Abstract
Given that the aetiology of biliary atresia (BA) is complex and that there is a multiplicity of possible pathogenic mechanisms then it is perhaps not surprising that the evidence for effect of a number of different agents is contradictory. Post-operative cholangitis for instance is common, bacterial in origin and various antibiotic regimens have been tested (although none in a randomized trial) but continuation beyond the early post-operative period does not appear to offer any greater protection. There is an inflammatory reaction in about 25–35% of cases of BA illustrated by abnormal expression of class II antigen and upregulation of ICAM, VCAM and E-selectin with an infiltrate of immune-activated T cells (predominantly CD4 + Th1 and Th17) and NK cells and a systemic surge in inflammatory cytokines (e.g. TNF-α, IL-2, IL-12). This has potential as a therapeutic target and is the main hypothesis behind the rationale use of steroids. The first report of steroids was published in 1985 by Karrer and Lilly as “blast” therapy to treat recalcitrant cholangitis, followed by a multiplicity of small-scale uncontrolled studies suggesting benefit. To date there has been one randomized placebo-controlled study with a low-dose (prednisolone 2 mg/kg/day) regimen (2007); one with a high-dose (IV prednisolone 4 mg/kg/day regimen) (2014); two prospective high-dose open-label studies (2013); a prospective comparison of low- and high-dose regimen and a large (380 infants) retrospective comparison. The most recent meta-analysis (2016) identified a significant difference in clearance of jaundice at 6 months (OR 1.59, 95% CI 1.03–2.45, P = 0.04), in patients treated with high-dose steroids, particularly if < 70 days at surgery. Ursodeoxycholic acid (UDCA) may increase choleresis or change the ratio of endogenous bile acids to a less hydrophobic and, therefore, less toxic millieu. UDCA may protect cholangiocyte membranes against damage and perhaps reduce the tendency to fibrogenesis. Biochemical benefit has been shown in a single crossover trial in older BA children who had cleared their jaundice. Other potential adjuvant therapies include immunoglobulin therapy, anti-viral agents and Chinese herbs although real evidence of benefit is lacking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary atresia (BA) comes to the surgeon as a possibly unique disease presenting in infancy with established obliteration of the biliary tree. It may be the result of a developmental flaw in bile duct development and be analogous to number of other congenital atresias such as jejunal and oesophageal atresias. But, in some, it is also feasible that there may be destruction and obliteration of a fully formed bile duct around about the time of birth. Either way, the disease is irreversible leading to liver fibrosis and in consequence portal hypertension, ascites and end-stage liver failure.
The management of BA is as straightforward as surgery can be with excision of damaged obliterated extrahepatic ducts and biliary reconstruction onto a transected portal plate (Kasai portoenterostomy—KPE), the aim being a reconfiguration with the primitive intrahepatic bile ducts and thereby usually restoration (albeit usually partial) of bile flow. If this does not work then liver transplantation (LT) will be required.
Clinical context of adjuvant therapy
We believe BA to be an example of aetiological heterogeneity and Fig. 1 illustrates a schematic of this idea postulating different mechanisms for the same end-point or phenotype (Fig. 2).
Thus, four variants can be defined:
-
(a)
BA splenic malformation (BASM) syndrome
These infants (usually female) are defined by association with a constellation of unusual anomalies including polysplenia (sometimes asplenia), vascular anomalies (preduodenal portal vein, absence of the cava), situs inversus and cardiac anomalies [1]. There appears to be a primary failure of extrahepatic bile duct development—the gallbladder is invariably atrophic and the CBD is absent. This developmental failure probably occurs early from 20- to 40-day gestation to account for the other anomalies. A maternal diabetic in utero milieu appears to predispose to this variant [1, 2].
-
(b)
Cystic biliary atresia
This can be defined as cystic change in an otherwise obliterated biliary tract and is seen in about 5–10% of most large series [3] and are certainly different radiologically and histologically from congenital choledochal cysts [4]. The cyst itself may contain bile implying onset after establishment of continuity between intra- and extrahepatic bile ducts at 10–12-week gestation. Most can be detected antenatally on the maternal ultrasound scan (from around 16–18 weeks) [3].
-
(c)
CMV IgM + ve-associated biliary atresia
Recently, we have defined a group of infants with BA who have IgM antibodies to CMV [5]. Clinically they were older at diagnosis and came to KPE later. Biochemically, they had higher bilirubin and AST levels, with larger spleens than age-matched IgM-ve BA infants. Their extrahepatic duct appearance was more inflammatory with prominent nodes. This inflammatory appearance carried over to the histological appearance within the liver with an obvious mononuclear cell infiltrate consisting of largely CD4 + Th1 + T cells (Fig. 3) [6]. Crucially, none of the liver biopsies in our clinical series stained positive for actual presence of CMV, implying that the virus had fled the scene, perhaps illustrating a “hit and run” mechanism with the subsequent immune-response directed against its own cholangiocytes.
This group appears to fulfil most of the requirements to support an immune-destructive pathogenesis following viral triggering [7].
