Abstract
Purpose
Intraoperative ultrasound (iUS) is a valuable tool—inexpensive, adds minimal surgical time, and involves minimal risk. The diagnostic predictive value of iUS is not fully characterized in Pediatric Neurosurgery. Our objective is to determine if surgeon-completed iUS has good concordance with post-operative MRI in estimating extent of surgical resection (EOR) of pediatric brain tumors.
Methods
We reviewed charts of all pediatric brain tumor resections (single institution 2006–2013). Those with iUS and postoperative imaging (<1 week) were included. The surgeon’s estimation of the EOR based on iUS and the post-operative neuroimaging results (gold standard) were collected, as well as information about the patients/tumors.
Results
Two hundred two resections were reviewed and 58 cases were included. Twenty-six of the excluded cases utilized iUS but did not have EOR indicated. The concordance of interpretation between iUS and post-operative MRI was 98.3 %. Of 43 cases where iUS suggested gross total resection, 42 were confirmed on MRI (negative predictive value (NPV), 98 %). All 15 cases where iUS suggested subtotal resection were confirmed on MRI (positive predictive value (PPV), 100 %). Agreement between iUS and post-operative imaging had an overall Kappa score of 0.956, signifying almost perfect agreement.
Conclusion
The results from this study suggest that iUS is reliable with both residual tumor (PPV—100 %) and when it suggests no residual (NPV—98 %) in tumors that are easily identifiable on iUS. However, tumors that were difficult to visualize on iUS were potentially excluded, and therefore, these results should not be extrapolated for all brain tumor types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraoperative imaging is a valuable adjunct to intra-cranial neurosurgery, for the purposes of locating tumors and estimating the extent of surgical resection (EOR) [2, 5, 7, 10, 17]. In pediatric neurosurgery, therapeutic surgical goals often include complete tumor resection. Intraoperative ultrasound (iUS) and intraoperative magnetic resonance imaging (iMRI) are both used for the purpose of estimating tumor location and EOR in pediatric patients. iMRI is considered by many to have superior, gold-standard image quality and is therefore a preferred diagnostic modality by some groups [5]. However, iMRI is much more expensive and requires specialized workspaces and personnel in comparison to iUS [2, 7, 10, 17, 18]. Additionally, iMRI does not operate in real-time, thus preventing monitoring of change and manipulation of tissues inside the surgical field [12, 13]. As a result, surgeons must pause the surgery every time they wish to obtain images using iMRI, consequentially adding to the overall length of procedures [5, 17, 18]. As well, although rare, iMRI does have potential risks such as endotracheal tube dislodgement, electromagnetically induced burns, and operative site infection [8].
In contrast, iUS provides surgeons with real-time imaging, which allows images to be obtained quickly and potentially repeatedly during the same tumor resection [5, 10, 12, 13, 16, 17].Moreover, the real-time imaging capability of iUS allows surgeons to manipulate and interact with the surgical environment. These abilities, coupled with the relatively low implementation and maintenance costs of iUS, provides institutions with a cost-effective and efficient intraoperative neuronavigation alternative, which requires less total time in the operating room [5, 10, 17, 18].Unfortunately, a potentially significant downside of iUS is the lower image quality it provides surgeons compared to iMRI [5, 7, 13, 18]. Lower image quality can ultimately lead surgeons to misinterpret scans and tumor margins, potentially resulting in leaving residual tumor behind [16].
The diagnostic predictive value of iUS for estimating the extent of surgical resection in pediatric brain tumors has yet to be fully characterized [2, 5, 10, 16]. The objective of this study is to determine if surgeon-completed iUS has good concordance with post-operative imaging in estimating the EOR of pediatric brain tumors. Despite iUS’s lower image quality compared to iMRI, we hypothesized that surgeon completed iUS has good concordance with immediate post-operative magnetic resonance imaging (MRI) in estimating EOR in pediatric brain tumor resections.
Methods
After obtaining institutional ethics approval, we retrospectively reviewed the charts of all pediatric brain tumor resections performed at our institution between 2006 and 2013. Only patients who received both iUS and immediate post-operative MRI or computed tomography (CT) (< 1 week post-op) were included in the study. In any cases where the immediate post-operative imaging was ambiguous (with regards to defining if there was residual tumor), the subsequent MRI (between 1 and 7 months post-surgery) was also reviewed to fully define operative extent of resection.
