Abstract
Tuberous sclerosis complex (TSC) is a genetic disorder arising from mutations in the TSC1 or TSC2 genes. The resulting over-activation of the mammalian target of rapamycin (mTOR) signalling pathway leaves patients with TSC susceptible to the growth of non-malignant tumours in multiple organs. Previously, surgery was the main therapeutic option for TSC. However, pharmacological therapy with mTOR inhibitors such as everolimus and sirolimus is now emerging as an alternate approach. Everolimus and sirolimus have already been shown to be effective in treating subependymal giant cell astrocytoma (SEGA) and renal angiomyolipoma (AML), and everolimus is currently being evaluated in treating TSC-related epilepsy. In November 2013 a group of European experts convened to discuss the current options and practical considerations for treating various manifestations of TSC. This article provides evidence-based recommendations for the treatment of SEGA, TSC-related epilepsy and renal AML, with a focus on where mTOR inhibitor therapy may be considered alongside other treatment options. Safety considerations regarding mTOR inhibitor therapy are also reviewed. With evidence of beneficial effects in neurological and non-neurological TSC manifestations, mTOR inhibitors may represent a systemic treatment for TSC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
mTOR inhibitors have been shown to be effective in treating TSC-associated subependymal giant cell astrocytoma (SEGA) and renal angiomyolipoma. |
mTOR inhibitors have shown promising therapeutic effects in a number of neurological and non-neurological manifestations of TSC in ongoing studies. |
Further studies are needed to determine the optimal dosing regimen and treatment duration for mTOR inhibitors across different age groups and indications. |
Long-term safety studies of mTOR inhibitors have shown that patients reported ≥1 adverse event, all mild to moderate in severity, with upper respiratory tract infections and stomatitis/mucositis occurring most commonly. |
1 Introduction
Tuberous sclerosis complex (TSC) is a genetic disorder caused by mutation of the TSC1 or TSC2 genes [1]. Mutations in these genes result in the overactivation of the mammalian target of rapamycin (mTOR) signalling pathway, leading to loss of control of cell growth and cell division. Patients with TSC, therefore, have a predisposition to non-malignant tumour formation in multiple organs such as the skin, brain, kidneys, lungs, heart, liver and eyes [2, 3]. It is possible to diagnose TSC during fetal development, when cardiac rhabdomyomas are seen in prenatal ultrasounds and confirmed by additional fetal magnetic resonance imaging (MRI) showing typical TSC-associated brain lesions such as cortical dysplasia and subependymal nodules (SENs) [4]. Diagnostic criteria were recently revised during an International Consensus Conference, and surveillance recommendations were also provided [5, 6]. As neurological manifestations occur frequently and early during the course of the disease, the paediatric neurologist plays a key role in clinical surveillance and in the monitoring of systemic treatment [7].
mTOR inhibitors (sirolimus and everolimus) are a class of drugs acting by specifically inhibiting the mTOR pathway by reducing the phosphorylation of downstream mTOR effectors [8, 9]. Their pharmacodynamic and pharmacokinetic properties are similar; however, everolimus was developed to improve sirolimus’ pharmacokinetics, and everolimus is currently the only drug approved for the treatment of TSC-related manifestations [2].
Clinical studies have shown that mTOR inhibitors are effective in treating subependymal giant cell astrocytoma (SEGA) and renal angiomyolipoma [1–14]. Further clinical studies are ongoing to evaluate mTOR inhibitor use in treating epilepsy in patients with TSC (e.g. the EXIST-3 trial). There is also clinical evidence that mTOR inhibitors may have a positive therapeutic effect on other TSC-related manifestations such as cardiac rhabdomyomas, pulmonary lymphangioleiomyomatosis and skin manifestations.
In November 2013, a panel of European experts met to discuss the current evidence-based options and current clinical practice for treating manifestations of TSC. The conclusions from this meeting form the basis of this report, addressing the treatment options for SEGA, TSC-related epilepsy and renal angiomyolipomas, with a focus on the use of mTOR inhibitors.
2 Management of Subependymal Giant Cell Astrocytoma (SEGA)
SEGAs occur in up to 20 % of patients with TSC and are more likely to develop during childhood and adolescence than in adulthood. Screening TSC patients for the presence of, or changes in, SEGAs from diagnosis is critical in preventing serious sequelae [6, 15]. Brain MRIs should be carried out every 1–3 years in asymptomatic TSC patients under the age of 25 years to monitor for new lesions; patients with existing SEGA who are still asymptomatic should undergo MRI more frequently, and should be educated about the potential for new symptoms. Patients who developed asymptomatic SEGA in childhood should also undergo periodic imaging to ensure that there is no growth [6]. In cases of tumour growth or acute deterioration of the patient’s neurological status, neuroimaging should be more frequent.
Recommendations for the management of symptomatic subependymal giant cell astrocytoma (SEGA)
-
Surgical resection of the tumour is the current standard of care [15–17].
