the colorectum [1, 12]. Neoplastic tumors develop rapidly, based on accumulated somatic mutations of these genes.
Initially, Al Tassan et al. reported a British Caucasian family comprising three affected siblings with multiple colorectal adenomas and carcinoma, who displayed heterozygous MYH biallelic mutations without inherited defects of the APC gene or mismatch repair genes [1]. Subsequent studies disclosed autosomal recessive inheritance of MAP. Biallelic germline mutations of the MYH gene were identified in patients with classic familial adenomatous polyposis (FAP) who contained no discernable mutations in the APC gene and those with 15 to 99 adenomas at the frequency of 7 to 42% [6, 8, 19, 21, 24, 26, 27]. Most previous studies based on European Caucasian patients reported p.Y165C and p.G382D as the most frequent germline mutations of MYH [2, 6, 8, 15, 17, 19, 21]. However, insufficient information is available on genotypic and phenotypic characteristics of MAP in Asian patients at present.
In the present study, we screened for germline mutations of MYH among patients with 10 to 99 adenomatous colorectal polyps and FAP patients with no APC germline mutations in Korea.
Materials and methods
Patients
The study population consisted of 2 subgroups, including 46 patients with 10 to 99 adenomatous polyps in the colorectum and 16 FAP patients (≥100 polyps in the colorectum) with no identified APC germline mutations. The inclusion criteria for enrolling patients with multiple polyps were: (1) those receiving colonoscopy at the National Cancer Center between March 2000 and October 2005; (2) histologically confirmed adenomas or adenocarcinomas (between 10 and 99) in the colorectum; (3) available blood samples for mutation screening; (4) available clinical data, including surgical records and pathology reports; (5) subjects who were not diagnosed with hereditary nonpolyposis colorectal cancer (CRC), Peutz–Jegher’s syndrome, or juvenile polyposis. Clinical data were retrospectively reviewed, including colonoscopy records, pathology records, and surgical records.
Previously, we identified a germline mutation of the APC gene in 78 of 100 (78.0%) patients with FAP registered in Korean Hereditary Tumor Registry [9, 28]. Blood samples for MYH screening were available for 16 out of 22 FAP patients with no identified germline mutations of the APC gene. Accordingly, 62 patients (46 with multiple polyps and 16 FAP) were included in the present study, and demographic parameters and disease characteristics of these 62 patients are summarized in Table 1. Informed consent was obtained from all patients before blood collection and screening for MYH mutations.
Screening for MYH mutations
Total genomic DNA was extracted from blood samples using Ficoll–Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) and the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Fragments encompassing the entire coding sequence of the MYH gene were amplified with polymerase chain reaction (PCR), as described previously [1]. Amplification was performed in a total volume of 25 μl containing 100 ng of genomic DNA, 10 pmol of each primer, 250 μM each dNTP, 0.5 units of Taq polymerase, and the reaction buffer provided by the supplier (QIAGEN, Hilden, Germany). Samples were denatured for 5 min at 94°C in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA), followed by 35 cycles at 94°C for 30 s, 54–62°C for 30 s, and 72°C for 1 min, with a final elongation of 10 min at 72°C. Annealing temperatures for each exon were as follows: 54°C for exon 15; 55°C for exons 2 and 7; 57°C for exons 3, 12, 13, and 14; 60°C for exons 5, 6, 10, and 16; and 62°C for exons 1, 4, 8, 9, and 11. All 16 exons of the MYH gene were bi-directionally sequenced in duplicate reactions using the Taq dideoxy terminator cycle sequencing kit and an ABI 3700 DNA sequencer (Applied Biosystems). Samples displaying mutations in direct sequencing were re-amplified, ligated into PCR–TOPO vectors (Invitrogen), and subcloned using the TA cloning system (Invitrogen) for sequencing and confirmation of the mutation.
To evaluate the MYH mutations detected in 62 study patients, we additionally screened 96 normal control individuals.
