Abstract
Introduction
Ventriculo-gallbladder shunt (VGS) has been recognized as a last-resort alternative to treat hydrocephalus when the peritoneum and/or other distal sites can no longer receive shunts. In some specific conditions, it may be conceded as a first-line treatment.
Case presentation
We report the case of a 6-month-old girl with progressive post-hemorrhagic hydrocephalus who presented a concomitant chronic abdominal symptom. Specific investigations ruled out acute infection and led to the diagnosis of chronic appendicitis. Both problems were managed in a one-stage salvage procedure consisting of laparotomy sanctioning to treat the abdominal pathology and seize the opportunity to perform a VGS as a first option since the abdomen is prone to ventriculoperitoneal shunt (VPS) failure.
Conclusion
Only few cases have reported the use of VGS as the first option to handle uncommon complex cases due to abdominal or cerebrospinal fluid (CSF) conditions. We wish to draw attention to VGS as an effective procedure not only in children with multiple shunt failures but also as first-line management in some selected cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The peritoneum remains the first receptacle choice for CSF diversion. However, in some instances, the VPS is rendered infeasible due to local complications. In these cases, the VGS, which is usually used as a last resort, becomes a viable option. Herein, we performed a VGS, on a 6-month-old girl, for progressive hydrocephalus secondary to neonatal intraventricular hemorrhage (IVH) as a first therapeutic option. Her peritoneum was found to be prone to VPS failure.
Case presentation
We present the case of a 6-month-old girl with a history of preterm birth and neonatal intensive care unit (NICU) stay for respiratory distress, grade III IVH and necrotizing enterocolitis (NEC) handled conservatively. Furthermore, both her jugular veins were compromised by the implementation of Broviac lines. At follow-up, we noticed a progressive increase in head circumference. Computed tomography (CT) scan (Fig. 1) demonstrated significant communicating hydrocephalus indicating CSF diversion.
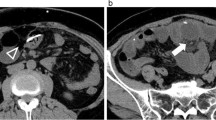
On admission, the patient was afebrile, alert with good activity. Physical examination findings were normal besides macro crania and wide tense anterior fontanel. However, her mother consistently mentioned episodic abdominal pain and mild fever. Complete blood count, C-reactive protein, and procalcitonin were normal. Stool test and urinalysis did not identify any potential pathogens. Abdominal CT scan and ultrasound (Fig. 2) unveiled thickening of the appendicular wall and mesenteric infiltration. Although rare, chronic appendicitis was the most likely diagnosis.
Considering that peritoneum might be hostile to VPS and her neonatal complications, we had few options for CSF. Since we had to open the abdomen for surgical exploration, the gallbladder seemed a simple accessible option for distal end placement. Surgery was planned with a pediatric surgeon. A coelioscopic first look showed extensive intraperitoneal adhesions; therefore, we converted to a median supra-umbilical laparotomy. After adhesiolysis and mobilizing the caecum, the appendix appeared to be inflammatory with a cluster of mesenteric lymph nodes. An appendectomy was then performed. The pediatric surgeon presented the dome of the gallbladder into surgical view. The distal catheter was then embedded into the fundus throw a 5-mm incision and fixed with a purse-string suture around it. A 30-cm-loop of the distal catheter was left free in the abdominal cavity to fit with future growth. The infant was discharged home in stable condition 7 days after surgery. Histological findings confirmed the diagnosis of chronic appendicitis.
The current 60 months post-operatively follow-up finds normal neurological development and complete relief of abdominal symptoms. Moreover, control X-ray and sonographies (Fig. 3) were obtained, and no migration of the catheter’s tip has been noted.
Discussion
Management of hydrocephalus, when the peritoneum is possibly hostile to CSF shunting, can be challenging. The gallbladder proved to be an effective and safe alternative and has been increasingly popular since first described by Smith et al. (1958) [1].
In previous studies, VGS has always been considered for cases in which VPS or ventriculo arterial shunt (VAS) are no longer feasible as a second or third-line salvage treatment [2,3,4,5,6].
To our knowledge, there are only five pediatric cases reporting VGS as a de novo procedure (Table 1). West et al. [6] described a patient with short bowel syndrome related to NEC, where VAS and VPS were not possible. Pal and Jindal [4] reported a similar case to ours with good clinical outcomes and revision-free until 3 years postoperatively. Aldana et al. [2] reported a case of an infant with no previous shunt but with multiple prior abdominal surgeries. Pancucci et al. [5] recorded a case of a 4-month infant presented with obstructive hydrocephalus caused by optic-chiasmal hypothalamic glioma. They stated that a high protein CSF rate predicts VPS failure since it may be responsible for ascites and non-resorptive peritoneum. Our child developed a progressive hydrocephalus and abdominal signs related to chronic appendicitis. Considering peritoneum hostility, poor venous access, and history of neonatal respiratory distress, we opted for VGS as a first-line and definitive treatment.