-
If patients experience symptoms of increased intracranial pressure this requires urgent imaging (preferably MRI) and consideration of:
-
Surgery (either immediate shunting and postponement of SEGA removal or immediate tumour surgery where appropriate expertise is available).
-
mTOR inhibitor therapy (in cases not amenable to respective surgery) [18].
-
Recommendations for the management of asymptomatic, but growing SEGA
-
Option 1 mTOR inhibitor therapy (if no contraindications, e.g. known hypersensitivity to the active substance of everolimus) [19]; this may induce SEGA regression and also act on other TSC manifestations [6, 15].
-
Option 2 surgery (first choice only if complete removal of the tumour is possible).
-
Option 3 medical treatment is recommended in cases of SEGA regrowth/recurrence after surgery.
2.1 Applying Clinical Evidence to Clinical Practice
Surgery is the first-choice therapy for symptomatic SEGA [15, 16]. The risk-benefit of surgery should be evaluated by the neurosurgeon, together with a multidisciplinary team, before a treatment decision is made. Evidence from the largest European study to date suggested that delaying surgery might result in significant morbidity [20]. However, surgery is not always appropriate and can be hazardous, especially in patients aged ≤3 years or those with bilateral tumours and/or large lesions [20]. SEGA regrowth can also occur in cases where only part of the tumour was removed surgically, requiring repeated surgery along with the increased risk of postoperative complications [20].
The efficacy and safety data for mTOR inhibitors in the management of SEGA from phase I/II and III clinical trials are summarised in Table 1 [10–13, 20–25]. Tumour regrowth has been reported after discontinuation of mTOR inhibitor therapy; therefore, treatment may need to be sustained for a persistent benefit [10]. In patients for whom surgery is not an option, therapy with the mTOR inhibitor everolimus may be effective. A phase I/II study showed that primary SEGA volume reduced by ≥30 % from baseline (treatment response) in 75 % of patients, and these positive results were confirmed by the EXIST-1 study, a randomized phase III controlled trial, that showed a ≥50 % reduction of SEGA volume in 35 % of treated patients and in up to 47 % of patients in the extension phase (Fig. 1) [10, 12, 21, 26]. mTOR inhibitor therapy could still play a role in patients undergoing surgery to potentially reduce tumour volume before the resection is performed. This approach could be particularly useful in patients with bilateral lesions or those with a tumour that is in an unusual location or growing aggressively [16]; however, no experiences have been published yet and little is known about it in practice. Furthermore, the exact risk of bleeding after a neoadjuvant intervention is still unknown, and there can also be an increased risk for infections or delayed wound healing. However, if surgery is considered after pre-emptive treatment with mTOR, there is a recommended interval of at least 2–3 weeks prior to surgery [17].
MRI scans of SEGA taken before everolimus treatment was initiated and after 23 months of everolimus treatment in a patient who was not a candidate for surgery. Subependymal nodule reduction was accompanied by diminished ataxia and improvements in cognition and behaviour. The patient has been seizure-free since May 2012. (Images provided by Jozwiak S). MRI magnetic resonance imaging, SEGA subependymal giant cell astrocytoma
Patient age is one of the factors to be considered when choosing between surgery and mTOR inhibitor therapy. Children aged ≤3 years may have an increased risk for poor outcomes after surgery for SEGA [20], making mTOR inhibitor therapy an attractive option. When surgery can be performed and it appears to be curative (i.e. there is the possibility of a complete removal of the lesion), it may be preferred in childhood to avoid long-term pharmacotherapy. Long-term pharmacotherapy may be necessary to maintain the clinical benefits, but because SEGA growth often slows spontaneously around the third or fourth decade of life [27, 28], long-term follow-up studies will inform us on the optimal treatment duration of SEGA in the context of TSC. Further studies into the optimal duration of therapy and dose maintenance are needed.
3 The Management of Epilepsy in Patients with Tuberous Sclerosis Complex (TSC)
Epilepsy onset is often in the first year of life in the form of focal seizures (62 %), which can coexist or evolve into infantile spasms. In infants and young children, both high seizure frequency and interictal epileptic activity (especially spike activity during sleep stages II) may lead to irreversible, severe psychomotor retardation and/or behavioural deterioration [29, 30]. However, not all TSC patients have early-onset epilepsies. Some patients experience an age-dependent evolution of seizure types and epileptic syndromes, with the possibility of evolving toward epileptic encephalopathies such as Lennox–Gastaut syndrome [31]. The personalization of treatment, targeting the most troublesome seizures for each patient, such as atonic seizures and drop attacks, is essential [32].
Recommendations for the management of tuberous sclerosis complex (TSC)-related epilepsy
-
Antiepileptic drugs (AEDs): vigabatrin therapy is the first-choice treatment in infants with focal seizures and/or infantile spasms.
-
Therapy should be started if ictal discharges occur (with or without clinical manifestations) in infants and children with TSC [32].
-
Surgery should be evaluated early.
-
Ketogenic diet (KD) or low glycaemic index diet.
-
Vagal nerve stimulation (VNS).
