Abstract
Purpose
Tuberous sclerosis complex (TSC) is a rare autosomal dominant genetic disorder that affects multiple organ systems. Mutations in the TSC1 and TSC2 genes result in the constitutive hyperactivation of the mammalian target of rapamycin (mTOR) pathway, contributing to the growth of benign tumors or hamartomas in various organs. Due to the implication of mTOR pathway dysregulation in the disease pathology, increasing evidence supports the use of mTOR inhibitors for treating multiple manifestations of TSC.
Methods
In this study, we conducted a retrospective analysis of clinical findings and treatment data from 38 patients diagnosed with tuberous sclerosis who were followed up in the Pediatric Oncology Clinic between 2010 and 2020. We collected information on patients’ ages, genders, affected sites, familial history, imaging findings, presence of tumors, and treatments.
Results
Among the patients, nine individuals with TSC manifestations were treated with mTOR inhibitors. Specifically, everolimus was successfully administered to five patients with inborn cardiac rhabdomyoma causing hemodynamic impairment. In addition, two patients with refractory seizures received everolimus in combination with anti-epileptic drugs. A patient with renal angiomyolipomas larger than 3 cm was treated with everolimus, while a patient with extensive facial angiofibroma received topical sirolimus. All patients tolerated the mTOR inhibitors well, and the side effects were deemed acceptable.
Conclusion
The utilization of mTOR inhibition in TSC is expected to become more prevalent in clinical practice, as current research is anticipated to provide a better understanding of the therapeutic roles of these treatments in TSC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder that commonly leads to the development of hamartomas in multiple major organ systems [1]. The pathogenesis is driven by uncontrolled mTOR activation caused by de novo or inherited mutations in the TSC1 (Tuberous sclerosis complex 1) or TSC2 (Tuberous sclerosis complex 2) genes [2]. TSC1 is located on chromosome 9 (9q34), while TSC2 is found on chromosome 16 (16p13.3), and they encode the proteins hamartin and tuberin, respectively [3]. These proteins play a role in inhibiting the mTOR pathway, which regulates cell growth and differentiation. Defects in hamartin and tuberin proteins lead to the activation of the mTOR pathway [4]. Consequently, affected patients experience neurological manifestations and develop brain, lung, kidney, and skin lesions. Updated TSC consensus recommendations established in 2012 recommend using systemic treatment with mammalian target of rapamycin (mTOR) inhibitors in specific cases, offering an opportunity to simultaneously address multiple manifestations of TSC [5]. This retrospective study aims to evaluate the clinical findings and treatment data of patients diagnosed with tuberous sclerosis who were followed up in our clinic.
Methods
The study included a total of 38 patients who were diagnosed with tuberous sclerosis and followed up in the Pediatric Oncology Clinic between 2010 and 2020. The patients’ records were retrospectively examined, and information such as ages, genders, affected sites, familial history, imaging findings, presence of tumors, and treatments was recorded. The diagnosis was made based on the updated international diagnostic criteria for TSC in 2012 (Table 1) [6]. The data extracted from the files were entered into the Statistical Package for the Social Sciences 22.0 for Windows (SPSS Inc.; Chicago, IL, USA) program. The patients with tuberous sclerosis were analyzed regarding grouping, percentages, and differences.
Patients and results
Of the 38 patients included in the study, 20 (52.6%) were male, and 18 (47.4%) were female. The median age at the time of diagnosis was 6 months, ranging from 1 day to 14 years, and the median follow-up period for the patients was 7 years, ranging from 0.5 to 10 years. Eight patients (21%) received their diagnosis during the neonatal period. Ten patients (26.3%) had a positive family history of TSC, and nine patients (23.6%) had consanguineous parents. The most common presenting symptom was seizures, observed in 23 patients (60.5%). Additionally, ten patients presented with cardiac findings, while five exhibited skin lesions.