Results
In 46 patients with multiple polyps, the mean age of diagnosis was 58.6 years (range, 34–81 years), and the mean number of colorectal polyps was 16.4 (range, 10–71 polyps). Biallelic germline mutations of the MYH gene were detected in 2 (4.3%) out of 46 patients with multiple polyps. A 39-year-old male patient displayed heterozygous biallelic mutations, specifically, p.G272E and p.A359V (Fig. 1). He received total proctocolectomy with ileal pouch-anal anastomosis for rectal cancer and 36 colorectal polyps evenly distributed throughout the colorectum in 2002. His two siblings received colonoscopic polypectomy for colorectal polyps at the respective ages of 43 and 41 years.
Sequencing chromatograms showing biallelic MYH germline mutations of a 39-year-old man in the present study. Codons encoding the variant sequences are underlined. Two missense mutations are identified, specifically, substitution of adenine for guanine in exon 10 (a) and cytosine for thymine in exon 12 (b)
The other patient with biallelic MYH mutations (p.Q253X and p.Q440P) was a 58-year-old female. She received right hemicolectomy for ascending colon cancer and 18 colonic polyps located in the ascending and hepatic flexure colon in 2003. Two polyps located in the sigmoid colon were removed during preoperative colonoscopy examination, and the 16 remaining polyps were resected en bloc with cancer in surgical resection. In a follow-up colonoscopy conducted in 2004, another 21 polyps were detected and removed. Fifteen polyps were located in the transverse colon, two in the descending colon, and four in the sigmoid colon. The patient received colonoscopic polypectomy again for five polyps (four transverse, one sigmoid) in the remnant colon in 2005. There was no history of colonic polyps or CRC in the family. Both patients were subjected to gastroduodenoscopy. No upper gastrointestinal polyps were identified in either patient. The clinical characteristics and mutation profiles of these patients are summarized in Table 2.
Among 46 patients with multiple polyps, 14 patients had more than 14 adenomatous polyps in the colorectum. The frequency of biallelic MYH germline mutations in these 14 patients with 15 to 99 polyps in this study was 14.3% (2 of 14). No biallelic MYH mutations were present among the 32 patients with 10 to 14 polyps, the 16 FAP patients with no identified APC germline mutations, or 96 normal control individuals.
Monoallelic MYH variants were identified in 3 (4.8%) of 62 study patients. Two patients with multiple polyps displayed a monoallelic p.A359V and p.IVS10-2A>G mutation, respectively, whereas an FAP patient contained p.P18L-p.G25D. In the 96 normal controls, monoallelic MYH variants were identified in eight individuals, specifically, p.A359V in four, p.P18L-p.G25D in three, and p.Q253X in one patient.
Discussion
Frequency of germline MYH mutations and genotypic characteristics
The frequency of biallelic germline mutations of the MYH gene is reported as between 7 and 42% in patients with multiple colorectal adenomas. This variation in frequency depends on diverse phenotypes, such as the number of polyps, and methods of mutation screening [2, 6, 8, 19, 21, 26, 27]. In the present study, the frequency of biallelic MYH germline mutations was 14.3% (2 of 14) in patients with 15 to 99 polyps. In contrast, no biallelic MYH germline mutations were observed in 32 patients with 10 to 14 polyps or 16 FAP patients with no identified APC germline mutations. However, this difference was not significant. The incidence of biallelic mutations was higher in patients with 15 to 99 adenomatous colorectal polyps (ranging from 16 to 42%), compared to FAP patients with no identified APC mutations [6, 21]. However, another studies reported similar or reversed frequencies in these two patient groups, and the differences are currently a controversial subject [2, 15, 27].
Most investigations on MYH mutations have been conducted in Western countries. Limited data on MAP in Asia are currently available. Miyaki et al. reported one (2.9%) patient with a homozygous biallelic MYH mutation (R231C) out of 35 Japanese patients with 20 to 100 colorectal adenomas. To our knowledge, this is the only biallelic mutation of MYH confirmed in East Asian patients to date [14]. Another group reported no MYH mutations in 30 Korean patients displaying multiple adenomatous polyps in the colorectum with a mean number of 10 adenomas [10]. In the present study, screening of 32 patients with 10 to 14 colorectal polyps disclosed no biallelic germline mutations of the MYH gene.