Patients for whom there was no indication of EOR in their operative report were not included in the study. This allowed the comparison of EOR reported intraoperatively, determined by the surgeon and iUS, with the findings of post-operative diagnostic neuroradiology examinations. Variables collected included the location, size, and histological type of the tumor resected along with patient demographic information. The surgeon’s estimation of the EOR (based on iUS) and the post-operative imaging estimation of EOR was collected.
The patients in this study underwent iUS using an Aloka Prosound Alpha 9, generally using a convex (UST-9120–3.75–10 MHz) probe. At our institution, the iUS is completed and interpreted by the attending surgeon, and images are not retained or subsequently reviewed. Although there is a learning curve for the use of iUS [7, 13], the neurosurgical team at our institution is well accustomed to this technique in brain tumors and other intraoperative indications.
Results
Overall, 58 cases out of a total of 201 pediatric brain tumor resections fit our inclusion criteria and were reviewed. Of the 143 tumor resections that were not included in our study, 118 were excluded because there was no mention of iUS in their surgical records. The remaining 25 cases had iUS used during the procedure, however did not have adequate information to be included in the study, primarily lack of documentation of iUS-determined EOR. Reasons why cases were excluded are as follows: (1) iUS was used to positively identify the lesion before resection and not used again to define EOR (13/25); (2) iUS was used to identify the tumor pre-resection; however, the scan was stated to be of poor quality and was not utilized again (3/25); (3) iUS was used at some point during the procedure; however, there was inadequate information in the surgical note describing the capacity which the iUS was utilized (4/25); (4) iUS was used to estimate EOR, but immediate postoperative neuroimaging was not performed (3/25); and (5) the EOR was reportedly difficult to determine by iUS and was not reported (2/25). Pathology diagnosis and tumor location of the excluded cases is summarized in Tables 1 and 2, respectively. Fischer’s exact test found no differences between counts of cases included vs. excluded, in terms of pathology diagnosis. However, there were a greater number of tumor location intraventricular tumors (third and lateral ventricles) excluded, in comparison to the included cases (p = 0.01). There were no other discernable differences between the pathologies and locations of tumors included and excluded in the study.
The mean age of the patients included in the study population was 7.8 years old, with the age of children ranging between 3 months and 19 years. The study population consisted of 34 males (59 %) and 24 females (41 %). There were 34 (59 %) infratentorial tumors and 24 (41 %) supratentorial tumors. Tumor pathology is summarized in Table 1. A total of four neurosurgeons performed the surgeries and completed the iUS studies included in this study.
The concordance of interpretation between iUS and post-operative imaging was 98.2 % (57 of 58 cases). Of the 43 cases where iUS suggested a gross total resection (GTR), 42 cases were later confirmed to be GTR on postoperative neuroimaging (Table 2). This constitutes iUS having a negative predictive value (the patient does not have disease when the test signals no disease—NPV) of 97.7 %. In the 15 cases that iUS suggested a subtotal resection, all 15 cases were confirmed on postoperative neuroimaging to be subtotal resection (Table 3). Thus, the positive predictive value (the patient has residual disease when the test signals disease—PPV) for iUS was 100 %.
A Kappa statistic was used to assess the agreement between iUS and post-operative neuroimaging. Agreement between the two imaging modalities had an overall Kappa score of 0.956, which equates to “almost perfect agreement” strength between the neuroimaging techniques [19].
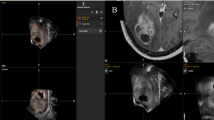
The one non-concordant case received immediate surgery to remove the residual tumor. This tumor was a calcified extraventricular neurocytoma (WHO Grade II) and was located in the left frontal lobe. Representative post-operative imaging for this case is presented in Fig. 1.
Discussion
iUS is a valuable tool in pediatric brain tumor resection procedures that allows surgeons to quickly identify residual tumor in a changing surgical environment, but the predictive ability of iUS and inter-test agreement with postoperative imaging has yet to be fully determined [2, 5, 10, 16]. This study aimed to determine the congruence between iUS and immediate postoperative imaging.
