Abstract
Pediatric brain tumors, particularly those affecting the brainstem, present a significant challenge due to their intricate anatomical location and diverse classification. This review explores the classification, anatomical considerations, and surgical approaches for pediatric brainstem tumors, focusing on recent updates from the World Health Organization (WHO) classification. Brainstem tumors encompass a spectrum from diffuse gliomas to focal intrinsic and exophytic types, each presenting unique clinical and surgical challenges. Surgical strategies have evolved with advancements in neuroimaging and surgical techniques, emphasizing approaches such as neuroendoscopy and tailored incisions to minimize damage to critical structures. Despite the complexities involved, recent developments offer promising outcomes in tumor resection and patient management, highlighting ongoing advancements in neurosurgical care for pediatric brain tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overview
With an incidence rate of 46% and a rising trend, pediatric brain tumors are the second most common category of cancer in children and the primary cause of cancer-related deaths in children. Based on both genetic and histological characteristics, pediatric low-grade gliomas (pLGGs) are a variety of diverse tumors categorized by the recently updated World Health Organization (WHO) Classification of Central Nervous System (CNS) Tumors [10]. The brainstem complexity is widely known, and there are many anatomical structures around to protect it. Superiorly the brainstem is protected by diencephalon, laterally by the petrous bone, anteriorly by the clivus, and posteriorly by the cerebellum. The brainstem facilitates the integration of all brain activities, including motor, sensory, sympathetic, and parasympathetic. Due to the brainstem’s intricate structure, surgery there is exceedingly challenging and demands precise skill.
Brainstem tumors account for up to 18% of pediatric brain tumors and 25% of posterior cranial fossa tumors, occurring more frequently in children. There is no gender preference. These tumors typically appear between the ages of 5 and 10 years old. A second peak in occurrence is seen in adults aged 30 to 40. The anatomy of the brainstem and “safe entry zones” have been the subject of multiple publications in recent years [2]. A small number of these focus on surgical techniques for brainstem tumors in children, while most address cavernoma surgery [11]. Based on their slow-growing radiographic behavior and nonmalignant features, these tumors are categorized as either grade I or II by the WHO Classification of Tumors of the Central Nervous System (CNS) [11]. Virchow-Robin gaps, brainstem features, and different fiber configurations can sometimes allow tumors to grow large without causing many symptoms. This might allow diffuse pontine malignancies to develop inside the pons without penetrating the medulla or mesencephalon. Midbrain tumors tend to grow toward the thalamus and do not invade the pons. Medulla tumors often develop caudally toward the spinal cord or into the fourth ventricle without entering the pons.
Classification
Brainstem tumors, as studied and proposed by Choux and widely accepted worldwide, are classified as follows: type I, diffuse brainstem gliomas; type II, focal intrinsic tumors (solid or cystic); type III, exophytic; and type IV, cervicomedullary [3].
Diffuse brainstem gliomas (type I)
Diffuse tumors account for up to 80% of all brainstem tumors. These disorders impact several nuclei and pathways, resulting in bilateral paralysis of cranial nerves VI and VII, leading to hemiparesis and tetraparesis. Most malignant astrocytomas (WHO grade III or IV) have fast clinical progression and histology [9]. Individuals with these tumors have a poor prognosis; the majority pass away within the first 2 years following diagnosis. However, specific cases may benefit from radiation and chemotherapy.
Focal intrinsic tumors (type II)
The course of focal and diffuse tumors differs. The symptomatology of the latter is indolent, and the lesions progress slowly. In contrast to diffuse tumors, local lesions have well-defined boundaries and can be either solid or cystic. Localized tumors, which are mostly low-grade gliomas, typically cause significantly less edema.
Surgery is recommended if the tumor is superficial; if it is deep, conservative care should be given hoping the tumor may create a pathway for future removal.
Exophitic (type III)
Another element that makes its excision easier to achieve is the possibility of a cystic component. Most of them constitute low-grade astrocytomas. They are large tumors with a significant portion originating from the brainstem.
Cervicomedullary (type IV)
Typically, these lesions do not expand cranially into the fourth ventricle and do not penetrate the pons. They could reach into the spinal cord caudally. When they grow close to the spinal cord, they can cause syringomyelia due to changes in cerebrospinal fluid dynamics. Topography assists with surgical methods; however, these scenarios often involve substantial morbidity.
Molecular biology
Despite the majority of pediatric LGGs being associated with a particular histological diagnosis according to the 2016 WHO categorization system, new research on molecular genetics and epigenetic profiling demonstrates that there may be some overlap with pediatric high-grade gliomas (HGGs) and other LGG entities. It has been determined that genetic changes that stimulate the Ras-mitogen-activated protein kinase (MAPK) signaling pathway are characteristic of both pLGGs and some low-grade glioneuronal tumors (LGGNTs) [7].