Apart from p.IVS10-2A>G, the five variants of MYH detected in our experiments have not been reported previously according to the MUTYH database in The Human Gene Mutation database (http://archive.uwcm.ac.uk/uwcm/mg/hgmd/search.html). Heterozygous biallelic germline mutations, including p.IVS10-2A>G, were confirmed only in two Japanese patients with familial gastric cancer, and monoallelic variants in three Japanese patients with CRC [14, 24]. These results support ethnic and geographic differences in the mutational spectrum of the MYH gene, consistent with previous findings [2, 14, 19, 26].
p.P18L-p.G25D mutation was found in one FAP patient and three normal controls in this study. When we found p.P18L-p.G25D mutation in the first direct sequencing, we could not know whether p.P18L and p.G25D mutations are on the same allele or not. After we screened each allele by cloning-sequencing analysis, we found that these two mutations were on the same allele.
Phenotypic characteristics
In the present investigation, both patients with biallelic MYH germline mutations were diagnosed with CRC at 39 and 58 years, respectively, and received surgical resection. The risk of CRC in MAP is reported as 50 to 60% at the time of diagnosis, and almost all patients develop CRC by 65 years of age [2, 4, 15]. Similar to attenuated FAP, the age of onset of CRC in MAP is later than that in FAP, predominantly occurring between the fifth and seventh decades of life [2, 3, 12, 27]. Treatment regimes for patients with MAP remain to be established. A previous study demonstrated that colonoscopy with polypectomy was not sufficient in MAP therapy, and prophylactic colectomy was recommended, based on the high frequency of cancer observed in the patients [11]. In contrast, another report showed that colonoscopic polypectomy was suitable in controlling MAP, and the beginning age and interval of colonoscopy surveillance, as advised for AFAP, was sufficient for MAP therapy [2, 3, 5]. Further prospective studies are required to establish the management of biallelic MYH mutation carriers.
Our study population comprised 11 monoallelic carriers, including 1 patient with 36 colorectal polyps, 1 with 13 polyps, 1 with FAP, and 8 of the 96 normal controls. Limited data are available on the phenotypic characteristics of monoallelic MYH mutation carriers. However, the risk of CRC in monoallelic carriers is significantly lower than that in biallelic carriers [2, 3, 8, 21]. Two recent studies reported an increased risk of CRC in monoallelic MYH mutant carriers with one of the two hot-spot mutations, p.Y165C or p.G382D [7, 25]. A population-based cohort study further demonstrated a threefold increased risk of CRC in monoallelic carriers and a 50-fold increased risk in biallelic carriers [7]. Another meta-analysis reported a relative risk of 1.27 for CRC in monoallelic MYH mutation carriers and 117 in biallelic carriers [25].
Gastroduodenoscopy disclosed no upper gastrointestinal tract manifestations in the two MAP patients with biallelic MYH mutations in our study. Congenital hypertrophy of the retinal pigment epithelium (CHRPE) was detected in a patient displaying FAP with no identified APC mutations, but a monoallelic MYH mutation (p.P18L-G25D). Upper gastrointestinal tract polyps are the most frequently reported extracolonic manifestation (ECM) in MAP (ranging from 4 up to 31%) [15, 19, 21]. The majority of upper gastrointestinal tract polyps reported were duodenal adenomas, followed by fundic gastric polyposis. A number of other ECMs, including osteoma, CHRPE, dental cysts, and malignancies in breast and thyroid, have been reported, but it is unclear whether these ECMs associated with FAP are also typical symptoms in MAP [2, 6, 15, 21].
In conclusion, we have identified biallelic germline mutations of the MYH gene in 2 (14.3%) out of 14 Korean patients with 15 to 99 colorectal polyps.