Cutaneous manifestations
Hypomelanotic macules were observed in all of our patients. The lesions exhibited characteristics of angiofibroma, appearing as pink-red papules were localized on the face and observed in 15 (39.4%) patients. Two (5.2%) patients presented with a “shagreen” patch and fibrous plaque. One of the patients, a 15-year-old male, underwent treatment for extensive facial angiofibroma with topical 0.1% sirolimus for approximately two years. As no topical form of sirolimus is available in Turkey, the hospital’s pharmacy department prepared a topical 0.1% sirolimus formulation using sirolimus solution (Rapamune®, 1 mg/ml, Pfizer, Turkey). The solution was compounded with soft white paraffin using basic pharmacy equipment. Remarkably, the patient did not experience any cutaneous adverse effects during the course of the topical sirolimus treatment. After using the 0.1% preparation for 3 months, there was a noticeable reduction in both the erythema and size of the angiofibromas (Fig. 1A, B).
Renal manifestations
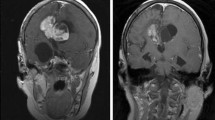
Renal angiomyolipoma (RAML) was detected in 15 patients (39%), while multiple renal cysts were observed in five patients (13%). Seven patients (18%) presented with both angiomyolipomas and renal cysts. Renal ultrasonography was conducted annually for follow-up purposes. In all patients except one, the size of renal angiomyolipomas did not increase by more than 0.5 cm during the follow-up period. However, one patient experienced a significant increase in the size of renal angiomyolipomas, with the lesions exceeding 3 cm. In this case, a 17-year-old female patient, treatment involved oral administration of everolimus at a dosage of 10 mg per day for a duration of 2 years. After 1 year of treatment, a remarkable reduction in volume exceeding more than 50% was recorded (Fig. 2A-D).
Cardiac manifestations
A total of 21 patients (55%) were diagnosed with cardiac rhabdomyoma. Three of these cases were identified through prenatal fetal echocardiography, while six patients were diagnosed during the neonatal period due to symptoms such as murmur, arrhythmia, and cyanosis. Routine echocardiography for tuberous sclerosis screening detected cardiac rhabdomyomas in 12 asymptomatic patients. In one patient, surgery was performed at the age of 6 weeks due to severe left ventricular outlet obstruction and hemodynamic impairment. Among the remaining five symptomatic patients, treatment involved oral administration of everolimus at 0.25 mg twice daily, 2 days per week. Treatment was adjusted to maintain blood everolimus levels between 5 and 15 ng/mL. Remarkably, all of these patients responded favorably to mTOR inhibitor treatment within a few days (Fig. 3A, B). The duration of treatment in these patients ranged from 3 to 12 months, after which it was discontinued. Although two patients experienced minimal progression of cardiac rhabdomyomas after discontinuing everolimus, they remained asymptomatic. Close monitoring was implemented for these patients, and no treatment restart was necessary.
Patients who underwent surgery or received medical treatment are currently being followed up and remain asymptomatic. In the case of asymptomatic patients, spontaneous regression of rhabdomyomas was observed.
Neurologic manifestations
Brain magnetic resonance imaging (MRI) revealed normal findings in only two patients (5%), while cortical/subcortical tubers and subependymal nodules were detected in the remaining 36 patients (95%). None of the patients were found to have subependymal giant cell astrocytoma. Among the patients, 33 (87%) exhibited epileptiform abnormalities with seizures, and one patient presented with infantile spasms. All these patients received anti-epileptic therapy.
In two patients whose convulsions could not be controlled with polytherapy, everolimus was administered. After 6 months, electroencephalographic data showed a decrease in seizure frequency for both patients. With the addition of everolimus to their treatment, these patients no longer experienced epileptic seizures and were able to transition from anti-epileptic polytherapy to monotherapy. They continued to receive a therapeutic dose of everolimus for 12 months. In one of these patients, everolimus was gradually tapered over six months and then discontinued after 18 months of treatment. However, the other patient experienced epileptic seizures during the tapering phase and required reintroducing a therapeutic dose of everolimus. After a few weeks, the seizures ceased, and everolimus was gradually tapered off after 30 months of treatment. Both of these patients are currently being followed up with anti-epileptic monotherapy.