-
(d)
Isolated biliary atresia
In the absence of any distinguishing features this forms the largest (70–80%) clinical grouping. Real evidence of what has caused BA in this group is minimal and must remain speculative [8]. Such infants could equally well have a developmental pathogenesis (but occurring later than the 1st trimester) or alternatively a secondary obliterative cholangiopathy, analogous to the CMV scenario. Some recent evidence towards the former was presented by a review of early liver biochemistry (day 1 and day 2 of life) in about half of a series of infants from Texas later shown to have BA [9]. This showed that all had elevated conjugated bilirubin by 24 h implying biliary obstruction at the time of their birth.

Reproduced with permission from [6]
CMV IgM + ve-associated biliary atresia: T cell sub-set study showing distribution of Th1, Th2, Th17 and Tregs in biliary atresia (a) with sub-set analysis by presumed aetiology for Th1 (b) and Th17 (c) cells showing proliferation of Th1 cells in CMV IgM-associated biliary atresia.
Adjuvant therapy
Corticosteroids
Inflammation is thought to be a key factor in the pathogenesis of some cases of BA. This could be a primary factor in the cholangiopathy for instance following exposure to viral antigen but it also could be secondary to leakage of bile salts, etc. from rupture of developmentally immature ductules into the surrounding liver parenchyma.
The earliest phase of the inflammatory process is signalled by abnormal expression of MHC class 2 antigens, with upregulation of pro-inflammatory cellular adhesion molecules ICAM and VCAM [10, 11]. Later there is a small cell infiltrate of activated CD4 lymphocytes and NK cells [10, 11]. More recent work from our laboratory showed that the T cell infiltrate is predominantly Th1 and Th17 cells with the former having a particular association in those with CMV IgM antibodies [6]. This inflammatory response can also be seen systematically with elevated plasma levels of sICAM, sVCAM together with cytokines such as IL2, IL-4, IL-18, TNF-α, γ-interferon [12]. The process persists and indeed may increase dramatically for cytokines such as TNF-α beyond the immediate operative period, being apparent up to 6 months later.
There may be other relevant effects of steroid therapy beyond the obvious anti-inflammatory properties. For instance, there is some experimental evidence that glucocorticoid treatment of both bile duct-ligated rats [13] and normal BALB/c mice [14] causes increased expression of ileal bile acid transporters thereby increasing enterohepatic recirculation together with suppression of actual bile acid synthesis by inhibition of a rate-controlling enzyme (in the rat Cyp7a1). Plasma bile acids, in both of these studies, rose showing that the ileum is the crucial element with over 95% of intestinal bile acids are reabsorbed.
Clinical evidence
Early clinical studies were reported during the 1980s, describing a so-called “blast-regimen”. This was a short course of a high-dose corticosteroid to try and reverse the effect of cholangitis and restore bile flow [15]. Following this, a multitude of non-randomized small-scale studies were published typically from Japanese centres [16, 17] suggesting benefit in a variety of doses and duration.
The first randomized, double-blind, placebo-controlled trial was published in 2007 from two English centres (London and Leeds) and involved 71 infants [18]. In retrospect, the regimen used was low dose (2 mg/kg/day oral prednisolone, reducing over a 4 week period). The primary outcome measure was defined as clearance of jaundice, but early liver biochemistry and native liver survival were also assessed. There was a significant difference in bilirubin levels at 1-month post-KPE but this did not carry-over into a significant difference in clearance. A further study was published in 2013 from the larger centre [19], but restricted to those infants who were < 70 days at KPE and adding a further group treated with a high-dose regimen (starting at 5 mg/kg/day). This now showed a significant difference between placebo and steroid groups in terms of clearance of jaundice to normal levels (52 vs 66% P = 0.037). Indeed a gradation of response could be seen between low- and high-dose regimens. Other biochemical differences were also observed including reduced AST levels and AST-to-platelet ratio index (APRi) at 1 month post-operatively. It did not translate into any change or improvement in native liver survival or the need for transplantation.
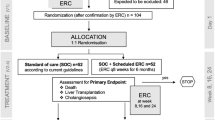
Subsequent data from London focused on the “age effect” alluded to in previous studies of steroids [20]. Thus, 104 infants with BA underwent KPE at < 70 days old, all receiving high-dose steroids. This group was then subdivided into consecutive age cohorts and the jaundice clearance at 6 months was assessed. This showed a significant trend overtime favouring early KPE (Fig. 4). For instance 100% of those in the KPE, < 40-day group cleared their jaundice compared to 60% in the 60–70-day group. Importantly, a significant improvement in overall native liver survival was shown for those operated on before 45 days (the median age in the sample). This study for the first time showed that high-dose steroid not only augments jaundice clearance but can also translate to improved native liver survival.