Many peer-reviewed articles in both adult and pediatric literature herald iMRI as a superior intraoperative neuroimaging modality [3, 5, 6, 11, 14, 15]. With very high postoperative imaging concordance rates (91–96 %), iMRI undoubtedly provides neurosurgeons with accurate intraoperative imaging results [3, 14]. However, a major downside of iMRI systems is the implementation and maintenance costs [7, 11]. Not only are iMRI systems expensive to purchase, they also require a specialized work environment, which can cost millions of dollars to build and maintain [11]. As a result, institutions (both in developed and developing nations) can find it difficult to afford this imaging modality, in terms of both direct costs as well as space and time [7]. In comparison, iUS has a relatively low implementation cost and does not require significant maintenance or a specialized work environment, with a concordance rate (98.3 %) that might approach those of published iMRI concordance rates (91–96 %), in terms of estimating GTR.
Another drawback of iMRI is that its utilization increases the time required for each surgical procedure and preoperative set-up [1, 3–5, 8, 15, 17, 18]. An article published on iMRI in a pediatric population noted that procedures using iMRI went, on average, over 1.5 h longer than surgeries without iMRI [15]. The set-up of the iUS in our operating room is done by our intraoperative nursing staff and so does not cause any surgical delays. In a prospective timing of five cases (and two different surgeons), iUS required less than 2 min per scan.
Despite our results suggesting that overall concordance between post-operative imaging and iUS is strong (98.3 %) with Kappa of 0.956 (“almost perfect agreement”), some literature indicates that tumor characteristics can complicate iUS interpretation [2, 9]. Mair et al. proposed a grading system for lesions on iUS, based upon how readily lesions are identified and the extent to which lesions are distinct from the surrounding brain [9].This grading system further refines which lesions iUS is most effective in distinguishing. Unfortunately, the one non-concordant case in our series was not represented in this grading scheme (extraventricular neurocytoma WHO grade II) [9]. Perhaps, future research could focus on further refinement of the iUS lesion grading scale, specifically in the pediatric population, to better outline which tumor types are best visualized on iUS.
The issue of tumor interpretability brought up by Mair et al. also highlights one of this study’s most prominent limitations. In at least five cases (and up to nine cases) that were excluded from this study, iUS image quality was poor and difficult to interpret. As a result, iUS was either not utilized again after initial identification or EOR was not estimated. These cases were excluded due to the lack of estimated EOR in the surgical note, but potentially could have demonstrated instances where iUS is ineffective at determining EOR. There were no notable differences in tumor pathological diagnoses of the excluded cases in comparison to the included cases. There were, however, a greater number of intraventricular tumors (third and lateral ventricles) represented in the excluded cases, which potentially suggests either a surgeon preference to not ultrasound that location, or possibly that tumors in that area were difficult to visualize. Regardless, the conclusions of our study do not appear to generalize at all to tumors in the supratentorial cerebral ventricles. No significant differences were noted between included and excluded cases in other areas of the brain. Nevertheless, omitting these patients creates a potential selection bias in our results. A prospective study design, with formal surgeon reporting of ease of tumor identification and determination of EOR, would likely address this bias. It is probable that in such a prospective study, the predictive power of iUS would be different than what this study determined.
In addition, this study has several other limitations. First, it is a retrospective analysis on a moderate sample size of pediatric brain tumor patients. This raises questions about patients that were excluded. Twenty-five tumor resections, in which iUS was utilized, were excluded from the study. In around half of these cases (13), iUS was used to identify the lesion and was not used again in the procedure. In these cases, it was not considered prudent to repeat iUS to estimate EOR, because the surgeons were confident of their estimation without iUS assistance. Another limitation to this study is that the estimations of concordance between iUS and postoperative neuroimaging presented in this study are not for iUS by itself. Surgeons have knowledge of the surgical field that is challenging to measure in this type of study. Consequentially, surgeons are naturally aware of their own impression of EOR, prior to use of iUS, and this might bias the surgeon’s interpretation of the ultrasound study (however this limitation could also be said for iMRI and other intraoperative imaging modalities). Because of the retrospective study design and our practice at that time (no storage of iUS study images), there is no opportunity to have iUS images independently reviewed. Finally, the study was completed at a single institution, by surgeons familiar with the use of intra-operative ultrasound. It is possible or even likely that there is a training effect of using intra-operative ultrasound, and the predictive values for the observations regarding EOR might only be valid for “ultrasound experienced” surgeons.
Conclusion
The results from this study suggest that when iUS is able to identify a brain tumor, it works well at estimating EOR (PPV = 100 %; NPV = 97.7 %). If a surgeon deems that the interpretability of iUS is of sufficient quality, it is likely that the intraoperative assessment of EOR will be congruent with postoperative imaging. However, these results should be taken with caution; numerous resection cases where iUS was utilized but EOR could not be ascertained were excluded from this study. Because the resection cases that iUS had the highest likelihood of succeeding in predicting EOR were selected for, it is probable that these results only translate to the subset of tumors that are easily identifiable on iUS. Continued investigation into the strengths and limitations of iUS needs to be conducted in a prospective manner.
