Abstract
Purpose
Pediatric low-grade gliomas (pLGG) are the most common brain tumors in children and achieving complete resection (CR) in pLGG is the most important prognostic factor. There are multiple intraoperative tools to optimize the extent of resection (EOR). This article investigates and discusses the role of intraoperative ultrasound (iUS) and intraoperative magnetic resonance imaging (iMRI) in the surgical treatment of pLGG.
Methods
The tumor registries at Tuebingen, Rome and Pretoria were searched for pLGG with the use of iUS and data on EOR. The tumor registries at Liverpool and Tuebingen were searched for pLGG with the use of iMRI where preoperative CR was the surgical intent. Different iUS and iMRI machines were used in the 4 centers.
Results
We included 111 operations which used iUS and 182 operations using iMRI. Both modalities facilitated intended CR in hemispheric supra- and infratentorial location in almost all cases. In more deep-seated tumor location like supratentorial midline tumors, iMRI has advantages over iUS to visualize residual tumor. Functional limitations limiting CR arising from eloquent involved or neighboring brain tissue apply to both modalities in the same way. In the long-term follow-up, both iUS and iMRI show that achieving a complete resection on intraoperative imaging significantly lowers recurrence of disease (chi-square test, p < 0.01).
Conclusion
iUS and iMRI have specific pros and cons, but both have been proven to improve achieving CR in pLGG. Due to advances in image quality, cost- and time-efficiency, and efforts to improve the user interface, iUS has emerged as the most accessible surgical adjunct to date to aid and guide tumor resection. Since the EOR has the most important effect on long-term outcome and disease control of pLGG in most locations, we strongly recommend taking all possible efforts to use iUS in any surgery, independent of intended resection extent and iMRI if locally available.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brain cancers are the most common solid tumors in children and lead to more years of life lost than other more common cancers. Therefore, improving outcomes for brain cancer is still, despite significant advances in the last decades, an unmet clinical need [1]. Low-grade gliomas (LGG) are the most frequent pediatric brain tumors and comprise multiple entities. Surgery is the first line of treatment in the majority, and the extent of resection (EOR) is the most important modifiable prognostic factor, such that prognosis and long-term outcome in patients who have had gross total resection (GTR) is better than those with incomplete resection [2, 3]. It is important to emphasize that pediatric LGG (pLGG) are a different collection of tumors than adult LGG, with different underlying molecular genetic characteristics [2, 4, 5]. Compared to adults, pLGG have lower rates of conversion to malignant tumors and have a low incidence of glioma related death. Secondly, treatment with radiation therapy can lead to inferior long-term outcomes [2, 6]. The primary goal in pLGG surgery is complete resection (CR) while preserving brain function. The latter is of utmost importance since the overall survival rates in pLGG patients are high and therefore functional impairments as the consequence of treatment have a long-lasting impact on the quality of life of the affected children and adolescents [6].
There are multiple aids to aim for CR. The use of intraoperative neuronavigation is one of them, but this is considered a major advantage for location of the bone flap and trajectory planning rather than for completeness of resection of tumor. As the tumor is removed the brain also moves (“brain shift”) which makes the technology to define the tumor margins at the end of surgery in many instances highly inaccurate. The gold standard to assess for complete tumor removal is the early postoperative MRI (done 1–2 days after surgery). Most accurate to confirm this purpose is the 3 months postoperative MRI scan when all surgery induced tissue changes or tumor related non-tumorous changes like edema have completely disappeared. Patients with residual tumor might need a second operation to complete the resection in case of regrowth originating from this residual [7].
To render surgery as effective as possible regarding completeness of tumor removal, intraoperative ultrasound (iUS) is the most commonly available intraoperative tool delivering real-time images enabling the surgeon to localize the tumor, assess vascularity, and determine the tumor margins and degree of resection [8, 9]. The benefits of being relatively cost-effective, portable, radiation-free, and providing real-time feedback have made this tool a very attractive option. There are however limitations regarding technology, such as the quality of machine algorithms to produce images, quality of ultrasound transducer, distance of tumor to probe, artifacts introduced by surgery or resection cavity and experience of the surgeon in interpretation of images, which are influencing the results and abilities of iUS [10]. Developments and advances in iUS equipment and software continue to make this modality more appealing. Several retrospective studies have shown good concordance with the postoperative MRI but only in selected tumor types and locations [11,12,13].
The gold standard for intraoperative imaging is the use of intraoperative MRI (iMRI), where an MRI is done while surgery is paused, with the possibility to proceed with the operation if residual tumor is detected and the functionality/eloquence of the brain allows for further resection. However, iMRI is not available to most children treated for pLGG. It involves a high initial investment in machinery and there is a specific setup of operative environment, with prolonged operating time and a higher number of staff, to achieve the goal of improved resection. In one series, ~ 28% of all pediatric tumor resections with iMRI received immediate further resection. This delivered GTR in > 95% of patients but added 2–4 h to the operation time [14]. In a specific analysis of iMRI in pLGG resection, the use of iMRI increased the rate of intended GTR from 41 to 71% compared to the cohort prior to introduction of iMRI and the remaining residual tumor volume was significantly lower [15].