We suggest classifying the predictor factors of VPS failure into two groups: CSF-related disorders and abdominal-related conditions. Indeed, CSF with a high protein concentration, specifically caused by optic-chiasmal hypothalamic glioma [5, 7,8,9], craniopharyngioma [10], plasminogene deficiency [3], and tuberculosis [5, 11], may be subject for proteinaceous ascites and therefore VPS failure. In these cases, the gallbladder is an efficient receptacle: CSF will be excreted and then absorbed in the intestinal tract. Nevertheless, the lytic action of bile may catabolize fibrinous adhesion around the distal shunt tip CSF [1, 5, 8, 11].
Abdominal-related conditions include multiple prior abdominal surgeries, peritoneal adhesions, ascites, pseudo-occlusion, and peritonitis [5, 12, 13]. Commonly, in these cases, surgeons use the gallbladder to drain CSF as last resort, electing the atrium or the pleural cavity for the second viable receptacle. This disinclination to VGS is not counselled by standard guidelines, but mainly as a consequence of potential complications (Table 2) and technical concerns [9, 11,12,13,14,15,16,17,18,19,20,21, 24,25,−26]. From our perspective, the VGS placement technique, as detailed by Morosanu et al. [19], was a little different compared to VPS. It could be planned with pediatric surgery teams. Furthermore, laparoscopic access makes this approach even more valuable [5, 10]. Neonatal history of NEC may be recognized as another abdominal-related cause predicting VPS failure by leading to an inflammatory peritoneal disease and a higher incidence of distal shunt obstructions [22].
Reisner et al. [23] suggest that prior to deciding on VGB, an extensive evaluation should be conducted including a liver function test, ultrasound imaging of the gallbladder, or preferably a CT scan of the abdomen. Bile culture and CSF volume assessment is not mandatory. When a CSF external drainage is previously placed, a surgeon should be aware that a high CSF flow may overwhelm the gallbladder [17]. Up to date, our child remained complication-free. If a revision is ever needed, a surgeon must be aware that retrieval of the distal end should be under visual control in order to prevent bile leakage.
Conclusion
VGS placement requires a conventional preoperative assessment and rigorous surgical technique. There have been five documented pediatric cases that have undergone VGS as de novo shunt system. Our case adds to the existing literature a detailed illustration and long outcome data. If future reports support the current results, surgical teams may legitimately resort to VGS earlier in selected cases.
Availability of data and material
Not applicable.
References
Smith GW, Moretz WH, Pritchard WL (1958) Ventriculo-biliary shunt; a new treatment for hydrocephalus. Surg Forum 9:701–705
Aldana PR, James HE, Postlethwait RA (2008) Ventriculogallbladder shunts in pediatric patients. J Neurosurg Pediatr 1:284–287. https://doi.org/10.3171/PED/2008/1/5/284
Ketoff JA, Klein RL, Maukkassa KF (1997) Ventricular cholecystic shunts in children. J Pediatr Surg 32:181–183. https://doi.org/10.1016/s0022-3468(97)90175-5
Pal K, Jindal V (2007) Ventriculo cholecystic shunt in the management of hydrocephalus. Indian Pediatr 44:435–437
Pancucci G, Plaza-Ramirez E, Driller C, Miranda-Lloret P, Botella-Asunción C (2019) Laparoscopy-assisted placement of a ventriculobiliary shunt: a technical note. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 35:1397–1400. https://doi.org/10.1007/s00381-019-04173-5
West KW, Turner MK, Vane DW, Boaz J, Kalsbeck J, Grosfeld JL (1987) Ventricular gallbladder shunts: an alternative procedure in hydrocephalus. J Pediatr Surg 22:609–612. https://doi.org/10.1016/s0022-3468(87)80110-0
Gil Z, Beni-Adani L, Siomin V, Nagar H, Dvir R, Constantini S (2001) Ascites following ventriculoperitoneal shunting in children with chiasmatic-hypothalamic glioma. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 17:395–398. https://doi.org/10.1007/s003810100460
Olavarria G, Reitman AJ, Goldman S, Tomita T (2005) Post-shunt ascites in infants with optic chiasmal hypothalamic astrocytoma: role of ventricular gallbladder shunt. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 21:382–384. https://doi.org/10.1007/s00381-004-0996-1