References
Al-Tassan N, Chmiel NH, Maynard J et al (2002) Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat Genet 30:227–232
Aretz S, Uhlhaas S, Goergens H et al (2006) MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 119:807–814
Croitoru ME, Cleary SP, Di Nicola N et al (2004) Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 96:1631–1634
Farrington SM, Tenesa A, Barnetson R et al (2005) Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet 77:112–119
Fornasarig M, Minisini AM, Viel A, Quaia M, Canzonieri V, Veronesi A (2006) Twelve years of endoscopic surveillance in a family carrying biallelic Y165C MYH defect: report of a case. Dis Colon Rectum 49:272–275
Gismondi V, Meta M, Bonelli L et al (2004) Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 109:680–684
Jenkins MA, Croitoru ME, Monga N et al (2006) Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomark Prev 15:312–314
Jones S, Emmerson P, Maynard J et al (2002) Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C→T:A mutations. Hum Mol Genet 11:2961–2967
Kim DW, Kim IJ, Kang HJ et al (2005) Mutation spectrum of the APC gene in 83 Korean FAP families. Human Mutat 26:281
Kim H, Kim HJ, Chi SG (2006) Absence of MutY homologue mutation in patients with multiple sporadic adenomatous polyps in Korea. World J Gastroenterol 12:951–955
Leite JS, Isidro G, Martins M et al (2005) Is prophylactic colectomy indicated in patients with MYH-associated polyposis? Colorectal Dis 7:327–331
Lipton L, Halford SE, Johnson V et al (2003) Carcinogenesis in MYH-associated polyposis follows a distinct genetic pathway. Cancer Res 63:7595–7599
Michaels ML, Miller JH (1992) The G0 system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J Bacteriol 174:6321–6325
Miyaki M, Iijima T, Yamaguchi T et al (2005) Germline mutations of the MYH gene in Japanese patients with multiple colorectal adenomas. Mutat Res 578:430–433
Nielsen M, Franken PF, Reinards TH et al (2005) Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 42:e54
Roldan-Arjona T, Wei YF, Carter KC et al (1997) Molecular cloning and functional expression of a human cDNA encoding the antimutator enzyme 8-hydroxyguanine-DNA glycosylase. Proc Natl Acad Sci USA 94:8016–8020
Russell AM, Zhang J, Luz J et al (2006) Prevalence of MYH germline mutations in Swiss APC mutation-negative polyposis patients. Int J Cancer 118:1937–1940
Sakumi K, Furuichi M, Tsuzuki T et al (1993) Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J Biol Chem 268:23524–23530
Sampson JR, Dolwani S, Jones S et al (2003) Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 362:39–41
Shibutani S, Takeshita M, Grollman AP (1991) Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349:431–434
Sieber OM, Lipton L, Crabtree M et al (2003) Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med 348:791–799
Slupska MM, Baikalov C, Luther WM, Chiang JH, Wei YF, Miller JH (1996) Cloning and sequencing a human homolog (hMYH) of the Escherichia coli mutY gene whose function is required for the repair of oxidative DNA damage. J Bacteriol 178:3885–3892
Sobin LH, Wittenkind CH (eds) (2002) International Union Against Cancer. TNM classification of malignant tumors, 6th edn. Wiley, New York
Tao H, Shinmura K, Hanaoka T et al (2004) A novel splice-site variant of the base excision repair gene MYH is associated with production of an aberrant mRNA transcript encoding a truncated MYH protein not localized in the nucleus. Carcinogenesis 25:1859–1866
Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM (2006) Association of MUTYH and colorectal cancer. Br J Cancer 95:239–242
Venesio T, Molatore S, Cattaneo F, Arrigoni A, Risio M, Ranzani GN (2004) High frequency of MYH gene mutations in a subset of patients with familial adenomatous polyposis. Gastroenterology 126:1681–1685
Wang L, Baudhuin LM, Boardman LA et al (2004) MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology 127:9–16
Won YJ, Park KJ, Kwon HJ et al (1999) Germline mutations of the APC gene in Korean familial adenomatous polyposis patients. J Hum Genet 44:103–108
Acknowledgment
D-W Kim and I-J Kim equally contributed to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, DW., Kim, IJ., Kang, HC. et al. Germline mutations of the MYH gene in Korean patients with multiple colorectal adenomas. Int J Colorectal Dis 22, 1173–1178 (2007). https://doi.org/10.1007/s00384-007-0289-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-007-0289-8