A high-dose prednisone regimen was also tested in a placebo-controlled trial in the North American multicentre (n = 14) steroids in biliary atresia randomized trial (START) [21]. It compared placebo (n = 70) against a regimen of IV methylprednisolone (4 mg/kg/day) for 2 weeks tapering down to oral prednisolone (2 mg/kg/day) for a further 9 weeks (n = 70). The primary endpoint was serum bilirubin < 1.5 mg/dL (≡ 25 µmol/L) at 6 months post-KPE. The secondary outcome measure was native liver survival at 6 months. They reported an overall non-significant increase in jaundice clearance at 6 months (49 vs 59%) in the steroid group.
Sub-set analysis in the START trial looking at only those < 70 days at KPE (n = 76) confirmed an increased proportion of those to clear their jaundice (72 vs 57%), but again not to statistical significance (P = 0.36). On review of the study design it appears that it was powered to require a difference of 25% in the primary outcome measured. This unrealistic estimation was based upon a previously published American retrospective study from Salt Lake City [22] where comparison with a no steroid, no antibiotic group had a very poor outcome with 11/13 needing transplant before their first birthday.
The largest study is a non-randomized cohort study from Shanghai, China [23], which compared the outcome of low- and high-dose steroids in two consecutive periods 2004–2006 and 2007–2009. In total, 380 (n = 253 in high-dose group) infants underwent KPE. Although there was a significant difference in mean age at KPE (74 versus 66 days; P = 0.03) there was also a significant difference in the proportion to clear their jaundice (39 versus 53%) in favour of the steroid group.
To try and resolve this uncertainty and provide an increase in the level of evidence there have been several systematic reviews. A recent meta-analysis was published by Chen et al. in 2015 which looked at 259 patients undergoing steroid therapy post-KPE [24]. Of the studies meeting the inclusion criteria two were RCTs and five were observational studies, published from 1968 to 2014. They identified from their analysis that there was a significant difference in jaundice clearance in those with moderate to high-dose steroid versus placebo at 6 months post-KPE (Fig. 5). They also suggested that longer regimens failed to elicit any further significant benefit and, therefore, a shorter course may be more prudent to avoid drug-related complications.

Reproduced with permission from [24]
Meta-analysis of steroids in biliary atresia. Forest plot showing improved clearance of jaundice with steroids.
Prednisolone is the most frequently prescribed steroid in most studies [18, 19, 21, 25] with a usual starting dose of 4 or 5 mg/kg/day. Some protocols begin this with intravenous methyl prednisolone [21, 25] although there is little evidence to suggest this has any extra effect. Dexamethasone has also been recommended by one English centre beginning oral dexamethasone (0.3 mg/kg twice daily for 5 days, 0.2 mg/kg twice daily for 5 days, and 0.1 mg/kg twice daily for 5 days), beginning on post-operative day 5 [26].
Of course, there are many potential side effects of steroids though none has actually been reported in the papers presented so far. Possible side effects include increased risk of infection, poor wound healing, hyperglycemia, hypertension, gastrointestinal bleeding, poor growth, and an inadequate response to routine immunizations. The START trial suggested an increased but non-specific incidence of side effects but was unable to identify anything more tangible [21].
In practice, high-dose steroid use is ubiquitous in the UK, Japan and much of Europe [27]. However, its use is probably declining in the USA because of the negative influence of the START trial.
Intravenous immunoglobulin therapy
The inflammatory component of BA has already been highlighted and is a clear target for adjuvant therapy. Whereas steroid use has been studied for many years culminating in placebo-controlled trials, the use of immunoglobulins has not. Intravenous immunoglobulin therapy is known to reduce inflammatory cytokines and increase anti-inflammatory regulatory T cells and is an established part of treatment in multiple autoimmune, immunodeficiency and inflammatory conditions. Fenner et al. [28] using a Rhesus-rotavirus model of BA in mice showed that there was diminished bilirubin levels together with less expression of VCAM-1 on portal epithelium and cytokine production by T cells using high-dose IgG therapy. In North America the Safety Study of IntraVenous Immunoglobulin (IVIG) Post-Portoenterostomy in Infants with Biliary Atresia (PRIME) trial is currently underway (https://clinicaltrials.gov/ct2/show/NCT01854827). This is an open-label multicentre phase 1 and 2 trial assessing the feasibility, tolerability and safety of intravenous immunoglobulin post-KPE. Infants will receive three doses of IV IgG over a 60-day period and be followed up for 360 days.
Ursodeoxycholic acid (UDCA)
UDCA is a hydrophilic secondary bile acid making up 1–4% of total bile acids in humans. Its medicinal benefits were first identified during the Tang Dynasty in China as the traditional drug Shorea, and used to treat liver disease. Shorea is derived from the bile of adult black bears which naturally contains a high concentration of UDCA.
It has proven efficacy in adult cholestatic conditions such as primary biliary cirrhosis and sclerosing cholangitis and is used in some relevant childhood pathological conditions such as the liver dysfunction of cystic fibrosis and prolonged parenteral nutrition and PFIC [29, 30].