A Cochrane review highlighted a lack of good quality evidence to support the use of any particular intraoperative imaging technology over another [16]. There was poor quality evidence regarding which technologies have the greatest efficacy and there was a lack of information about the impact on neurological function of more radical surgical resection. There are no ongoing or new trials evaluating iUS or iMRI that will influence treatment guidelines and policy.
This article is intended to demonstrate and analyze the use and value of iUS or iMRI in the surgical treatment of pLGG patients based on accumulated experience from 4 large pediatric neuro-oncology centers across Europe and South Africa. All institutions are using iUS as routine, with two centers having iMRI in routine use as well, with sufficient previous experience in the field [9, 10, 14, 15].
Materials and methods
Patients were included from the existing pediatric tumor registries in each hospital. The data were retrospectively collected and anonymized. All centers had institutional approval for retrospective data collection.
For the iUS data, children aged 0–18 years were included when histological diagnosis of pLGG was confirmed after surgery; iUS was used for either location and/or extent of resection of the tumor. Inclusion was done at Fondazione Policlinico Universitario A. Gemelli Roma (Italy) from 2017 till 2023, Steve Biko Academic Hospital Pretoria (South Africa) from 2020 till 2024 and Universitätsklinik Tuebingen (Germany) from 2014 till 2020. iUS was performed with MyLabTwice® microconvex probe (Esaote, Italy) in Roma; with Philips Epic Elite (Amsterdam, The Netherlands) probes L15-7io hockey stick and C8-5 curvilinear in Pretoria; and with Siemens Acuson (Siemens, Erlangen, Germany), BK5000 and BK Active, BK Medical, Copenhagen, Denmark) with linear 15-MHz Hockeystick probe and 10-MHz neurosurgery curvilinear probe in Tuebingen.
In the Rome, Pretoria, and Tuebingen iUS experience, iUS was methodically performed before opening the dura mater to assess the lesion site and its features. Brain parenchyma was insonated on standard planes repeatedly during different phases of tumor resection, and again once resection was completed and after dural closure, which could simplify the comparison with preoperative MRI. In addition, the Pretoria group’s imaging protocol also includes routinely performing ultrasound imaging of the optic nerve sheath at the beginning and at the end of the procedure. Concordance of iUS images with preoperative MRI is essential at this stage of surgery and may be time-consuming, especially in early experience with iUS. In general, for frontal craniotomies, coronal, and sagittal planes are used, while coronal and axial planes are useful for temporal craniotomies. Usually, curved probes (5–8 MHz) are used for large and deep lesions, although they might lack resolution. On the other hand, linear probes (6–15 MHz) have higher spatial resolution and are used for small and superficial tumors [17]. The curved array probe with a 14-mm ray of curvature allows for imaging in smaller craniotomy access and the ability to change the focus of the ultrasound beam provides the combined advantage of using linear and phased-array probes.
Additionally, hybrid systems using preoperative MRI and iUS data allows real-time neuronavigated iUS, thanks to the magnetic coregistration of preoperative MRI and iUS, that could further aid the interpretation of iUS and orientation of the surgeon. In deep-seated lesions, iUS was also used to guide the surgical trajectory. It is important to re-scan the operative field by iUS during the course of resection with known and visible residual tumor to monitor progress of resection, appearance of artifacts and their difference to confirmed residual tumor. This improves the surgeons’ orientation, gives her/him a feeling for the residual disease and thus improves assessment at the end. After the macroscopic complete resection of the lesion according to the microsurgical view, or at the end of intended subtotal resection due to functional limitations, US was repeated to assess the EOR on the same planes explored before resection and concordance with postoperative MRI was defined, as described elsewhere [10].
For the iMRI data, children aged 0–18 years were included when iMRI was used for control of resection, histological diagnosis of pLGG was confirmed after surgery and preoperative intent of treatment was CR. Inclusion was done at Alder Hey Liverpool NHS trust (UK) from 2010 till 2023 and Universitätsklinik Tuebingen (Germany) from 2014 till 2020. iMRI was performed by 3 T-MRI in Liverpool (Philips Healthcare, Amsterdam, the Netherlands) and by 1.5 T-MRI in Tuebingen (Espree, Siemens Medical Systems). Imaging included the brain tumor protocol with T1, T2, FLAIR, DWI and contrast-enhanced images [18, 19]. The EOR on iMRI scan findings was based on evaluation performed by the radiologist and the operating neurosurgeon in consensus during iMRI acquisition.
Variables that were reviewed included patient demographics, location of the tumor (supratentorial or posterior fossa), EOR, pathological diagnosis and follow-up of tumor where available.
Results
Intraoperative ultrasound
The iUS data of pLGG includes 111 operations in total (in 111 patients), 51 from Tuebingen, 35 from Rome, and 25 from Pretoria. The details of these operations can be found in Table 1. Patients included and analyzed contained all cases in a given time period, also those where subtotal resection due to functional limitations was the primary intention to treat. iUS was used for both purposes: to maximize the rate of CR in the group of patients where CR was the intention or to minimize the amount of residual tumor in the group where subtotal resection was the defined intention to treat. Two examples of peroperative imaging with iUS are demonstrated in Figs. 1 and 2.
