Abstract
Embryonal tumor with multilayered rosettes (ETMR), C19MC-altered was introduced to the World Health Organization classification of central nervous system tumors in 2016. It is characterized by amplification or fusion of the chromosome 19 microRNA cluster (C19MC) locus at 19q13.42. Medulloepithelioma also an ETMR but lacks C19MC alteration. We report a rare case of spinal medulloepithelioma in a 2-year-old boy and review the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryonal tumor with multilayered rosettes (ETMR), C19MC-altered was introduced to the World Health Organization classification of central nervous system tumors (WHO CNS) in 2016 [1]. It is defined as an ETMR and amplification or fusion of the chromosome 19 microRNA cluster (C19MC) locus at 19q13.42. In the absence of C19MC amplification, a tumor with ETMR histological features should be diagnosed as ETMR, not otherwise specified; a tumor with medulloepithelioma histological features should be diagnosed as medulloepithelioma [1, 2]. Medulloepithelioma arises mainly within the cranium [1].
Intramedullary spinal cord tumors account for 1–2% of all pediatric CNS tumors. Diffuse astrocytic tumors and ependymomas are common in the spinal cord, but embryonal tumors are extremely rare [1]. We report a spinal medulloepithelioma without C19MC alteration in a 2-year-old child.
Case report
A 2-year-old boy was admitted to our hospital because of progressively worsening thigh and buttock pain over the previous month. His medical and family histories were unremarkable. Physical examination revealed difficulty walking and standing because of mild bilateral lower limb weakness and pain. He was slightly constipated but had no obvious bladder or rectal dysfunction.
Radiology
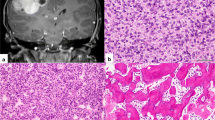
Magnetic resonance imaging (MRI) showed a well-defined tumor on the ventral side of the thoracolumbar spinal cord extending from T7 to S1. The tumor appeared isointense on T1-weighted imaging and slightly hyperintense on T2-weighted imaging (Fig. 1a, b). It enhanced modestly after administration of gadolinium contrast (Fig. 1c).
Magnetic resonance imaging. a Preoperative sagittal T1-weighted imaging shows a hypointense tumor on the ventral side of the thoracolumbar spinal cord extending from T7 to S1. b The tumor is hypointense on T2-weighted imaging. c Modest tumor enhancement is visualized on contrast-enhanced sagittal T1-weighted imaging. d Postoperative contrast-enhanced sagittal T1-weighted imaging shows complete resection of the tumor except for the attachment to the filum terminale
Surgery
The patient underwent laminectomies from L1 to S1 for tumor resection using intraoperative neurophysiological monitoring with motor evoked potentials. The tumor was soft and hemorrhagic and had an extramedullary component (Fig. 2a, b). It was resected in a piecemeal fashion to minimize retraction on the spinal cord. Near-total resection was achieved; the attachment to the filum terminale involved one nerve root and was intentionally left behind (Fig. 2c, d).
Pathology
Hematoxylin and eosin staining showed a biphasic pattern of densely packed undifferentiated cells forming multilayered rosettes and perivascular pseudorosettes as well as a component of sparse clear cytoplasmic cells without rosette formation (Fig. 3a, b). The clear cytoplasmic cells stained strongly and diffusely positive for synaptophysin (Fig. 3c). Glial fibrillary acidic protein and epithelial membrane antigen staining was uniformly negative. Tumor cells in both patterns were positive for LIN28A (Fig. 3d). The Ki-67 labeling index in hot spot regions was approximately 90% (Fig. 3e). Fluorescence in situ hybridization testing showed no C19MC amplification (Fig. 3f) [3]. The histopathological diagnosis was medulloepithelioma.
