Abstract
Introduction
The management of hydrocephalus in paediatric patients where the peritoneum has failed can be challenging. One option is to perform a ventriculo-cholecystic shunt. However, little is known about the capacity of the gall bladder to accommodate cerebrospinal fluid (CSF).
Methods
A retrospective case series was performed to include all paediatric patients who received a ventriculo-cholecystic shunt at a single centre, Sheffield Children’s Hospital.
Results
We identified three patients who had a ventriculo-cholecystic shunt inserted. The shunt survived past 1 year in two patients, who had pre-operative external ventricular drain (EVD) outputs of 8 and 10 ml/h respectively. One patient shunt failed at day four post-op due to distal dysfunction, his pre-operative EVD was over 30 ml/h.
Conclusions
When considering a patient for a ventriculo-cholecystic shunt, caution should be taken if a high CSF output is known, for example, as per an EVD measurement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the management of paediatric hydrocephalus, the preferential placement of distal cerebrospinal fluid (CSF) shunt tubing is into the peritoneum, followed by the right atrium. However, when the peritoneum and right atrium are compromised and endoscopic third ventriculostomy (ETV) has failed, it is unclear how best to proceed. The pleural cavity is often utilised in adults; however, there are concerns regarding pleural effusion and potential respiratory failure in children [1,2,3,4]. Younger age has been shown to be associated with a higher risk of symptomatic effusions following the insertion of a ventriculo-pleural shunt [3,4,5]. There have been attempts at various anatomical locations for the distal shunt tubing, such as the ureter [6], superior sagittal sinus [7], and sternum [8]. There is growing evidence demonstrating that ventriculo-cholecystic (VC) shunts are a safe and effective alternative [9,10,11,12,13,14,15,16,17]. We present our experience of VC shunts inserted as a joint case between Neurosurgery and Paediatric Surgery.
Case 1
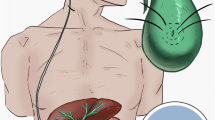
Premature infant born at 28 + 4 weeks (1019 g) subsequently developed grade IV intraventricular haemorrhage and necrotising enterocolitis managed by a laparotomy. The hydrocephalus was primarily treated with a ventriculo-peritoneal (VP) shunt, which failed due to extensive intra-abdominal adhesions. A ventriculo-atrial (VA) shunt was inserted on numerous occasions with no long-term success. Therefore, the right atrium was not a viable option. Furthermore, an ETV was carried out which failed. In total, the patient underwent 14 operations for the treatment of his hydrocephalus prior to the insertion of a VC shunt (Fig. 1) at 2 years of age. Before this procedure, his EVD output was approximately 8 ml/h. A VC shunt was inserted, and at 22-month follow-up, there have been no further shunt revisions or gall bladder–related complications.
Case 2
A 2-year-old male presented with hydrocephalus secondary to a posterior fossa ependymoma. Following treatment for his tumour, he required a VP shunt for longer-term management of his hydrocephalus. He underwent multiple distal shunt revisions and it was concluded after repeated failure that the peritoneum was non-absorptive. An ETV was performed; however, this failed. The right atrium was unsuitable due to an infected portacath tubing in situ, with S. aureus–positive blood cultures. Long-term central access was required to receive regular chemotherapy and thought to be not ideal to have two separate tubes in the right atrium. Furthermore, there were concerns that having a colonised central line in situ and treatment with chemotherapy would put the patient at high risk of VA shunt infection, so a VC shunt was inserted. Pre-operatively, his external ventricular drain (EVD) output was approximately 10 ml/h. At 12-month follow-up, there have been no further distal shunt revisions and no gall bladder–related complications.
Case 3
An 8-year-old male with congenital hydrocephalus who had a VP shunt in situ since infancy presented with a distended abdomen and with clinical signs of shunt dysfunction. His peritoneum was found to be overfilled with CSF due to non-absorption. Neuroendoscopy was performed and his third ventriculostomy from previous surgery was found to be patent. A VA shunt was inserted; however, this was complicated by a yeast infection. Due to concerns of fungal infective endocarditis, it was decided that a VC shunt would be inserted semi-electively after a period of antifungal treatment. His pre-operative hourly EVD output was excessively high, 30–35 ml/h. At day 4 post-VC shunt insertion, he deteriorated with signs of shunt dysfunction. Intra operatively, his gall bladder was noted to be tense and distended so the VC shunt was removed. After two further surgeries, he was eventually discharged with a functioning VA shunt.
Discussion
We have described three cases where the insertion of a VC shunt has been placed. In two cases, this has been successful in the management of complex paediatric hydrocephalus where traditional forms of CSF diversion and ETV have failed. A further case is described where a VC shunt was placed but failed due to the high volume of CSF overwhelming the drainage capacity of the gall bladder.
Operative technique for distal insertion
The VC shunt is a planned joint case between the neurosurgery and paediatric surgery teams. A right subcostal incision is made to approach the fundus of the gall bladder. The distal end of the shunt catheter is also identified. A purse string suture is applied to the gallbladder fundus and a small cholecystostomy is made. A 5-cm atrial catheter closed at the tip with a distal slit valve is inserted into the gallbladder. A straight connector is used to join this catheter to the distal shunt catheter. Purse sutures are used to secure the straight connector to the gall bladder wall. This is a similar technique to that described by Bassi et al. [9].