Midbrain lesion—anatomical aspects and approaches
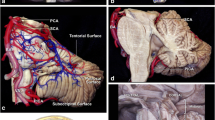
Anatomically the midbrain is divided by the pontomesencephalic sulcus from the pons and by a sulcus between the optic tract and cerebral peduncle from the diencephalon. Three anatomical features of the midbrain should be well known: the third and fourth cranial nerve nuclei, and the pyramidal tract, which is situated in the anterolateral part of the midbrain [13]. The lower half of the superior colliculus and the upper half of the inferior colliculus correspond to the level of the third cranial nerve’s nucleus. The inferior part of the inferior colliculus is caudal to the trochlear nucleus. These nuclei are situated 9.5 mm medial to the surface of the lateral mesencephalic sulcus on average and next to the midline. The mesencephalic lateral sulcus extends from the medial geniculate body superiorly to the pontomesencephalic sulcus inferiorly. This sulcus is considered the posterior boundary of the ventral lateral midbrain.
The fourth cranial nerve has a smaller intrinsic part and passes via the contralateral cerebellomesencephalic fissure, whereas the third cranial nerve travels throughout the entire central region of the mesencephalon. The third cranial nerve exits the midbrain at the medial sulcus of the cerebral peduncle and travels towards the oculomotor triangle before entering the cavernous sinus. Branches of the mesencephalic perforating basilar artery provide blood to the midbrain.
The crus, substantia nigra, and medial lemniscus are supplied by the anterolateral midbrain arteries, also known as peduncular branches. Collicular arteries and branches of the superior cerebellar artery constitute the posterior arteries, which encircle the quadrigeminal plate in a plexus [12].
Anterior midbrain
Both open and minimally invasive endoscopic approaches are performed to treat or biopsy midbrain lesions. Development of tumors in the front region of the midbrain often occurs in two directions: toward the interpeduncular cistern and the third ventricle. An interfornicial, transcallosal, transchoroidal, or transforaminal approach is utilized for malignancies developing into the third ventricle. Tumors, almost all of which are low-grade astrocytomas, can be removed with a neuroendoscope paired with an ultrasonic aspirator when the lesion is less than 2 cm.
The Transsylvian pathway can access anterior and anterolateral lesions of the midbrain via the standard pterional, orbito-fronto-zygomatic, or temporal route.
Sano introduced the temporopolar technique in the 1980s, enabling an anterior–posterior opening across the temporal lobe and visualization of the anterolateral interpeduncular fossa. Additionally, Krause introduced the subtemporal transtentorial technique as an alternative method. However, this technique carries risks such as ophthalmoparesis from damage to the third and fourth cranial nerves along the tentorial incisure, as well as venous infarction from damage to the Labbé complex vein. Nevertheless, it offers excellent visualization of the incisural area, including the basilar artery, interpeduncular cistern, brainstem, and rostral ventral surface of the pons. Since only the middle three-fifths of the peduncle are occupied by the corticospinal tract’s fibers, this area is considered a safe entry zone to the anterolateral midbrain [1].
Considering that these tumors are frequently exophytic, they can be removed by incising the tumor at the lesion’s starting point site instead of needing to penetrate the brainstem. In cases of localized tumors requiring incision of the brainstem, a diamond-tipped scalpel is utilized. Pyramidal tract damage must be avoided by performing parallel and lateral incisions to the third cranial nerve.
Central midbrain
The tumors in the intermediate midbrain can develop into the fourth ventricle or the pineal area. The supraoccipital telovelar approach is employed when they extend toward the fourth ventricle. As they expand into the pineal area, we propose the suboccipital infratentorial supra cerebellar route, which is further categorized into median and paramedian pathways. Coagulation of veins is unnecessary when the lesions are lateral. Caution is advised in the area just below the inferior colliculus due to the proximity of the fourth cranial nerve. With exophytic lesions, we may address the tumor directly without needing to incise the brainstem. However, for small cavernomas, we employed three different access routes: the lateral mesencephalic sulcus, the upper and lower pericollicular, or both. A midbrain incision below the inferior colliculus, known as an infracollicular access, and above the trochlear nerve, known as a supracollicular access, are the two “safe zones” that may be accessible from the pericollicular access point. A transverse incision is performed in the supracollicular approach, right above the superior colliculus, and it should be limited by the aqueduct. The medial longitudinal fasciculus and the nucleus of cranial nerves III and IV may sustain harm from further expansion in such a way.
For lesions extending to the fourth ventricle, incising the cerebellum’s quadrangular lobe provides better access to the cerebellar mesencephalic fissure.
Posterior midbrain
The smallest brain tumors with the potential to cause fatal hydrocephalus in patients are those located in the quadrigeminal plate, accounting for approximately 5% of juvenile brainstem cancers. Endoscopic third ventriculostomy is the most effective treatment for hydrocephalus. Due to the risk of bleeding beyond the biopsy site, it is advisable to avoid endoscopic biopsy whenever possible. The majority of these lesions are low-grade astrocytomas, including mixed gliomas, pilocytic and non-pilocytic astrocytomas, and occasionally more aggressive tumors such as anaplastic astrocytomas [8].
Surgery may be necessary if a tumor grows. Two approaches have been used in these cases: the transtentorial occipital approach, preferred by Poppen, and the supracerebellar infratentorial route, chosen when they grow towards the superior part of the fourth ventricle. There are four “safe zones” for midbrain surgery: the perioculomotor area for anterior region injuries, the lateral mesencephalic sulcus to the intermediate midbrain, supracollicular access, and infracollicular access.