Alongside improving liver biochemistry, UDCA has been suggested to have an immunomodulatory effect though actual evidence from adult studies is conflicting [31]. There is a documented inverse relationship between increasing serum concentrations of UDCA and decreasing “toxic” endogenous bile salts. This is thought to be protective to hepatocytes and cholangiocytes adding a third rationale for potential benefit in BA [32]. So, although there may be documented theoretical benefits for UDCA in BA, actual scientific evidence is thin.
A French group from Lille performed a prospective uncontrolled crossover trial assessing the effect of UDCA in stable patients post-KPE [33]. Sixteen children met the inclusion criteria, having cleared their jaundice post-KPE and had been on UDCA (25 mg/kg/day TDS) for at least 1 year. A discontinuation/reintroduction methodology was used. Thirteen of the 16 children were restarted on UDCA within 6 months of stopping: the main reason for resumption being worsening of their liver enzyme profile, with one child having recurrence of jaundice. All of these improved on reintroduction of the drug. Although UDCA showed beneficial effects on liver biochemistry, this study did not observe a pronounced effect on clinical status.
In contrast, a larger but retrospective and largely uncontrolled study has been published from Cairo, Egypt, which looked at a cohort of 141 infants of which 108 had received UDCA [34]. They suggested that this group had had a worse outcome. However, this may just reflect poor results—only 19 (13%) infants overall cleared their jaundice and there is an obvious tendency to try anything if you think your ship is sinking.
Antibiotics
Cholangitis remains a very serious post-operative complication following KPE, reportedly affecting between 40 and 93% [35,36,37]. The common causative organisms have been identified as Klebsiella spp., Escherichia coli, Pseudomonas aeruginosa, Escherichia cloacae, A. baumani, Streptococcus mitis and Salmonella typhi. The mechanism is most likely to be an ascending cholangitis via the Roux loop into the intrahepatic duct system, primitive and distorted though it is. Other contributory mechanisms may also be involved including bacterial overgrowth of bacteria in the gut, translocation from lymphatics and haematogenous spread via the portal vein.
The practice of prescribing prophylactic antibiotics to try and reduce the incidence of cholangitis is universal but extremely variable. There is no consensus as to what drug to give, for what duration and indeed if there is even any clinical benefit. A recent systematic review by Decharun et al. [38] sought to systematically review current evidence on prophylactic antibiotics and their prevention of cholangitis post-KPE. Four studies met the eligibility criteria: one randomized control trial [39] and three retrospective cohort studies [40,41,42]. A lack of high-quality evidence for antibiotic prophylaxis is evident when from 87 abstracts reviewed, only one RCT was found. The primary outcome measures were the incidence of cholangitis, recurrent cholangitis and transplant-free survival and a total of 329 patients were included in total (196 receiving antibiotics, 133 control).
There was wide variation in results from the smaller studies, with the largest [42] recruiting from a Dutch national cohort (n = 214). This latter study did not identify any reduction in cholangitis rates; however, interestingly it was associated with a higher 4-year transplant-free survival rate (54% in antibiotic group, 34% in the control group).
The randomized control trial by Bu et al. [39] concentrated on antibiotic prevention of recurrent cholangitis. Nineteen children, all of who had had one episode of cholangitis were randomized to receive either trimethoprim–sulfamethoxazole (Septrin™) (n = 9) or oral neomycin (n = 10). A historical control group (n = 18) of patients who did not receive antibiotics was also used. The authors found that rates of recurrent cholangitis were significantly lower in both antibiotic groups, with no significant difference between the two. Neomycin delayed the first episode of recurrence and was associated with a higher survival rate.
This review does reinforce the high incidence of cholangitis in children post-KPE and the expectation that most episodes will occur in the first year post-operatively. A sensible approach to duration of antibiotic therapy may be to give them concomitantly steroids due to immunosuppression risks or if a longer course is desired to stop at 1 year post-KPE, when the cholangitis risk significantly decreases.
Native liver survival decreases markedly if there are repeated episodes of cholangitis especially within the first 2 years of surgery. For example, in one recent study of 76 infants, Koga et al. showed that liver transplantation was ultimately required by all jaundice-free children who had had cholangitis within 3 months of the KPE [43].
Cholangitis occurring after some years of infection-free life in children who have cleared their jaundice should make one suspicious of an actual mechanical problem with the Roux loop [44]. These children should be fully investigated with radionuclide imaging, ultrasound and MRCP. Balloon enteroscopy may also have a diagnostic role enabling visualization of the Roux limb. Dilatation proximal to the retrocolic tunnel is the key diagnostic feature and if there is sufficient evidence of this then laparotomy should be embarked upon.
Ganciclovir and valganciclovir
We have proposed that cytomegalovirus IgM + ve-associated BA is a distinct variant of BA with a worse prognosis [5]. This negative effect of CMV has been confirmed by a large Chinese study [45] although it is still controversial. Perinatal viral exposure may lead to destruction of fully formed bile ducts but by the time of KPE evidence of the actual virus appears scant.