All patients tolerated the mTOR inhibitors well, and the side effects were deemed acceptable. Two patients developed short-term grade II oral mucositis. Omega-3-responsive transient hypertriglyceridemia developed in other 2 patients. The characteristics of patients treated with mTOR inhibitors are summarized in Table 2.
Discussion
The current management approach for TSC primarily addresses symptoms through pharmacologic, surgical, or behavioral interventions. Animal studies have shown the potential benefits of utilizing mTOR inhibitors for managing various TSC symptoms, and these findings have been successfully translated into clinical trials, resulting in significant symptom relief. Specifically, everolimus, a first-generation mTOR inhibitor, has received FDA (Food and Drug Administration) approval for three indications related to TSC: subependymal giant cell astrocytomas, renal angiomyolipomas, and epilepsy [7].
Other TSC symptoms that may potentially benefit from this class of medication are currently being investigated, including cutaneous manifestations. Hofbauer et al. reported a case of TSC with facial angiofibromas that showed improvement after systemic sirolimus treatment following a renal transplant [8]. This prompted further investigation into the effect of sirolimus on angiofibromas. However, systemic sirolimus is expensive and can cause oral mucositis and hypercholesterolemia [9]. As an alternative, topical application of sirolimus was considered.
Wataya-Kaneda et al. [10] were the first to utilize topical sirolimus, either by crushing pills and mixing them with vaseline or using a sirolimus solution. In our case, the patient was treated with sirolimus solution compounded in soft white paraffin. After 3 months of application, the facial angiofibromas showed a reduction in size and paler appearance, with no observed adverse reactions. However, the angiofibromas increased in size and redness a few weeks after discontinuing the treatment. Cinar et al. reported that the efficacy of topical sirolimus diminished over time, but repetitive usage was effective based on their prospective study evaluating the effectiveness and tolerability of topical sirolimus [11]. This suggests that the effect of topical sirolimus is transient.
In our patient, topical sirolimus was restarted, and a repeat treatment protocol is ongoing. Topical sirolimus is a promising treatment option for facial angiofibromas due to its minimal side effects and ease of application. It is anticipated that physicians will increasingly utilize this treatment in the future.
The renal lesions are frequently observed in TSC and their frequency increases with age. The most common renal lesions are angiomyolipomas. Kingswood et al. reported the effects of everolimus on renal angiomyolipoma in patients with TSC who were being treated for subependymal giant cell astrocytoma in the EXIST-1 trial, which largely involved a pediatric population. They found angiomyolipoma response rates of 53.3% for everolimus and 0% for placebo after a median treatment duration of 9.6 and 8.3 months, respectively. After 1 year of treatment, more than 80% of patients achieved a reduction in renal angiomyolipoma volume of over 50% [12]. Everolimus was also evaluated for managing renal angiomyolipoma in the large phase 3 EXIST-2 trial. The angiomyolipoma response rate after approximately 8 months of treatment was 42% in patients taking everolimus compared with 0% in patients receiving a placebo. This response rate increased to 54% in patients treated with everolimus for a median of 29 months and 58% after the open-label extension phase [13,14,15]. Based on the results from the core phase of EXIST-2, mTOR inhibitors are recommended as the first-line treatment for cases with asymptomatic TSC-associated AML larger than 3 cm in size [5].