Neuroendoscopic approach
Diagnosis using MRI alone can sometimes be challenging. In selected cases, a neuroendoscopic approach for biopsy may be considered, particularly when hydrocephalus is present [4, 5, 8]. Case 1 illustrates a 1-year-old patient presenting with clinical signs of eye deviation. An MRI scan revealed a diffuse lesion intrinsic to the brainstem (Figs. 1, 2, and 3).
After an endoscopic third ventriculostomy, on the same surgery, a biopsy was performed in the region of the middle segment of the floor of the third ventricle (Figs. 4 and 5). Histopathological analysis showed a low-grade glioma.
Case 2 [6] illustrates a 9-year-old girl with a headache complaint and clinical sign of Parinaud’s syndrome. An MRI scan showed an exophytic lesion at the posterior region of midbrain (Figs. 6, 7, and 8).
After an endoscopic third ventriculostomy, on the same surgery, a biopsy was performed in the region of the cerebral aqueduct entrance (Figs. 9, 10 and 11). Histopathological analysis showed a low grade glioma.
Conclusions
Midbrain surgery remains a significant challenge for neurosurgeons, particularly in pediatric patients. However, advancements in our understanding of the intrinsic and extrinsic anatomy of the brainstem, coupled with the availability of more advanced tools such as high-resolution microscopes and ultrasonic aspirators with thinner tips, have allowed us to remove a considerable amount of brainstem tumors from various topographies within this complex cerebral structure. As a result, we have seen acceptable mortality and morbidity rates in these procedures.
Data availability
No datasets were generated or analysed during the current study.
References
Bricolo A, Turazzi S (1995) Surgery for gliomas and other mass lesions of the brainstem. Adv Tech Stand Neurosurg 22:261–341. https://doi.org/10.1007/978-3-7091-6898-1_5
Cantore G, Missori P, Santoro A (1999) Cavernous angiomas of the brain stem. Intra-axial anatomical pitfalls and surgical strategies. Surg Neurol 52:84–93. https://doi.org/10.1016/s0090-3019(99)00036-1. (discussion 93–84)
Choux MLG (2000) Brainstem tumors. In: Choux MDRC, Hockley A (eds) Pediatric neurosurgery. Churchill Livingstone, New York, pp 471–491
Dezena RA (2017) General principles of endoscopic neurosurgery. In: Atlas of endoscopic neurosurgery of the third ventricle. Springer, Cham. https://doi.org/10.1007/978-3-319-50068-3_2
Dezena RA, Dezena RA (2020) Historical aspects of hydrocephalus and its treatments. In: Endoscopic third ventriculostomy: classic concepts and a state-of-the-art guide 3–23. https://doi.org/10.1007/978-3-030-28657-6
Dezena RA, Reis RGD, de Oliveira Jr JP, Amboya RCM, Nyarko OY (2020) Endoscopic third ventriculostomy and tumor biopsy. Archives of Pediatric Neurosurgery 2(1 (January-April)):58–58
Jones DT, Hutter B, Jäger N et al (2013) International Cancer Genome Consortium PedBrain Tumor Project. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 45(08):927–932. https://doi.org/10.1038/ng.2682
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S, Canadian Pediatric Neurosurgery Study G (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155(254–259):e251. https://doi.org/10.1016/j.jpeds.2009.02.048
Mauffrey C (2006) Paediatric brainstem gliomas: prognostic factors and management. J Clin Neurosci: Off J Neurosurg Soc Austral 13:431–437. https://doi.org/10.1016/j.jocn.2005.05.015
Packer RJ, Pfister S, Bouffet E, Avery R, Bandopadhayay P, Bornhorst M, Bowers DC, Ellison D, Fangusaro J, Foreman N (2016) Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol 19:750–761. https://doi.org/10.1093/neuonc/now209
Recalde RJ, Figueiredo EG, de Oliveira E (2008) Microsurgical anatomy of the safe entry zones on the anterolateral brainstem related to surgical approaches to cavernous malformations. Neurosurgery 62:9–15. https://doi.org/10.1227/01.neu.0000317368.69523.40. (discussion 15–17)
Rhoton AL Jr (2000) Cerebellum and fourth ventricle. Neurosurgery 47:S7–S27. https://doi.org/10.1097/00006123-200009001-00007
Yagmurlu K, Rhoton AL Jr, Tanriover N, Bennett JA (2014) Three-dimensional microsurgical anatomy and the safe entry zones of the brainstem. Neurosurgery 10(Suppl 4):602–619. https://doi.org/10.1227/NEU.0000000000000466. (discussion 619–620)
Author information
Authors and Affiliations
Contributions
R.A.D., M.M.C., D.G.S., L.G.A.F., L.F.A.P., G.B.A., and S.P.P.S. wrote the main manuscript text L.C.M.M., L.B.X. and S.P.P.S. prepared figures 1-11. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dezena, R.A., Correia, M.M., Fujita, L.G.A. et al. Upper brainstem pediatric low-grade gliomas: review and neuroendoscopic approach. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06527-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00381-024-06527-0