Antiviral therapy has, therefore, been suggested for this group of infants because of its negative effect on outcome. Principally the agents include ganciclovir (Fig. 6) and its oral prodrug valganciclovir. Ganciclovir triphosphate is a competitive inhibitor of deoxyguanosine triphosphate (dGTP) and is incorporated into viral DNA and where it preferentially inhibits viral DNA polymerases. It is widely used for “congenital CMV infection”. For instance, IV ganciclovir was used in 100 infants with congenital CMV disease affecting their central nervous system [46] and was shown to reduce disease progression. Its use was, however, associated with myelosuppression (neutropenia) in two-thirds so should not be underestimated and, furthermore, it can only be given intravenously.
By contrast, its use in infants with CMV IgM + ve-associated BA has been only infrequently reported. Fischler et al. [47] from Sweden first reported this in 2009 in infants with CMV-associated cholestasis. They identified six infants in their centre with CMV infection (CMV IgM + ve/CMV in urine). Of these, two had BA, one had septo-optic dysplasia and three had intrahepatic cholestasis attributed to CMV hepatitis. One of the BA patients was diagnosed at < 3 weeks of age suggesting congenital infection. Ganciclovir was given at 5 mg/kg BD IV for 2 weeks then reduced to 5 mg/kg IV daily thereafter. The first BA patient received ganciclovir pre-KPE for 3 weeks then post-KPE for a further 2 weeks. The second BA patient had an isolated 4 week course. The response was measured biochemically, virologically and by clinical outcome. The results in this very small group of CMV BA patients treated with ganciclovir were unclear. The first infant displayed improvement in markers of cholestasis. Unfortunately, the second infant did not benefit from KPE and subsequently died from complications following a liver transplant.
Shah et al. from Mumbai, India, reported success using oral valganciclovir for CMV-associated BA in one case [48]. One patient who had not cleared their jaundice a month post-KPE was given a 6-week course of valganciclovir. The infant’s bilirubin and CMV viral load subsequently improved to normal values.
Only treatment of CMV-associated BA has been published. Whether there is any effect with other viruses purported to be causal in BA is not known. Schukfeh et al. [49] from Hannover, Germany indeed suggested that there was no change in prognosis whether viral RNA or DNA could be isolated or not. So, 28 of 70 (40%) infants with BA were positive for hepatotropic viruses (typically REOvirus, 29%). There was no difference at all in long-term outcome. However, the actual native liver survival used as the marker of outcome seems rather low by comparison to other centres (23% at 7 years).
The current lack of data makes it impossible to draw any firm conclusions regarding the efficacy of either ganciclovir or valganciclovir in CMV IgM + ve-associated BA. We are currently using ganciclovir as an open-label drug in those infants with IgM + ve antibodies and high viral load. Remaining challenges to be overcome will be determination of time of onset of infection and a safe method of delivery of the drug and isolation.
Liver fibrosis
BA is characterized as having a particularly aggressive tendency to liver fibrosis characterized by excessive amounts of extracellular matrix (ECM) although its origin is still unclear.
Myofibroblasts appear to be the key source of pathologic ECM in damaged liver and much adult-based research has focused on trying to delineate their origin and the signalling pathways responsible for activation, deactivation, and elimination (Fig. 7). During the development of hepatic fibrosis, a number of different cellular populations may contribute to the myofibroblast pool and include resident hepatic stellate cells and portal fibroblasts, mesothelial cells, and bone marrow-derived mesenchymal cells and fibrocytes.

Reproduced with permission from [52]
The central role of the myofibroblast in liver fibrosis.
Hepatic progenitor cells associated with the ductular reaction may be the source of ECM deposition, specifically in biliary-induced fibrosis and there is some recent evidence incriminating a novel population of Prominin 1-expressing cells from within the periportal region [50, 51] which activate fibroblast growth factor (FGF) and transforming growth factor-β (TGFβ). Collagens are the main extracellular matrix components involved in fibrosis with two principle types found in normal liver (I and III—80% of all collagen).
Can anything be done to staunch this process? The evidence is very limited although the investment in research has been great [52]. The goal of fibrosis inhibition (or even reversal) also depends on appropriate and effective targeting of a drug to the liver and methods of this have ranged from attachment to ursodeoxycholic acid (as this is almost exclusively metabolized by hepatocytes) and asialoglycoprotein receptor-mediated targeting (which are primarily expressed on hepatocytes and minimally on extrahepatic cells) to using the adeno-virus as a vector. The actual molecules may be transcription factors, therapeutic genes or peptides targeting receptor molecules on myofibroblasts [53]. Currently, there are no European Medicines Agency (EMEA) or US Food and Drug Administration (FDA)-approved anti-fibrotic therapies available to treat patients with liver fibrosis though this is not from want of trying.
Chinese herbs and Kampo medicine
Both Japanese and Chinese centres routinely prescribe the Chinese herb mixture “Inchinko-to” (TJ135) to infants post-KPE [54]. Though clearly not as controlled as conventional medicines, the predominant contents are Artemisia Capillaris spike, Gardenia fruit and Rhubarb rhizome. There is some experimental evidence of inhibition of hepatic stellate activity in rats in isolated cell culture cell [55] and, therefore, inhibition of the key myofibroblast transformation found in fibrosis [52].