We initiated everolimus treatment in our asymptomatic patient, who experienced a significant increase in the size of renal angiomyolipomas (RAMLs) (> 3 cm). If these tumors continue to grow, TSC-RAML can lead to arterial hypertension and pose a risk of life-threatening hemorrhage, which is the primary cause of TSC-associated mortality in such cases [16]. After one year of treatment, our patient achieved a reduction of more than 50% in the volume of RAMLs. However, previous studies suggest that regrowth of TSC-RAML may occur after the discontinuation of mTOR inhibitor therapy, and the optimal duration for this therapeutic approach is yet to be defined [17, 18]. Luo et al. reported that low-dose everolimus maintenance therapy is an effective strategy for controlling TSC-RAML following full-dose induction therapy [19]. All of their RAML patients underwent low-dose everolimus maintenance therapy for at least 6 months after receiving standard-dose everolimus induction therapy. Our patient also underwent low-dose oral everolimus maintenance (5 mg/day) for 6 months following 1 year of induction therapy (10 mg/day). She is currently in the tapering period, and her lesions have decreased in volume by more than 50% and remain stable.
Intracardiac rhabdomyoma, a benign cardiac tumor, is observed in nearly 50% of TSC patients [20]. Cardiac rhabdomyomas are among the earliest TSC lesions to appear and can be detected on prenatal ultrasound as early as 22 weeks of gestation [7]. They typically regress within the first 3 years of life and are mostly asymptomatic. However, in some cases, they may cause arrhythmia, valvular defects, or cardiac failure, necessitating prenatal and postnatal surveillance until regression occurs [21]. Symptomatic patients can be managed with medication, and surgery is rarely required. In our clinic, we identified cardiac rhabdomyomas in 21 (55%) of the patients undergoing follow-up for tuberous sclerosis. One patient with severe left ventricular outlet obstruction and hemodynamic disorder underwent surgery. This patient was diagnosed in 2010 when mTOR inhibitors were unavailable as a treatment option at our clinic. Five of our patients received everolimus treatment after prenatal or neonatal diagnosis due to rhabdomyomas causing hemodynamic impairment. All of these patients responded remarkably well to the treatment.
Neurologic manifestations of TSC include epilepsy, cortical tubers, subependymal nodules, giant cell astrocytomas, intellectual disability, autism spectrum disorder, and behavioral problems. Cortical tubers and subependymal nodules, considered hamartomatous lesions, are observed in 80–90% of patients [22]. Cortical tubers are most commonly localized in the frontal and temporal areas and are believed to contribute to epilepsy and behavioral problems. Epilepsy is the predominant medical disorder in TSC, affecting up to 96% of individuals [23]. During the presentation, two of our patients had normal brain MRI findings, while cortical tubers and subependymal nodules were detected in other patients. Most of these patients exhibited epileptiform abnormalities and experienced seizures. Various antiseizure medications can be utilized for epilepsy in TSC. However, refractory epilepsy remains a significant concern, as seizures persist in over 60% of patients [24]. Although no mTOR inhibitors are currently approved specifically for treating TSC-associated seizures, recent clinical evidence has shown promise in this regard [25]. The EXIST-3 trial demonstrated the efficacy of everolimus in patients with treatment-resistant focal seizures [26]. We successfully controlled refractory seizures in two patients by adding everolimus to their anti-epileptic regimen. These findings indicate suggest that adjunctive treatment with everolimus may be an effective option for reducing refractory seizures.
In conclusion, a multidisciplinary approach involving oncologists, neurologists, cardiologists, nephrologists, psychiatrists, and genetic counselors is crucial for the successful management of TSC. mTOR inhibitors are increasingly utilized not only for the hamartomatous and oncologic manifestations of TSC but also as adjunctive therapy for neurologic manifestations. Future research will aim to define the optimal use of mTOR inhibitors in TSC, including indications and dosages for both short- and long-term treatment.