References
Barua E, Johnston J, Fujii J, Dzwonczyk R, Chiocca E, Bergese S (2009) Anesthesia for brain tumor resection using intraoperative magnetic resonance imaging (iMRI) with the polestar N-20 system: experience and challenges. J Clin Anesth 21:371–376
Chacko AG, Kumar NK, Chacko G, Athyal R, Rajshekhar V (2003) Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours–a comparative study with computed tomography and histopathology. Acta Neurochir 145:743–748 discussion 748
Coburger J, Konig R, Seitz K, Bazner U, Wirtz CR, Hlavac M (2014) Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg 120:346–356
Czyz M, Tabakow P, Weiser A, Lechowicz-Glogowska BE, Zub LW, Jarmundowicz W (2014) The safety and effectiveness of low field intraoperative MRI guidance in frameless stereotactic biopsies of brain tumours-design and interim analysis of a prospective randomized trial. Neurosurg Rev 37:127–137
El Beltagy MA, Aggag M, Kamal M (2010) Role of intraoperative ultrasound in resection of pediatric brain tumors. Childs Nerv Syst 26:1189–1193
Gerganov VM, Samii A, Giordano M, Samii M, Fahlbusch R (2011) Two-dimensional high-end ultrasound imaging compared to intraoperative MRI during resection of low-grade gliomas. J Clin Neurosci 18:669–673
Ivanov M, Wilkins S, Poeata I, Brodbelt A (2010) Intraoperative ultrasound in neurosurgery - a practical guide. Br J Neurosurg 24:510–517
Jankovski A, Francotte F, Vaz G, Fomekong E, Duprez T, Van Boven M, Docquier MA, Hermoye L, Cosnard G, Raftopoulos C (2008) Intraoperative magnetic resonance imaging at 3-T using a dual independent operating room-magnetic resonance imaging suite: development, feasibility, safety, and preliminary experience. Neurosurgery 63:412–424 discussion 424-416
Mair R, Heald J, Poeata I, Ivanov M (2013) A practical grading system of ultrasonographic visibility for intracerebral lesions. Acta Neurochir 155:2293–2298
Moiyadi A, Shetty P (2011) Objective assessment of utility of intraoperative ultrasound in resection of central nervous system tumors: a cost-effective tool for intraoperative navigation in neurosurgery. J Neurosci Rural Pract 2:4–11
Ramina R, Coelho Neto M, Giacomelli A, Barros Jr E, Vosgerau R, Nascimento A, Coelho G (2010) Optimizing costs of intraoperative magnetic resonance imaging. A series of 29 glioma cases. Acta Neurochir 152:27–33
Roth J, Beni-Adani L, Biyani N, Constantini S (2006) Classical and real-time neuronavigation in pediatric neurosurgery. Childs Nerv Syst 22:1065–1071
Roth J, Biyani N, Beni-Adani L, Constantini S (2007) Real-time neuronavigation with high-quality 3D ultrasound SonoWand in pediatric neurosurgery. Pediatr Neurosurg 43:185–191
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003
Shah MN, Leonard JR, Inder G, Gao F, Geske M, Haydon DH, Omodon ME, Evans J, Morales D, Dacey RG, Smyth MD, Chicoine MR, Limbrick DD (2012) Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr 9:259–264
Shinoura N, Takahashi M, Yamada R (2006) Delineation of brain tumor margins using intraoperative sononavigation: implications for tumor resection. J Clin Ultrasound 34:177–183
Ulrich NH, Burkhardt JK, Serra C, Bernays RL, Bozinov O (2012) Resection of pediatric intracerebral tumors with the aid of intraoperative real-time 3-D ultrasound. Childs Nerv Syst 28:101–109
Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA (2002) Neuronavigation by intraoperative three-dimensional ultrasound: initial experience during brain tumor resection. Neurosurgery 50:804–812 discussion 812
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Conflict of interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singhal, A., Ross Hengel, A., Steinbok, P. et al. Intraoperative ultrasound in pediatric brain tumors: does the surgeon get it right?. Childs Nerv Syst 31, 2353–2357 (2015). https://doi.org/10.1007/s00381-015-2805-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2805-4