Clinically, only small-scale studies have been published. Tamura et al. [56] reported a prospective study of 21 children post-KPE who had cleared their jaundice but who had persistent elevated liver enzymes and γ-glutamyl transpeptidase. Inchinko-to was given to 12 for up to 3 years while the remainder continued with their standard regimen. Liver enzymes, bile acids and markers of liver fibrosis were measured sequentially. There were no side effects of treatment. In the Inchinko-to group, markers of liver fibrosis (e.g. hylauronic acid) were significantly decreased at 1 and 3 years without change in liver enzymes, bile acids or bilirubin.
Colchicine
Colchicine, formerly widely used in gout, and initially derived from the Autumn crocus (Colchicum autumnale) is known to impede the microtubule-mediated transport of procollagen [57] and has an anti-fibrotic effect in bile duct-ligated rats in reducing collagen deposition [58]. There has been a single randomized placebo-controlled trial of its use in BA though shamefully the results have never been fully published. Thus, oral colchicine (25 µg/kg/day) was studied at Kings College Hospital during the 1980s and 1990s with some results available only in abstract form [59]. So at a mean of 4 years of age, there was no difference in outcome or in other indices of morbidity. However, key data on serial liver biopsies were never reported.
Miscellaneous
Phenobarbitone and its derivatives have a long history of empirical use in paediatric hepatology and have been within our Kings College Hospital protocol since the 1980s (prescribed as phenobarbitone 15 mg daily increasing to 45 mg in steps of 15 mg/week). Similarly, the bile acid sequestrant, cholestyramine (1 sachet t.d.s.), is also part of our protocol, again empirically without any real evidence of benefit. Its use probably started following publication of a randomized French trial of 80 infants post-KPE looking at both agents individually together with a comparative control group [60]. Ironically, no significant differences were identified.
Conclusions
The fundamental therapy for BA remains surgical. No medical therapy has been found which can alter or reverse the pathological processes which cause or have been implicated in the genesis of BA without surgery. The real question, however, is can such processes be influenced following impaired restoration of bile flow, as is almost invariable after KPE. To re-iterate, if there has been no flow established (persistent unpigmented stool and a rising bilirubin) post-KPE then these children are destined for transplant or death.
There is a range of adjuvant therapies reported (Fig. 8) with steroid use being the most studied. I believe it is clear that high-dose steroids (whether it be prednisolone, prednisone or dexamethasone) do have a positive effect (of the order of 10–15%) in infants who have livers which are not irretrievably scarred by the detrimental effects of advanced age at surgery (arbitrarily 70 days +). No study has shown a negative effect on jaundice clearance and no study has shown sufficiently serious side effects to warrant not giving them. We await more precise identification of those subsets where its use may be irrelevant on theoretical groups (e.g. the non-inflammatory livers of syndromic infants).
The use of UDCA is widespread, and is probably based on its proven efficacy in “analogous” adult cholestatic conditions such as sclerosing cholangitis and primary biliary cirrhosis rather than any body of evidence in children. This too also appears to be free of any serious side effects.
The use of antibiotic is unquestioned in the early post-operative period though again, not actually based on any kind of trial data in children. Prophylactic use beyond 1 month for up to 1 year is also common but seems completely without evidence.
It is obvious that with a failure rate of 40–60% of children undergoing KPE that there is a huge potential for improving results in biliary atresia. It is no use relying on transplantation as a “get out of jail card” in this disease. While undoubtedly effective as a new organ with unlimited potential the operation has mortality and much morbidity; the donor pool is limited and for many born into this world it is a completely unrealistic option. Urgent clarification of the role of adjuvant therapy will only happen in rare diseases by collaboration and pooling of patients and data. Your future patients demand nothing less!