-
3.1 Applying Clinical Evidence to Clinical Practice
The AED vigabatrin has demonstrated good efficacy in both TSC-related spasms and focal seizures during the first year of life [33]. Vigabatrin is an inhibitor of GABA transaminase but also reduces mTOR overactivation, thus offering a potential explanation for its high disease specificity [34]. Prompt AED treatment (soon after or even before seizure onset) might also minimise long-term neurocognitive sequelae [30]. However, irreversible visual field defects/loss has been associated with long-term exposure to vigabatrin therapy [35]. AED combination therapy, prescribed to take advantage of multiple drug mechanisms-of-action and to address multiple seizure types is often necessary, but also carries a risk of adverse events (AEs) [32]. Although vigabatrin may be the preferred treatment for infantile spasms in patients with TSC, adrenocorticotropic hormone (ACTH) or ACTH analogues could be considered the second-line treatment option for vigabatrin-refractory spasms [36].
Surgery is a relevant option in the management of AED-resistant epilepsy in TSC patients and should be evaluated early after the onset of seizures. A systematic review revealed that seizure freedom was achieved in 59 % of patients [37]. Success of surgery is increased by early intervention, accurate localisation of the epileptogenic zone and the possibility of its complete resection (absence of eloquent cortex involvement) [38]. An overview of localization techniques is provided in Pittau et al. [38].
The efficacy and safety data for mTOR inhibitors in the management of TSC-associated epilepsy from phase I/II and III everolimus clinical trials are summarised in Table 2 [10, 12, 13]. At present everolimus is not approved for use in this indication but a prospective, placebo-controlled, phase III clinical trial to evaluate the efficacy and safety of everolimus in patients with TSC who have refractory partial-onset seizures is ongoing and the first results are expected in 2016 [22]. According to the opinion of the expert clinicians, everolimus therapy needs to be continuous in order to obtain clinically meaningful seizure reduction. Again, the optimal duration of treatment is yet to be established. In a small study of children aged ≤3 years with TSC and AED-resistant epilepsy treated with everolimus, reductions in seizure frequency (at least 50 %) were reported in four of five children with active seizures at baseline [39]. As patients with epilepsy are likely to be receiving AEDs, it is important to be aware that the combination of everolimus with AEDs requires caution because interactions with CYP3A4/p-glycoprotein (p-GP) inducers and/or inhibitors have been reported (Table 3) [19].
The use of VNS, the KD, and a low glycaemic index diet for AED-resistant epilepsy in TSC patients have been retrospectively studied [40–42]. Overall, 72 % of patients reported ≥50 % seizure reduction with VNS, with the seizure reduction effects persisting for up to 36 months [40]. VNS does not preclude subsequent improvement in seizure burden with intracranial epilepsy surgery [43]. On the KD, over 90 % of children had a >50 % reduction in their seizures at 6 months and 67 % had a >90 % reduction; many children were seizure free for ≥5 months [41]. KD has multiple mechanisms of action, including an inhibition of the mTOR pathway [44]. The low glycaemic index diet was developed as a more acceptable alternative to the classic KD, and has been reported to have beneficial effects in reducing seizure frequency, with 47 % of patients experiencing a >50 % reduction in seizure frequency at 6 months [42].
4 The Management of Renal Angiomyolipomas
Angiomyolipomas occurring in the kidneys are an important non-neurological manifestation of TSC [45]. However, they do not always require immediate treatment and small renal angiomyolipomas may only require regular monitoring. The vascular component of these tumours makes them susceptible to bleeding, and larger renal angiomyolipomas often develop aneurysms that may rupture. Overall, estimates suggest that 25–50 % of TSC patients with renal angiomyolipomas will experience haemorrhages [46].
Premature decline of renal function (as measured by glomerular filtration rate; GFR) is now a recognised problem in adults with TSC, occurring in up to 40 % TSC patients [47]; it is unclear what proportion is related to angiomyolipoma burden and its complications. In the absence of renal angiomyolipomas, GFR and blood pressure should be assessed at least annually. Renal angiomyolipomas should be monitored using MRI/ultrasound every 1–3 years. Large renal angiomyolipomas (>3 cm) require more frequent assessment and should be assessed using MRI/ultrasound at least annually [24].
Recommendations for the treatment of renal angiomyolipomas
-
mTOR inhibitor therapy is the first-line therapy in the short term for asymptomatic, growing angiomyolipomas (>3 cm) [6].
-
If mTOR inhibitor therapy is not available, embolization is the second choice for growing renal angiomyolipomas.
-
Emergency embolisation is the first-line therapy for renal angiomyolipomas with acute bleeding or angiomyolipoma aneurysm [6].
-
Surgery is considered the last-resort treatment, given the potential for loss of renal function.