References
-Curatolo P, Bombardieri R, Jozwiak S (2008) Tuberous sclerosis. Lancet 372:657–668
-European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315
-van Slegtenhorst M, de Hoogt R, Hermans C et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808
-Schwartz RA, Fernández G, Kotulska K et al (2007) Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol 57:189–202
Krueger DA, Northrup H (2013) Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49:255–265
Krueger DA, Northrup H (2013) Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 49:243–254
-Uysal SP, Şahin M (2020) Tuberous sclerosis: a review of the past, present, and future. Turk J Med Sci 50:1665–1676
-Hofbauer GF, Marcollo-Pini A, Corsenca A et al (2008) The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. Br J Dermatol 159:473–475
-Merkel S, Mogilevskaja N, Mengel M et al (2006) Side effects of sirolimus. Transplant Proc. 38:714-5
-Wataya-Kaneda M, Tanaka M, Nakamura A et al (2011) A topical combination of rapamycin and tacrolimus for the treatment of angiofibroma due to tuberous sclerosis complex (TSC): a pilot study of nine Japanese patients with TSC of different Disease severity. Br J Dermatol 165:912–916
-Cinar LS, Kartal D, Bayram AK et al (2017) Topical sirolimus for the treatment of angiofibromas in tuberous sclerosis. Indian J Dermatology Venereol Leprology 83(1):27–32
-Kingswood JC, Jozwiak S, Belousova ED et al (2014) The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, phase 3 trial EXIST-1. Nephrol Dial Transplant 29:1203–1210
-Bissler JJ, Kingswood JC, Radzikowska E et al (2013) Everolimus for Angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381:817–824
-Bissler JJ, Kingswood JC, Radzikowska E et al (2016) Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant 31:111–119
-Bissler JJ, Radzikowska E, Zonnenberg BA et al (2016) Everolimus for renal angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: final long-term results from EXIST-2. Munich: Presented at the 31st European Association of Urology (EAU) Annual Congress
-Kapoor A, Girard L, Lattouf JB et al (2016) Evolving strategies in the treatment of Tuberous Sclerosis Complexassociated Angiomyolipomas (TSC-AML). Urology 89:19–26
-Cai Y, Guo H, Wang W et al (2018) Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: a two years trial. Orphanet J Rare Dis 13:43
-Bissler JJ, McCormack FX, Young LR et al (2008) Sirolimus for Angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358:140–151
-Luo C, Ye WR, Zu XB et al (2021) Low-dose Everolimus maintenance therapy for renal Angiomyolipoma Associated with Tuberous Sclerosis Complex. Front Med 8:744050
-Hinton RB, Prakash A, Romp RL et al (2014) International Tuberous Sclerosis Consensus Group. Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the International Tuberous Sclerosis Consensus Group. J Am Heart Assoc 3(6):e001493
-Staley BA, Vail EA, Thiele EA (2011) Tuberous sclerosis complex: diagnostic challenges, presenting symptoms, and commonly missed signs. Pediatrics 127:e117–e125
-DiMario FJ Jr (2004) Brain abnormalities in tuberous sclerosis complex. J Child Neurol 19:650–657
-Thiele EA (2004) Managing Epilepsy in tuberous sclerosis complex. J Child Neurol 19:680–686
-Chu-Shore CJ, Major P, Camposano S et al (2010) The natural history of Epilepsy in tuberous sclerosis complex. Epilepsia 51:1236–1241
-Franz DN, Capal JK (2017) mTOR inhibitors in the pharmacologic management of tuberous sclerosis complex and their potential role in other rare neurodevelopmental disorders. Orphanet J Rare Dis 51:1–9
-French JA, Lawson JA, Yapici Z et al (2016) Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, doubleblind, placebo-controlled study. Lancet 388:2153–2163
Author information
Authors and Affiliations
Contributions
A wrote the main manuscript. D prepared Fig. 2a-d All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeşil, Ş., Kurucu, B., Hamamcı, M.B. et al. Treatment of tuberous sclerosis complex manifestations in children with mTOR inhibitors. Childs Nerv Syst 40, 831–837 (2024). https://doi.org/10.1007/s00381-023-06218-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06218-2