References
Davenport M, Savage M, Mowat AP, Howard ER (1993) Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery 113:662–668
Davenport M, Tizzard SA, Underhill J et al (2006) The biliary atresia splenic malformation syndrome: a 28 year single center retrospective study. J Pediatr 149:93–400
Caponcelli E, Kinsely AS, Davenport M (2008) Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg 43:1619–1624
Lobeck IN, Sheridan R, Lovell M, Dupree P et al (2017) Cystic biliary atresia and choledochal cysts are distinct histopathologic entities. Am J Surg Pathol 41:354–364
Zani A, Quaglia A, Hadzic N et al (2015) Cytomegalovirus associated biliary atresia: an aetiological and prognostic subgroup. J Pediatr Surg 50:1739–1745
Hill R, Quaglia A, Hussain M et al (2015) Th-17 cells infiltrate the liver in human biliary atresia and are related to surgical outcome. J Pediatr Surg 50:1297–1303
Sokol RJ, Mack C (2001) Etiopathogenesis of biliary atresia. Semin Liver Dis 21:517–524
Hartley JL, Davenport M, Kelly DA (2009) Biliary atresia. Lancet 374(9702):577–585
Harpavat S, Finegold MJ, Karpen SJ (2011) Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics 128:e1428–33
Davenport M, Gonde C, Redkar R et al (2001) Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg 36:1017–1025
Mack CL, Tucker RM, Sokol RJ et al (2004) Biliary atresia is associated with CD4 + Th1 cell-mediated portal tract inflammation. Pediatr Res 56:79–87
Narayanaswamy B, Gonde C, Tredger JM et al (2007) Serial circulating markers of inflammation in biliary atresia—evolution of the post-operative inflammatory process. Hepatol 46:180–187
Xiao Y, Yan W, Zhou K, Cao Y, Cai W (2016) Glucocorticoid treatment alters systemic bile acid homeostasis by regulating the biosynthesis and transport of bile salts. Dig Liver Dis 48:771–779
Out C, Dikkers A, Laskewitz A, Boverhof R et al (2014) Prednisolone increases enterohepatic cycling of bile acids by induction of Asbt and promotes reverse cholesterol transport. J Hepatol 61:351–357
Karrer FM, Lilly JR (1985) Corticosteroid therapy in biliary atresia. J Pediatr Surg 20:693–695
Muraji T, Higashimoto Y (1997) The improved outlook for biliary atresia with corticosteroid therapy. J Pediatr Surg 32:1103–1106
Muraji T, Nishijima E, Higashimoto Y, Tsugawa C (1997) Biliary atresia: current management and outcome. Tohoku J Exp Med 181:155–160
Davenport M, Stringer MD, Tizzard SA et al (2007) Randomized, double-blind, placebo-controlled trial of corticosteroids after Kasai portoenterostomy for biliary atresia. Hepatology 46:1821–1827
Davenport M, Parsons C, Tizzard S, Hadzic N et al (2013) Steroids in biliary atresia: single surgeon, single centre, prospective study. J Hepatol 59:1054–1058
Tyraskis A, Davenport M (2016) Steroids after Kasai procedure for biliary atresia: the effect of age at Kasai portoenterostomy. Pediatr Surg Int 32:193–200
Bezerra JA, Spino C, Magee JC et al (2014) Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA 311:1750–1759
Meyers RL, Book LS, O’Gorman MA et al. (2003) High-dose steroids, ursodeoxycholic acid and chronic intravenous antibiotics improve bile flow after Kasai procedure in infants with biliary atresia. J Pediatr Surg 38:406–411
Dong R, Song Z, Chen G et al (2013) Improved outcome of biliary atresia with postoperative high-dose steroid. Gastroenterol Res Pract. doi:10.1155/2013/902431 (Epub 24 Nov 2013)
Chen Y, Nah SA, Chiang L, Krishnaswamy G, Low Y (2015) Postoperative steroid therapy for biliary atresia: systematic review and meta-analysis. J Pediatr Surg 50:1590–1594
Petersen C et al (2008) Postoperative high-dose steroids do not improve mid-term survival with native liver in biliary atresia. Am J Gastroenterol 103:712–719
Stringer MD, Davison SM, Rajwal SR, McClean P (2007) Kasai portoenterostomy—12 year experience with a novel adjuvant therapy regimen. J Pediatr Surg 42:1324–1328
Japanese Biliary Atresia Society, Nio M, Muraji T (2013) Multicenter randomized trial of postoperative corticosteroid therapy for biliary atresia. Pediatr Surg Int 29:1091–1095
Fenner EK, Boguniewicz J, Tucker RM et al (2014) High-dose IgG therapy mitigates bile duct-targeted inflammation and obstruction in a mouse model of biliary atresia. Pediatr Res 76:72–80
Colombo C, Battezzati PM, Podda M et al (1996) Ursodeoxycholic acid for liver disease associated with cystic fibrosis: a double blind multicentre trial: the Italian group for the study of ursodeoxycholic acid in cystic fibrosis. Hepatology 23:1484–1490
De Marco G, Sordino D, Bruzzese E et al (2006) Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Aliment Pharmacol 24:387–394
van Milligen de Wit AW, Kuiper H, Camoglio L et al (1999) Does ursodeoxycholic acid mediate immunomodulatory and anti-inflammatory effects in patients with primary sclerosing cholangitis?. Eur J Gastroenterol Hepatol 11:129–136
Yamashiro Y, Ohtsuka Y, Shimizu T et al (1994) Effects of ursodeoxycholic acid treatment on essential fatty acid deficiency in patients with biliary atresia. J Pediatr Surg 29:425–428