4.1 Applying Clinical Evidence to Clinical Practice
The efficacy and safety data of mTOR inhibitors (sirolimus and everolimus) in the management of renal manifestations of TSC from phase I/II, II, and III clinical trials are summarised in Table 4 [11, 12, 23–25]. Everolimus is licensed for the treatment of renal angiomyolipomas in adults but not children. However, it has been shown to be very effective in shrinking renal angiomyolipomas in children, with minor and tolerable side effects in the short term (Fig. 2) [48]. Embolisation is first-line therapy for acutely haemorrhaging angiomyolipoma aneurysms. However, mTOR inhibitors may be favourable as a first-line therapy considering their effect on vascularity and avoiding risks of embolisation (Fig. 3). This implies that aneurysm size could be reduced due to shrinkage of vasculature associated with mTOR inhibitor therapy, and might explain why the marked shrinkage of renal angiomyolipomas is associated with a very low incidence of haemorrhage (none observed so far in EXIST-2) [11; Kingswood JC, personal communication]. Furthermore, recent results from the EXIST-2 trial show that mTOR inhibitors show continued efficacy and no new side effects after treatment for 2.45 years [49].
Renal MRI of a 21-year-old patient with several large angiomyolipomas in both kidneys. MRI performed at baseline, after 3 months, and after 17 months of treatment with everolimus, demonstrating a marked reduction of hamartoma size, most clearly depicted in the larger lesions. Reduction of the angiomyolipoma with everolimus therapy was accompanied by a reduction in seizure frequency and improvements in behaviour, making possible the tapering of antiepileptic drug therapy [Dill P, personal communication]. (Images provided by Dill P). MRI magnetic resonance imaging
Case study showing loss of vascularity in renal angiomyolipomas in a patient with TSC. The successful treatment of SEGA and renal angiomyolipomas with everolimus was accompanied by remission of subclinical epilepsy, with improvement in neurocognition and autistic behaviour [Kingswood JC, personal communication]. (Images provided by Kingswood JC). SEGA subependymal giant cell astrocytoma, TSC tuberous sclerosis complex
5 Management of Adverse Events (AEs) Associated with Mammalian Target of Rapamycin (mTOR) Inhibitor Therapy
The AEs associated with mTOR inhibitor therapy are well defined [50]. Practical guidance on how to manage the most common AEs is summarised in Table 5. As a general rule, AEs should be classified according to the Common Terminology Criteria for Adverse Events, and when an AE is grade 1 no dose changing is necessary, when it is grade 2 or 3 a temporary discontinuation might be necessary, and when toxicity is back to grade 1 or lower treatment can be started again, at the same dosage or at a lower one. Finally, in case of a grade 4 AE, permanent discontinuation is required. Although AE management might require a temporary or permanent interruption in therapy (with or without dose reduction) [19], clinicians should try to manage AEs to avoid permanent discontinuation of this treatment. Data suggest that mTOR inhibitor-related AEs might be dose-dependent and that dose reductions can minimise AEs yet maintain efficacy [51; Kotulska K, personal communication]. If a dose reduction is required, the suggested dose is approximately 50 % lower than the dose previously administered [19].
5.1 Skin Complaints
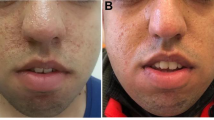
Skin complaints, including generalised, macular, maculo-papular, papular, allergic dermatitis rashes and urticaria, were common during clinical trials of everolimus (Table 5) [19]. Acne has also been observed and in these cases standard therapy can be provided to address the complaint [Kingswood JC, personal communication].
5.2 Renal Failure
Renal failure, including acute renal failure, has been observed in patients treated with everolimus (2.5 % of everolimus group vs. 0 % of placebo group) [11, 19]. Acute kidney injury (AKI) has been observed in the two major randomised controlled trials (EXIST-1 and EXIST-2); AKI was noted to be more common in those receiving placebo than in those on everolimus. In addition, EXIST-1 and EXIST-2 have shown that the GFR remains stable (within the ~2.75-year follow-up periods of these trials) unless it was already declining and <30 ml/min before starting everolimus [52].
Levels of proteinuria remained stable in patients enrolled in the EXIST-1 and EXIST-2 randomised controlled trials up to the ~2.75-year follow-up [52]. However, serum creatinine and proteinuria elevations have been reported in patients taking everolimus (respectively in 1 % of everolimus and 8 % of placebo group, and 4 % of everolimus and 8 % of placebo group), so monitoring of renal function (including measurement of blood urea nitrogen), urinary protein or serum creatinine is recommended prior to the start of therapy and periodically afterwards [19]. In these patients it can be difficult to ascertain the exact cause of renal damage, since TSC itself and the other drugs used in this condition might determine renal toxicity. Therefore, monitoring is particularly important among patients with additional risk factors for impaired renal function. The exacerbation or new onset of proteinuria is not necessarily an indication to stop mTOR inhibitor therapy in those who are benefiting from it, unless the patient develops nephrotic syndrome. Continued monitoring and referral to a nephrologist, to exclude other causes, is recommended [Kingswood JC, personal communication].