Pathological findings. a One part of the tumor was composed of densely packed undifferentiated cells forming multilayered rosettes and perivascular pseudorosettes. The other part was composed of sparse clear cytoplasmic cells without rosette formation (hematoxylin and eosin stain, × 100). b Multilayered rosettes (arrows) (hematoxylin and eosin stain, × 200). c Immunoreactivity to synaptophysin was strong in the clear cytoplasmic cells and negative in the undifferentiated cells (counter stained by hematoxylin, × 100). d The undifferentiated cells were positive for LIN28A (× 200). e The Ki-67 labeling index was 90% in hot spot regions (× 200). f Fluorescence in situ hybridization using a C19MC 19q13.42 probe (green signals) and a reference 19p13 probe (red signals) showed no C19MC amplification (× 1000)
Postoperative course
Postoperative MRI showed near-total resection (Fig. 1d), and his symptoms immediately recovered without new neurological deficit. He was treated with concurrent proton beam local irradiation (50.4 GyE in 28 fractions) and six cycles of chemotherapy (carboplatin and vincristine). As maintenance therapy, three courses of combination ifosphamide, cisplatin, and etoposide were administered. Ten months after surgery, the patient was asymptomatic and recurrence-free on follow-up MRI.
Discussion
Embryonal tumors are composed of small immature undifferentiated tumor cells that histologically resemble cells of the neural tube in the embryonic period. They occur mainly in infants. In the 2007 WHO CNS, this tumor group included medulloblastoma, CNS primitive neuroectodermal tumor (PNET), and atypical teratoid/rhabdoid tumor (AT/RT) [4]. Tumors are now classified according to their molecular characteristics. Therefore, the term CNS PNET was removed from the diagnostic lexicon in the 2016 WHO CNS because these rare tumors often show amplification of the C19MC region on chromosome 19. The presence of C19MC amplification now results in a diagnosis of ETMR, C19MC-altered [1, 2].
Histologically, tumors classified as ETMR, C19MC-altered show papillary and tubular arrangements of malignant single and multilayered columnar epithelium surrounded by an outer basement membrane [1]. Medulloepithelioma shares many clinical features with ependymoblastoma and embryonal tumor with abundant neuropil and true rosettes, as well as molecular features including amplification of 19q13.42 and expression of LIN28A [1, 5].
In our patient’s tumor, numerous rosettes composed of large cells were recognized, which were multilayered rather than true rosettes. Although the histopathological findings of LIN28A immunoreactivity and high Ki-67 labeling index were consistent with ETMR, C19MC gene amplification was not detected. Therefore, the tumor was finally diagnosed as medulloepithelioma. To the best of our knowledge, only two cases of spinal cord medulloepithelioma have been previously reported [6, 7].
Since the 2016 WHO CNS, medulloepithelioma has been mainly included in the ETMR category. Median age at diagnosis is 2 years with female predominance. Supratentorial location is most common [3, 8,9,10,11,12]. ETMR is highly malignant, and mean survival time is 12 months after combination therapy [1, 5, 9, 11]. The prognostic significance of C19MC amplification in patients with CNS embryonal tumors remains unclear. According to previous reports, patients with C19MC amplification might show worse prognosis than those without C19MC amplification [2,3,4, 11].
Although treatment has not been standardized because of the low incidence, surgical resection, radiation therapy, high-dose chemotherapy plus peripheral blood stem cell transplantation, and proton beam therapy have been reported useful [13,14,15,16,17].
To the best of our knowledge, only five cases of spinal cord ETMR have been reported (Table 1) [8, 12, 16, 18]. Most were located in the lumbar spinal cord and presented with lumbar pain, gait disorder, and bladder and/or rectal dysfunction. The outcome was poor in one patient with multiple lesions at diagnosis [9]; however, two underwent total resection with radiation and chemotherapy and survived more than 3 years [12, 16]. This suggests that prognosis may be more favorable for spinal ETMR than intracranial ETMR. Our patient underwent combination therapy and was alive at the 10-month follow-up without any sign of recurrence.
Prognosis of spinal ETMR may be better for two reasons. First, unlike intracranial lesions, radiation therapy can be used for spinal lesions, even in infants. Second, molecular features may differ between intracranial and spinal lesions. Our patient did not have C19MC amplification, so further molecular studies would be required to diagnose spinal ETMRs.