CSF volume versus gallbladder capacity
Pre-operatively, the volume of CSF collected per hour via the EVD should be evaluated. This may help identify patients who would not be suitable candidates for a VC shunt. Woodfield et al. described a case of a 1-year-old girl who had a failed VC shunt, where a CT abdomen showed a distended gall bladder and subsequent insertion of an EVD demonstrated a CSF production of 40 to 50 ml/h [18]. Similarly, in case 3, the pre-operatively EVD output was 30 to 35 ml/h of CSF. At the time of the revision intraoperatively, his gallbladder was found to be tense and distended prior to removal of the distal shunt catheter. On the other hand, cases 1 and 2 had pre-operative EVD outputs of 8 and 10 ml/h, respectively, and they did not experience distal shunt dysfunction. CSF production is known to increase with age and weight [19, 20] and the mean output of CSF via EVD in infants and children is approximately 6.3–8.1 ml/h [19, 20]. However, it is not known exactly how much additional volume the gall bladder can be tolerated via CSF diversion. Assessing a patient’s gall bladder absorptive capacity and drainage via the bile duct may be difficult when considering a patient for a VC shunt. Nevertheless, gall bladder volume and common bile duct width are known to increase with the age [21,22,23]. Yoo et al. showed that for children aged 2 and 8 years old, the median gall bladder volumes were 5.6 cm3 and 7.7 cm3 respectively [23]. In patient’s age less than 1 year old, the gall bladder was shown to be particularly small with a median volume of 1.4 cm3 [23]. Furthermore, Zhang et al. demonstrated that the mean common bile duct diameters for children aged two to four and eight to ten were 2.1 mm and 3.9 mm respectively [22]. These studies suggest that younger patients may be less likely to tolerate a VC shunt. However, the volume of CSF output via the EVD may potentially be a bigger predictor of VC shunt failure. In our experience, cases 1 and 2 were both aged 2 years old, and the VC shunt has been successful, whereas case 3 was aged eight and the VC shunt had to be removed due to distal shunt dysfunction. Therefore, caution should be taken in patients who produce significantly high volumes via the external ventricular drain. Although not common practice in the UK, there are neuro-endoscopic procedures that could reduce the volume of CSF produced by the choroid plexus [24,25,26,27], which may improve the success of VC shunt function. Medical treatment using drugs such as Acetazolamide can also help reduce CSF production [28]. Nevertheless, our hypothesis is based on a small case series and there is scanty evidence with regard to the gall bladders’ capacity to accommodate CSF. Therefore, we cannot conclude whether or not a high EVD output would be a contraindication for a VC shunt in children.
Gall bladder–related complications
VC shunts may lead to an increased risk of gall stones at long-term follow-up [29]. Aldana et al. report a case of gall stones causing shunt dysfunction 5 years after the insertion of a VC shunt [12]; this was managed via conversion to a VP shunt. Furthermore, Surfield et al. described a similar case of gall stones 11 years after insertion of a VC shunt; the shunt was revised, and at 6 months, there were no further signs or symptoms of gall stone–related shunt dysfunction [30]. Gall bladder atony has been reported, which can be successfully treated medically with Cholecystokinin [13].
Conclusion
Our study demonstrates that ventriculo-cholecystic shunts can safely be used in paediatric patients with hydrocephalus. However, caution may be advisable in patients who are known to produce large volumes of CSF.
References
Ratliff M, Unterberg A, Bachli H (2016) Ventriculo-bipleural shunt as last resort in a 4-year-old child in whom a VP and VA shunt failed. J Neurosurg Pediatr 17:285–288
Irani F, Elkambergy H, Okoli K, Abou DS (2009) Recurrent symptomatic pleural effusion due to a ventriculopleural shunt. Respir Care 54:1112–1114
Hanak BW, Bonow RH, Harris CA, Browd SR (2017) Cerebrospinal fluid shunting complications in children. Pediatr Neurosurg 52:381–400
Jones RF, Currie BG, Kwok BC (1988) Ventriculopleural shunts for hydrocephalus: a useful alternative. Neurosurgery 23:753–755
Melamed EFCE, Krieger MD, Berry C, Yashar P, McComb JG (2016) Age as a novel risk factor for revision of ventriculo-pleural shunt in pediatric patients. Neurosurgery 63(Supplement 1):178–179
Pillai A, Mathew G, Nachimuthu S, Kalavampara SV (2017) Ventriculo-ureteral shunt insertion using percutaneous nephrostomy: a novel minimally invasive option in a patient with chronic hydrocephalus complicated by multiple distal ventriculoperitoneal shunt failures. J Neurosurg: 1-5