5.3 Amenorrhoea/Ovarian Toxicity
Temporary amenorrhoea was reported with everolimus during clinical trials [19]. A clinical experience shared during the meeting described the disease course and management of a 19-year-old female who received everolimus therapy for SEGAs, renal angiomyolipoma, skin and eye manifestations, and refractory epilepsy. This patient then developed an ovarian cyst that ultimately required surgical resection [Moavero R, personal communication]. The mTOR pathway is involved in the regulation of ovarian function (e.g. follicle quiescence/activation) [53]; therefore, not surprisingly, the mTOR inhibitor therapy has an effect on ovarian function and sirolimus has previously been reported to increase the risk of both oligomenorrhoea and ovarian cysts [54]. These reports highlight the potential for ovarian toxicity during mTOR inhibitor therapy in females, and the need for ovarian function/hormonal assessment during treatment [Moavero R, personal communication]. However, if diagnosed early, there should be no need for permanent treatment discontinuation. Increased awareness that this AE can occur in TSC patients receiving mTOR inhibitor therapy can help speed up diagnosis.
6 Other Safety Considerations with mTOR Inhibitor Therapy
6.1 Drug Level Monitoring
Although there are no evidence-based data to suggest serum levels to be achieved during everolimus treatment, actual knowledge suggests they should be maintained at between 5 and 15 ng/ml [2]. The ongoing study EXIST-3 will compare efficacy and safety of two trough ranges of everolimus as adjunctive therapy in patients with TSC and refractory partial onset seizures (NCT01713946).
6.2 Drug–Drug Interactions
Significant drug–drug interactions between everolimus and CYP3A4/p-GP inhibitors and inducers are summarised in Table 3 [19]. Interactions between everolimus and CYP3A4/p-GP inhibitors lead to increased everolimus availability; interactions with CYP3A4/p-GP inducers lead to reduced everolimus availability. It is important to monitor everolimus serum levels during treatment, and in particular every time a CYP3A4/p-GP inducer/inhibitor is added as a co-medication or their dosage is modified.
Recommendations: drug–drug interactions with mTOR inhibitors
CYP3A4/p-GP inhibitors
-
Avoid concomitant use of strong CYP3A4/p-GP inhibitors with everolimus (see Table 3).
-
Caution should be used when co-administering with moderate CYP3A4/p-GP inhibitors.
-
If co-administration with a moderate CYP3A4/p-GP inhibitor is required:
-
Reduce the everolimus dose to 2.5 mg daily; this is predicted to adjust the area under the curve (AUC) to the range observed without inhibitors. A later dose increase from 2.5 mg to 5 mg may then be considered based on patient tolerance. Note: these recommendations do not apply to patients who have a lower initial everolimus dose.
-
If discontinued, a washout period of approximately 2–3 days should be allowed before increasing the everolimus dose to the level used before the introduction of the moderate inhibitor.
-
CYP3A4/p-GP inducers
-
Avoid the concomitant use of strong CYP3A4/p-GP inducers (see Table 3).
-
If patients require co-administration of a strong CYP3A4/p-GP inducer consider:
-
Increasing the daily dose of everolimus in increments of ≤5 mg; this dose is predicted, based on pharmacokinetic data, to adjust the AUC to the range observed without inducers although there are no clinical data to support this approach. Note: these recommendations do not apply to young children who have a lower initial everolimus dose.
-
If discontinued, consider a washout period of 3–5 days before decreasing the everolimus dose to that used before initiation of the strong inducer
-
-
St John’s Wort (Hypericum perforatum) may decrease everolimus exposure unpredictably and should be avoided
6.3 Vaccinations
The immunosuppressive properties of everolimus may predispose patients to infections, including infections from those who have received live vaccines [19]. Although a study in renal patients suggested that everolimus treatment may allow/preserve the T-cell-dependent and independent secondary humoral immune response to pneumococcal polysaccharide vaccine [55], comprehensive evidence-based recommendations on immunisation schedules in infants and toddlers receiving everolimus are needed.
Recommendations: mTOR inhibitors and vaccinations
CYP3A4/p-GP inhibitors
-
Avoid the use of live vaccines and close contact with individuals who have received live vaccines (e.g. intranasal influenza, measles, mumps, rubella, oral polio, Bacillus Calmette–Guérin (BCG), yellow fever, varicella and TY21a typhoid vaccines) during everolimus therapy.
-
If possible, the childhood immunisation schedule should be completed prior to starting everolimus, and an accelerated vaccination schedule may be appropriate.
-
If this is not possible, the expert panel recommended stopping treatment for 2 weeks before and after vaccination
-
7 Conclusions
Currently there are two licensed indications for the use of everolimus in TSC, i.e. SEGA irrespective of age and renal angiomyolipomas in adults. Other potential indications are being evaluated in phase II or phase II/III studies. These include severe epilepsy (EXIST-3), neurocognitive deficit (TRON—A Study of Everolimus in the Treatment of Neurocognitive Problems in Tuberous Sclerosis [56]) and autistic spectrum behaviour (RAPIT—Efficacy of RAD001/Everolimus in Autism and NeuroPsychological Deficits in Children With Tuberous Sclerosis Complex [57]), and pulmonary lymphangioleiomyomatosis and skin involvement (EXIST-2). On the other hand, sirolimus has been approved for pulmonary lymphangioleiomyomatosis in the USA and Japan. Other potential indications being evaluated in phase II or phase II/III studies include autism spectrum disorder and/or intellectual disability associated with TSC [58] and, with a new topical formulation, angiofibroma and other skin lesions in patients with TSC [59, 60].