Willot S, Uhlen S, Michaud L, Briand G, Bonnevalle M et al (2008) Effect of ursodeoxycholic acid on liver function in children after successful surgery for biliary atresia. Pediatrics 122:1236–1241
Kotb MA (2008) Review of historical cohort: ursodeoxycholic acid in extrahepatic biliary atresia. J Pediatr Surg 43:1321–1327
Ernest van Heurn LW, Saing H, Tam PK (2003) Cholangitis after hepatic portoenterostomy for biliary atresia: a multivariate analysis of risk factors. J Pediatr 142:566–571
Luo Y, Zheng S (2008) Current concept about postoperative cholangitis in biliary atresia. World J Pediatr 4:14–19
Wu ET, Chen HL, Ni YH, Lee PI et al (2001) Bacterial cholangitis in patients with biliary atresia: impact on short-term outcome. Pediatr Surg Int 17:390–395
Decharun K, Leys CM, West KW, Finnell SME (2016) Prophylactic antibiotics for the prevention of cholangitis in patients with biliary atresia status post Kasai portoenterostomy: a systematic review. Clin Pediatr (Phila) 55:66–72
Bu L-N, Chen H-L, Chang C-J et al (2003) Prophylactic oral antibiotics in prevention of recurrent cholangitis after the Kasai portoenterostomy. J Pediatr Surg 38:590–593
Lally KP, Kanegaye J, Matsumura M et al (1989) Perioperative factors affecting the outcome following repair of biliary atresia. Pediatrics 83:723–726
Wu ET, Chen HL, Ni YH et al (2001) Bacterial cholangitis in patients with biliary atresia: impact on short-term outcome. Pediatr Surg Int 17:390–395
de Vries W, de Langen ZJ, Groen H et al (2012) Biliary atresia in the Netherlands: outcome of patients diagnosed between 1987 and 2008. J Pediatr 160:638–644
Koga H, Wada M, Nakamura H et al (2013) Factors influencing jaundice-free survival with the native liver in post-portoenterostomy biliary atresia patients: results from a single institution. J Pediatr Surg 48:2368–2372
Houben C, Phelan S, Davenport M (2006) Late-presenting cholangitis and Roux loop obstruction after Kasai portoenterostomy for biliary atresia. J Pediatr Surg 41:1159–1164
Shen C, Zheng S, Wang W et al (2008) Relationship between prognosis of biliary atresia and infection of cytomegalovirus. World J Pediatr 4:123–126
Kimberlin DW, Lin CY, Sánchez PJ et al (2003) Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 143:16–25
Fischler B, Svennsson JF, Nemeth A (2009) Early cytomegalovirus infection and the long-term outcome of biliary atresia. Acta Pediatr 98:1600–1602
Shah I, Bhatnagar S (2012) Biliary atresia and cytomegalovirus and response to valganciclovir. Indian Pediatr 49:484–486
Schukfeh N, Al-Gamrah A, Petersen C, Kuebler JF (2012) Detection of hepatotropic viruses has no impact on the prognosis after Kasai procedure. J Pediatr Surg 47:1828–1832
Mavila N, James D, Shivakumar P, Nguyen MV et al. (2014) Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology 60:941–953
Nguyen MV, Zagory JA, Dietz WH, Park A et al. (2017) Hepatic prominin-1 expression is associated with biliary fibrosis. Surgery. doi:10.1016/j.surg.2016.09.043 (Epub ahead of print)
Dobie R, Henderson NC. (2016) Homing in on the hepatic scar: recent advances in cell-specific targeting of liver fibrosis. F1000 Faculty Rev-1749. doi:10.12688/f1000research.8822.1 (Published online 19 Jul 2016)
Henderson NC, Arnold TD, Katamura Y et al (2013) Targeting of av integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19:1617–1624
Iinuma Y, Kubota M, Yagi M et al (2003) Effects of the herbal medicine inchinko-to on liver function in postoperative patients with biliary atresia—a pilot study. J Pediatr Surg 38:1607–1611
Imanishi Y, Maeda N, Matsui H et al (2004) Suppression of rat stellate cell activation and liver fibrosis by a Japanese herbal medicine, Inchinko-to (TJ135). Comp Hepatol 3(Suppl 1):S11. doi:10.1186/1476-5926-2-S1-S11 (Published online 14 Jan 2004)
Tamura T, Kobayashi H, Yamataka A et al (2007) Inchin-ko-to prevents medium-term liver fibrosis in postoperative biliary atresia patients. Pediatr Surg Int 23:343–347
Ehrlich HP, Bornstein P (1972) Microtubules in transcellular movement of procollagen. Nat New Biol 238:257–260
Poo JL, Feldmann G, Moreau A, Gaudin C, Lebrec D (1993) Early colchicine administration reduces hepatic fibrosis and portal hypertension in rats with bile duct ligation. J Hepatol 19:90–94
Hatziioannidou A, Cheeseman P, Trivedi P et al (1992) Double blind controlled trial of colchicine versus placebo in extrahepatic biliary atresia: interim results (abstract). Hepatology 16:60
Vajro P, Couturier M, Lemonnier F, Odievre M (1986) Effects of postoperative cholestyramine and phenobarbital administration on bile flow restoration in infants with extrahepatic biliary atresia. J Pediatr Surg 21:362–365
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davenport, M. Adjuvant therapy in biliary atresia: hopelessly optimistic or potential for change?. Pediatr Surg Int 33, 1263–1273 (2017). https://doi.org/10.1007/s00383-017-4157-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-017-4157-5