References
Louis DN, Ohgaki H, Wiestler OD et al. eds. (2016) World Health Organization histological classification of tumours of the central nervous system. Int Agency Res Cancer, Lyon
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Korshunov A, Remke M, Gessi M et al (2010) Focal genomic amplification at 19q13.42 comprises a powerful diagnostic marker for embryonal tumors with ependymoblastic rosettes. Acta Neuropathol 120:253–260. https://doi.org/10.1007/s00401-010-0688-8
Louis DN, Ohgaki H, Wiestler OD et al. eds. (2007) World Health Organization histological classification of tumours of the central nervous system. Int Agency Res Cancer, Lyon
Spence T, Sin-Chan P, Picard D, Barszczyk M et al (2014) CNS-PNETs with C19MC amplification and/or LIN28A expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol 128:291–303. https://doi.org/10.1007/s00401-014-1291-1
Takei H, Florez L, Moroz K, Bhattacharjee MB (2017) Medulloepithelioma: two unusual locations. Pathol Int 57:91–95. https://doi.org/10.1111/j.1440-1827.2006.02062.x
Karch SB, Urich H (1972) Medulloepithelioma: definition of an entity. J Neuropathol Exp Neurol 31:27–53
Eberhart CG, Brat DJ, Cohen KJ, Burger PC (2000) Pediatric neuroblastic brain tumors containing abundant neuropil and true rosette. Pediatr Dev Pathol 3:346–352. https://doi.org/10.1007/s100249910049
Gessi M, Giangaspero F, Lauriola L et al (2009) Embryonal tumors with abundant neuropil and true rosettes: a distinctive CNS primitive neuroectodermal tumor. Am J Surg Pathol 33:211–217. https://doi.org/10.1097/PAS.0b013e318186235b
Nobusawa S, Yokoo H, Hirato J et al (2012) Analysis of chromosome 19q13.42 amplification in embryonal brain tumors with ependymoblastic multilayered rosettes. Brain Pathol 22:689–697. https://doi.org/10.1111/j.1750-3639.2012.00574.x
Korshunov A, Sturm D, Ryzhova M et al (2014) Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol 128:279–289. https://doi.org/10.1007/s00401-013-1228-0
Horwitz M, Dufour C, Leblond P et al (2016) Embryonal tumors with multilayered rosettes in children: the SFCE experience. Childs Nerv Syst 32:299–305. https://doi.org/10.1007/s00381-015-2920-2
Molly PT, Yachnis AT, Rorke LB (1996) Central nervous system medulloepithelioma: a series of eight cases including two arising in the pons. J Neurosurg 84:430–436. https://doi.org/10.3171/jns.1996.84.3.0430
Li D, Hao S, Wang L et al (2018) Clinicoradiological features and surgical outcomes of primary intracranial medulloepitheliomas: a single-center experience and pooled analysis of individual patient data. J Neurosurg 1:1–15. https://doi.org/10.3171/2018.1.JNS172509
Hayase T, Morimoto A, Kawahara Y et al (2015) An infant with medulloepithelioma successfully treated by high-dose chemotherapy followedby autologous pepripheral blood stem cell transplantation without radiotherapy. J Pediatr Hematol Oncol 37:e394–e398. https://doi.org/10.1097/MPH.0000000000000381
Jaramillo S, Grosshans DR, Philip N et al (2018) Radiation for ETMR: literature review and case series of patients treated with proton therapy. Clin Transl Radiat Oncol 15:31–37. https://doi.org/10.1016/j.ctro.2018.11.002
Mayr L, Gojo J, Peyrl A et al (2020) Potential importance of early focal radiotherapy following gross total resection for long-term survival in children with embryonal tumors with multilayered rosettes. Front Oncol 10:584681. https://doi.org/10.3389/fonc.2020.584681
Wang B, Gogia B, Fuller GN et al (2018) Embryonal tumor with multilayered rosettes, C19MC-altered: clinical, pathological, and neuroimaging findings. J Neuroimaging 28:482–489. https://doi.org/10.1111/jon.12524
Acknowledgements
The authors thank Enago (https://www.enago.jp/) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakamura, K., Matsuda, Ki., Kabasawa, T. et al. A surgical case of pediatric spinal medulloepithelioma. Childs Nerv Syst 38, 473–477 (2022). https://doi.org/10.1007/s00381-021-05293-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05293-7