For many patients TSC is a systemic and progressive disease, with a majority of patients at risk for developing significant morbidity by middle age. It may, therefore, be more logical to use a lifetime risk score to decide whether an mTOR inhibitor should be prescribed rather than just base the decision on isolated individual organ complications. The decision should also take into account the limited knowledge of the effects of long-term mTOR inhibitor therapy initiated in young infants. The necessary epidemiological data needed to evaluate this approach are not yet available; however, studies like the Tuberous Sclerosis Registry to Increase Disease Awareness (TOSCA) registry, an international disease registry set up to assess manifestations, interventions and outcomes in patients with TSC, will help [61].
The identification and optimal treatment of neurological and non-neurological manifestations of TSC require a multidisciplinary approach, and strong collaboration between paediatricians, neurologists, neurosurgeons and nephrologists. Although surgery was once the only therapeutic option for treating TSC-related tumours, mTOR inhibitor therapy now provides a new, biologically targeted pharmacological treatment to be considered alongside other approaches. Moreover, due to the systemic nature of mTOR inhibitor treatment, it may be indicated in patients with one or more manifestations of TSC (e.g. some patients with epilepsy and/or skin complaints). Questions remain relating to proper dosage of mTOR inhibitors, the serum level to be achieved, the timing of treatment initiation and withdrawal, the length of treatment and the long term side effects. It is hoped that disease registries such as TOSCA and other clinical studies may help answer these questions.
References
Nellist M, van den Heuvel D, Schluep D, Exalto C, Goedbloed M, Maat-Kievit A, et al. Missense mutations to the TSC1 gene cause tuberous sclerosis complex. Eur J Hum Genet. 2009;17(3):319–28.
Curatolo P, Moavero R. mTOR inhibitors in tuberous sclerosis complex. Curr Neuropharmacol. 2012;10(4):404–15.
Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–68.
Moavero R, Coniglio A, Garaci F, Curatolo P. Is mTOR inhibition a systemic treatment for tuberous sclerosis? Ital J Pediatr. 2013;39:57.
Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49:243–54.
Krueger DA, Northrup H, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):255–65.
Curatolo P, Moavero R, de Vries PJ. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14(7):733–45.
Curran MP. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatric Drugs. 2012;14:51–60.
Franz DN. Everolimus: an mTOR inhibitor for the treatment of tuberous sclerosis. Expert Rev Anticancer Ther. 2011;11:1181–92.
Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–11.
Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817–24.
Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–32.
Krueger DA, Wilfong AA, Holland-Bouley K, Anderson AE, Agricola K, Tudor C, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74(5):679–87.
Franz DN, Agricola K, Mays M, Tudor C, Care MM, Holland-Bouley K, et al. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol. 2015;78:929–38. doi:10.1002/ana.24523.
Jóźwiak S, Nabbout R, Curatolo P, participants of the TSC Consensus Meeting for SEGA and Epilepsy Management. Management of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. 2013;17(4):348–52.
Amin S, Carter M, Edwards RJ, Pople I, Aquilina K, Merrifield J, et al. The outcome of surgical management of subependymal giant cell astrocytoma in tuberous sclerosis complex. Eur J Paediatr Neurol. 2013;17(1):36–44.
Roth J, Roach ES, Bartels U, Jóźwiak S, Koenig MK, Weiner HL, et al. Subependymal giant cell astrocytoma: diagnosis, screening, and treatment. Recommendations from the international tuberous sclerosis complex consensus conference 2012. Pediatr Neurol. 2013;49(6):439–44.
Perek-Polnik M, Jóźwiak S, Jurkiewicz E, Perek D, Kotulska K. Effective everolimus treatment of inoperable, life-threatening subependymal giant cell astrocytoma and intractable epilepsy in a patient with tuberous sclerosis complex. Eur J Paediatr Neurol. 2012;16(1):83–5.
Afinitor® (everolimus). Prescribing information. Novartis, revised July 2014. http://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf. Accessed 1 Sept 2014.
Kotulska K, Borkowska J, Roszkowski M, Mandera M, Daszkiewicz P, Drabik K, et al. Surgical treatment of subependymal giant cell astrocytoma in tuberous sclerosis complex patients. Pediatr Neurol. 2014;50(4):307–12.
Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–80.
ClinicalTrials.gov identifier: NCT01713946. A placebo-controlled study of efficacy and safety of 2 trough-ranges of everolimus as adjunctive therapy in patients with tuberous sclerosis complex (TSC) and refractory partial-onset seizures (EXIST-3). Accessed 1 Sept 2014.