West GA, Berger MS, Geyer JR (1994) Childhood optic pathway tumors associated with ascites following ventriculoperitoneal shunt placement. Pediatr Neurosurg 21:254–258; discussion 259. https://doi.org/10.1159/000120846
Ignacio RC, Schermerhorn SMV, Marrotte AJ, Prieto JM (2019) Laparoscopic ventricular-cholecystic shunt. J Pediatr Surg Case Rep 47:101233. https://doi.org/10.1016/j.epsc.2019.101233
Frim DM, Lathrop D, Chwals WJ (2001) Intraventricular pressure dynamics in ventriculocholecystic shunting: a telemetric study. Pediatr Neurosurg 34:73–76. https://doi.org/10.1159/000055998
Lyngdoh BT, Islam MS (2012) Ventriculocholecysto shunt: a solution to recurrent shunt complications in comorbid post-tubercular hydrocephalus with tubercular adhesive peritonitis. Acta Neurochir (Wien) 154:2267–2270. https://doi.org/10.1007/s00701-012-1506-y
Rivero-Garvía M, Pancucci G, Morcillo J, Millán A, Márquez-Rivas J (2015) Ventriculobiliary shunts, another option. Pediatr Neurosurg 50:152–156. https://doi.org/10.1159/000381030
Alraee S, Alshowmer S, Alnamshan M, Azzubi M (2020) Management of ventriculo-gallbladder shunt in the presence of gallstones. BMJ Case Rep 13:e234775. https://doi.org/10.1136/bcr-2020-234775
Barami K, Sood S, Ham S, Canady A (1998) Chemical meningitis from bile reflux in a lumbar-gallbladder shunt. Pediatr Neurosurg 29:328–330. https://doi.org/10.1159/000028748
Fountas KN, Kassam MA, Grigorian AA (2007) A rare, delayed complication of a ventriculogallbladder shunt. Case report and review of the literature. Neurosurg Focus 22:E12. https://doi.org/10.3171/foc.2007.22.4.14
Henderson D, Budu A, Horridge M, Jesurasa A, Sinha S, Ushewokunze S, Fisher R (2019) The ventriculo-cholecystic shunt: does CSF volume matter? Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 35:1557–1560. https://doi.org/10.1007/s00381-019-04317-7
Kulwin CG, Margaron FC, Leys CM, Boaz JC, Fulkerson DH (2014) Ventriculogallbladder shunt fracture: bile peritonitis. J Neurosurg Pediatr 13:94. https://doi.org/10.3171/2013.10.PEDS13289
Morosanu CO, Priscu A, Florian IS (2021) Evaluation of the ventriculocholecystic shunt-an overview of present practice in adult and pediatric hydrocephalus. Neurosurg Rev 44:2533–2543. https://doi.org/10.1007/s10143-021-01472-x
Scaife M, Abegglen R, Vila C, Stahlfeld K (2018) Abnormal presentation of ascending cholangitis. Clin Case Rep 6:1172–1173. https://doi.org/10.1002/ccr3.1357
Surfield GA, Klein RL (2006) Case report of symptomatic cholelithiasis after ventricular cholecystic shunt. J Pediatr Surg 41:1933–1934. https://doi.org/10.1016/j.jpedsurg.2006.07.017
Pierro A, Manalang LR, May PL, Cooke RW, Cudmore RE, Lloyd DA (1993) Necrotizing enterocolitis complicating the management of posthemorrhagic hydrocephalus. J Pediatr Surg 28:982–985. https://doi.org/10.1016/0022-3468(93)90497-9
Reisner A, Smith AD, Wrubel DM, Buster BE, Sawvel MS, Blackwell LS, Laxpati NG, Brahma B, Chern JJ (2021) Utility of ventriculogallbladder shunts in complex cases of hydrocephalus related to extreme prematurity. J Neurosurg Pediatr 27:511–517. https://doi.org/10.3171/2020.9.PEDS20522
Adegbite AB, Khan M (1982) Role of protein content in CSF ascites following ventriculoperitoneal shunting. Case report J Neurosurg 57:423–425. https://doi.org/10.3171/jns.1982.57.3.0423
Demetriades AK, Haq IZ, Jarosz J, McCormick D, Bassi S (2013) The ventriculocholecystic shunt: two case reports and a review of the literature. Br J Neurosurg 27:505–508. https://doi.org/10.3109/02688697.2013.771135
Parikh P, Carratola MC, Malik T (2013) Removal of a retained fragment of a ventriculo-gallbladder shunt in the common bile duct. Eur J Surg Sci 4(3):126. https://corescholar.libraries.wright.edu/surg/169
Author information
Authors and Affiliations
Contributions
MH: conceptualization. ZS: writing—original draft and editing. MB: review and editing. SM: review of literature. AB: methodology. MD: supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from a relative for the publication of this case report and accompanying images.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hadhri, M.M., Souei, Z., Boukhit, M. et al. Can we consider ventriculo-gallbladder shunt a first-line treatment in selected patients? Case report of a successful management. Childs Nerv Syst 39, 1963–1968 (2023). https://doi.org/10.1007/s00381-023-05923-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-05923-2