Toma AK, Tarnaris A, Kitchen ND, Watkins LD (2010) Ventriculosinus shunt. Neurosurg Rev 33:147–152 discussion 153
Ming Woo PY, Hung Pang PK, Chan KY, Ching Kwok JK (2015) Ventriculosternal shunting for the management of hydrocephalus: case report of a novel technique. Neurosurgery 11(Suppl 3):371–375 discussion 375
Demetriades AK, Haq IZ, Jarosz J, McCormick D, Bassi S (2013) The ventriculocholecystic shunt: two case reports and a review of the literature. Br J Neurosurg 27:505–508
Girotti ME, Singh RR, Rodgers BM (2009) The ventriculo-gallbladder shunt in the treatment of refractory hydrocephalus: a review of the current literature. Am Surg 75:734–737
Rivero-Garvia M, Pancucci G, Morcillo J, Millan A, Marquez-Rivas J (2015) Ventriculobiliary shunts, another option. Pediatr Neurosurg 50:152–156
Aldana PR, James HE, Postlethwait RA (2008) Ventriculogallbladder shunts in pediatric patients. J Neurosurg Pediatr 1:284–287
West KW, Turner MK, Vane DW, Boaz J, Kalsbeck J, Grosfeld JL (1987) Ventricular gallbladder shunts: an alternative procedure in hydrocephalus. J Pediatr Surg 22:609–612
Stringel G, Turner M, Crase T (1993) Ventriculo-gallbladder shunts in children. Childs Nerv Syst 9:331–333
Olavarria G, Reitman AJ, Goldman S, Tomita T (2005) Post-shunt ascites in infants with optic chiasmal hypothalamic astrocytoma: role of ventricular gallbladder shunt. Childs Nerv Syst 21:382–384
Ketoff JA, Klein RL, Maukkassa KF (1997) Ventricular cholecystic shunts in children. J Pediatr Surg 32:181–183
Pal K, Jindal V (2007) Ventriculo cholecystic shunt in the management of hydrocephalus. Indian Pediatr 44:435–437
Woodfield J, Magdum S (2013) Failure of peritoneal and gallbladder shunts in a child with craniopharyngioma. J Pediatr Neurosci 8:221–223
Yasuda T, Tomita T, McLone DG, Donovan M (2002) Measurement of cerebrospinal fluid output through external ventricular drainage in one hundred infants and children: correlation with cerebrospinal fluid production. Pediatr Neurosurg 36:22–28
Drake JM, Sainte-Rose C, DaSilva M, Hirsch JF (1991) Cerebrospinal fluid flow dynamics in children with external ventricular drains. Neurosurgery 28:242–250
Hernanz-Schulman M, Ambrosino MM, Freeman PC, Quinn CB (1995) Common bile duct in children: sonographic dimensions. Radiology 195:193–195
Zhang Y, Wang XL, Li SX, Bai YZ, Ren WD, Xie LM, Zhang SC (2013) Ultrasonographic dimensions of the common bile duct in Chinese children: results of 343 cases. J Pediatr Surg 48:1892–1896
Yoo JH, Kwak HJ, Lee MJ, Suh JS, Rhee CS (2003) Sonographic measurements of normal gallbladder sizes in children. J Clin Ultrasound 31:80–84
Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E, Mugamba J, Ssenyonga P, Donnelly R, Levenbach J, Monga V, Peterson M, MacDonald M, Cherukuri V, Warf BC (2017) Endoscopic treatment versus shunting for infant hydrocephalus in Uganda. N Engl J Med 377:2456–2464
Zandian A, Haffner M, Johnson J, Rozzelle CJ, Tubbs RS, Loukas M (2014) Endoscopic third ventriculostomy with/without choroid plexus cauterization for hydrocephalus due to hemorrhage, infection, Dandy-Walker malformation, and neural tube defect: a meta-analysis. Childs Nerv Syst 30:571–578
Weil AG, Fallah A, Chamiraju P, Ragheb J, Bhatia S (2015) Endoscopic third ventriculostomy and choroid plexus cauterization with a rigid neuroendoscope in infants with hydrocephalus. J Neurosurg Pediatr:1–11
Fallah A, Weil AG, Juraschka K, Ibrahim GM, Wang AC, Crevier L, Tseng CH, Kulkarni AV, Ragheb J, Bhatia S (2017) The importance of extent of choroid plexus cauterization in addition to endoscopic third ventriculostomy for infantile hydrocephalus: a retrospective North American observational study using propensity score-adjusted analysis. J Neurosurg Pediatr 20:503–510
Carrion E, Hertzog JH, Medlock MD, Hauser GJ, Dalton HJ (2001) Use of acetazolamide to decrease cerebrospinal fluid production in chronically ventilated patients with ventriculopleural shunts. Arch Dis Child 84:68–71
Fountas KN, Kassam MA, Grigorian AA (2007) A rare, delayed complication of a ventriculogallbladder shunt. Case report and review of the literature. Neurosurg Focus 22: E12
Surfield GA, Klein RL (2006) Case report of symptomatic cholelithiasis after ventricular cholecystic shunt. J Pediatr Surg 41:1933–1934
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Henderson, D., Budu, A., Horridge, M. et al. The ventriculo-cholecystic shunt: does CSF volume matter?. Childs Nerv Syst 35, 1557–1560 (2019). https://doi.org/10.1007/s00381-019-04317-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04317-7