Davies DM, de Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res. 2011;17(12):4071–81.
Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–51.
Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ Jr, Miles D, et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS One. 2011;6(9):e23379.
Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Everolimus for supependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014;15(13):1513–20.
Moavero R, Pinci M, Bombardieri R, Curatolo P. The management of subependymal giant cell tumors in tuberous sclerosis: a clinician’s perspective. Childs Nerv Syst. 2011;27(8):1203–10.
Torres VE, King BF, McKusick MA, Bjornsson J, Zincke H. Update on tuberous sclerosis complex. Contrib Nephrol. 2001;136:33–49.
Cusmai R, Moavero R, Bombardieri R, Vigevano F, Curatolo P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011;22(4):735–9.
Jóźwiak S, Kotulska K, Domańska-Pakieła D, Lojszczyk B, Syczewska M, Chmielewski D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. 2011;15(5):424–31.
Domańska-Pakieła D, Kaczorowska M, Jurkiewicz E, Kotulska K, Dunin-Wąsowicz D, Jóźwiak S. EEG abnormalities preceding the epilepsy onset in tuberous sclerosis complex patients—a prospective study of 5 patients. Eur J Paediatr Neurol. 2014;18(4):458–68.
Curatolo P, Jóźwiak S, Nabbout R, TSC Consensus Meeting for SEGA and Epilepsy Management. Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. 2012;16(6):582–6.
Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14(2):146–9.
Zhang B, McDaniel SS, Rensing NR, Wong M. Vigabatrin inhibits seizures and mTOR pathway activation in a mouse model of tuberous sclerosis complex. PLoS One. 2013;8:e57445.
Willmore LJ, Abelson MB, Ben-Menachem E, Pellock JM, Shields WD. Vigabatrin: 2008 update. Epilepsia. 2009;50(2):163–73.
Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013;6:CD001770.
Zhang K, Hu WH, Zhang C, Meng FG, Chen N, Zhang JG. Predictors of seizure freedom after surgical management of tuberous sclerosis complex: a systematic review and meta-analysis. Epilepsy Res. 2013;105(3):377–83.
Pittau F, Grouiller F, Spinelli L, Seeck M, Michel CM, Vulliemoz S. The role of functional neuroimaging in pre-surgical epilepsy evaluation. Front Neurol. 2014;5:31.
Kotulska K, Chmielewski D, Borkowska J, Jurkiewicz E, Kuczyński D, Kmieć T, et al. Long-term effect of everolimus on epilepsy and growth in children under 3 years of age treated for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Eur J Paediatr Neurol. 2013;17(5):479–85.
Zamponi N, Petrelli C, Passamonti C, Moavero R, Curatolo P. Vagus nerve stimulation for refractory epilepsy in tuberous sclerosis. Pediatr Neurol. 2010;43(1):29–34.
Kossoff EH, Thiele EA, Pfeifer HH, McGrogan JR, Freeman JM. Tuberous sclerosis complex and the ketogenic diet. Epilepsia. 2005;46(10):1684–6.
Larson AM, Pfeifer HH, Thiele EA. Low glycemic index treatment for epilepsy in tuberous sclerosis complex. Epilepsy Res. 2012;99(1–2):180–2.
Elliott RE, Carlson C, Kalhorn SP, Moshel YA, Weiner HL, Devinsky O, et al. Refractory epilepsy in tuberous sclerosis: vagus nerve stimulation with or without subsequent resective surgery. Epilepsy Behav. 2009;16(3):454–60.
McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–11.
Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):243–54.
Dixon BP, Hulbert JC, Bissler JJ. Tuberous sclerosis complex renal disease. Nephron Exp Nephrol. 2011;118(1):e15–20.
Kingswood JC, Demuth D, Nasuti P, Lucchese L, Gray E, Magestro M. Real-world assessment of renal involvement in tuberous sclerosis complex (TSC) patients in the United Kingdom (UK) [abstract 318]. Eur Urol Suppl. 2014;13(1):e318–a.
Kingswood JC, Jozwiak S, Belousova ED, Frost MD, Kuperman RA, Bebin EM, et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, Phase 3 trial EXIST-1. Nephrol Dial Transplant. 2014;29(6):1203–10.
Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everoliums for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant. 2016;31(1):111–9.
Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28(3):126–33.
Rugo HS, Pritchard KI, Gnant M, Noguchi S, Piccart M, Hortobagyi G, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25(4):808–15.
Bissler JJ, Kingswood JC, Zonnenberg BA, Frost M, Belousova E, Sauter M, et al. Effect of everolimus on renal function in patients with tuberous sclerosis complex (TSC): results from EXIST-1 and EXIST-2 [abstract TO006]. Presented at the 51st ERA-EDTA Congress, 31 May to 3 June 2014, Amsterdam, The Netherlands.
Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19(3):397–410.
Braun M, Young J, Reiner CS, Poster D, Krauer F, Kistler AD, et al. Low-dose oral sirolimus and the risk of menstrual-cycle disturbances and ovarian cysts: analysis of the randomized controlled SUISSE ADPKD trial. PLoS One. 2012;7(10):e45868.
Struijk GH, Minnee RC, Koch SD, Zwinderman AH, van Donselaar-van der Pant KA, Idu MM, et al. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int. 2010;78(9):934–40.
ClinicalTrials.gov identifier: NCT01954693. A study of everolimus in the treatment of neurocognitive problems in tuberous sclerosis (TRON). Accessed 1 Sept 2014.
ClinicalTrials.gov identifier: NCT01730209. Efficacy of RAD001/everolimus in autism and neuropsychological deficits in children with tuberous sclerosis complex (RAPIT). Accessed 1 Sept 2014.
ClinicalTrials.gov identifier: NCT01929642. Rapalogues for autism phenotype in TSC: a feasibility study (RAPT). Accessed 26 Jan 2016.
ClinicalTrials.gov identifier: NCT02634931. Long-term trial of topical sirolimus to angiofibroma in patient with tuberous sclerosis complex. Accessed 26 Jan 2016.
ClinicalTrials.gov identifier: NCT02635789. Phase III trial of topical formulation of sirolimus to skin lesions in patients with tuberous sclerosis complex (TSC). Accessed 26 Jan 2016.
Kingswood JC, Bruzzi P, Curatolo P, de Vries PJ, Fladrowski C, Hertzberg C, et al. TOSCA—first international registry to address knowledge gaps in the natural history and management of tuberous sclerosis complex. Orphanet J Rare Dis. 2014;26(9):182. doi:10.1186/s13023-014-0182-9.
Acknowledgments
The authors wish to acknowledge the expert contribution from Dr. De Wit (Erasmus MC, Rotterdam, The Netherlands) attending the VEnice Network In Clinical Excellence Masterclass meeting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The VEnice Network in Clinical Excellence Masterclass meeting organized and supported by Novartis, formed the basis for this publication. The authors are fully responsible for the content of this manuscript. Medical writing support used for this article in terms of final editing and formatting of the draft manuscript was provided by Excerpta Medica, funded by Novartis, and none of the authors received any funding for the preparation of this manuscript. The work of PC, AJ, KK, SJ, RN, MF, CH, RM was partially supported by the Seventh Framework Programme of European Commission within the Large-scale Integrating Project EPISTOP (Proposal No: 602391-2).
Conflict of interest
PC was on the study steering committee of EXIST-1 and 3 trials funded by Novartis. He is also on the Scientific Advisory Board of TOSCA, a natural history study of tuberous sclerosis, funded by Novartis, and has received honoraria from Novartis for participation in advisory board meetings. MB is the national coordinator (Norway) of the EXIST-1 trial and TOSCA (international disease registry of tuberous sclerosis), funded by Novartis. PED has received consulting fees from Novartis. JCF has received honoraria and support from Novartis for travel to the VENICE (VEnice Network In Clinical Excellence) TSC Masterclass in 2013. MF has received honoraria and travel support from Novartis, Cyberonics, UCB and Eisai. CH declares that he received an honorarium for his participation, and travel funding from Novartis, for the TSC meeting in 2013. AJ is a member of the Scientific Advisory Board of TOSCA, a natural history study of tuberous sclerosis, funded by Novartis, and has received honoraria from Novartis for her participation in advisory board meetings. SJ was on the study steering committee of EXIST-1 trial funded by Novartis. He is also on the Scientific Advisory Board of TOSCA, a natural history study of tuberous sclerosis, funded by Novartis, and has received honoraria from Novartis for participation in advisory board meetings. JCK has received support for presenting his research at international meetings and manuscript publication about findings from his research from Novartis. He has received honoraria for his advisory work and presentations from Novartis. Dr. Kingswood’s institution has received research grants from Novartis, for which Dr. Kingswood was a Principal Investigator. KK has received speaker’s honoraria from Novartis. AM is a member of the TOSCA Scientific Advisory Board, funded by Novartis. He has received payment from Novartis for lectures and participation in advisory board meetings. RM has received a consulting fee from Novartis. RN was on the study steering committee of EXIST 3 trials funded by Novartis. She is also on the Scientific Advisory Board of TOSCA, a natural history study of tuberous sclerosis, funded by Novartis, and has received honoraria from Novartis for participation in advisory board meetings. She has also received speaker’s honoraria from Eisai, Nutricia, Zogenix and Shire. BAZ has received funding for research, as well as consulting and speaking fees, from Novartis.
Ethical standards
The manuscript does not contain clinical studies of identifiable patient data.
Rights and permissions
About this article
Cite this article
Curatolo, P., Bjørnvold, M., Dill, P.E. et al. The Role of mTOR Inhibitors in the Treatment of Patients with Tuberous Sclerosis Complex: Evidence-based and Expert Opinions. Drugs 76, 551–565 (2016). https://doi.org/10.1007/s40265-016-0552-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0552-9