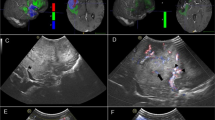
Pre- (A, B) and postoperative MRI (C, D) showing cerebellar pilocytic astrocytoma in a 16-year-old boy that was completely resected through suboccipital approach. iUS before resection showing a well-delineated hyperechoic tumor on axial and sagittal planes (E and F, respectively). iUS after resection confirms CR on axial and sagittal planes (G and H, respectively). Note the concordance of iUS with the detail of the MRI (in the upper left angle of the image) that is oriented as iUS for the sake of interpretation
Pre- (A, B) and postoperative MRI (C, D) showing a right parietal low-grade glioma in a 4-year-old boy that underwent partial resection guided by the intraoperative neurophysiological monitoring. iUS before resection showing the hyperechoic tumor with the superficial cystic portion (arrowhead) and faint margins of the deep component, on coronal and sagittal planes (E and F, respectively). iUS after partial resection showing the residual tumor (arrows) on coronal and sagittal planes (G and H, respectively)
In the Tuebingen Cohort of 51 patients, the intent was CR in 35 cases and STR due to obvious functional limitations in 16 patients, greatly depending on the anatomical region. Intraoperative neurophysiological monitoring was used if monitorable brain or brain stem function was involved at the site of surgery. In all 29 cases of supratentorial hemispheric and cerebellar hemispheric locations, the intention to treat was CR. According to the reading of 3 months postoperative MRI, CR was reached in 12/12 patients (supra-) and 15/17 patients (infratentorial). The remaining tissue volume in the 2 incomplete cerebellar cases was 0.1 ml and 0.4 ml respectively, therefore minimal. One of those cases was lost to follow-up, the other remained stable. In the 4th ventricle manifestations, CR was intended in 4 patients and only reached in 2 for functional reasons of ventricular floor infiltration. In all other locations (brainstem, supratentorial midline), we did define STR as goal of surgery, except for 2 cases in the thalamus. In one GTR was reached, in the other 6% of the tumor volume remained. The total number of patients in all locations with GTR was 30; follow-up was available for 29 patients. In two (6.9%) of these patients, there was recurrence of disease. The US-guided volume reduction in the 21 STR-designed cases ranged from 22 to 98%, median of 57% of initial tumor volume. Long-term follow-up regarding progression of residual disease after iUS-guided resection was available for 18 patients with a median follow-up of 51 months. In nine (50%) of these patients, there was progression. These results are significantly different (chi-square test, p < 0.01).
In the Rome Cohort, CR was the intent of surgery in 24 out of 28 supratentorial and in 5 out of 7 infratentorial tumors. Similar to the Tuebingen experience, intended STR for supratentorial tumors was performed to avoid obvious functional limitations (e.g., optic pathway glioma) or in case of large tumors involving the region of thalamus and basal ganglia. In the posterior cranial fossa, STR was performed in two cases of brainstem exophytic gliomas while CR was achieved in all cases of cerebellar hemispheric gliomas. This was similar to the Pretoria experience, where a combined structural and functional monitoring approach was used for all hemispheric and posterior fossa tumors, i.e., combining iUS guidance with intraoperative neurophysiological monitoring to achieve the goal of maximal safe resection. In Rome, no cases of recurrence were recorded after CR guided by iUS so far, with a median follow-up of 36 months.
Overall, a total concordance of post-iUS to postoperative MRI was noted in Rome and Pretoria, although a detailed volumetric analysis of the residual tumor was not available for STR like for the Tuebingen cases. Interestingly, iUS was also able to detect intraoperative complications, such as a focal intraparenchymal hemorrhage secondary to brain collapse after the resection of a large intraventricular tumor.
Intraoperative MRI
The iMRI data of pLGG with the intent of CR includes 182 operations in total, 164 operations (in 150 patients) from Liverpool and 18 operations (in 18 patients) from Tuebingen. The details of these operations can be found in Table 2. Two examples of peroperative imaging with iMRI are demonstrated in Figs. 3 and 4.
A and B show the preoperative images with a large contrast-enhancing lesion in the fourth ventricle in a 15-year-old boy, histology confirmed a DNET. Figures C and D are the peroperative images that show a small persistent contrast-enhancing zone in the roof of the fourth ventricle. E and F show the results after second-look surgery with further resection of the tumor. G and H are the latest available images postoperatively which show CR, done 6 years postoperatively
A and B show the preoperative images of an 8-year-old boy with a thalamic cystic contrast-enhancing lesion, histology confirmed a ganglioglioma. C and D show the peroperative images done to assess the progress of resection of tumor, whereafter further resection was proceeded. E and F are done at the end of surgery and show the EOR. CR was redeemed unsafe and a small deep-seated thalamic zone along the capsula interna was left in situ. G and H show the last postoperative imaging done 5 years after surgery, which show that there is no progression of disease
Long-term follow-up regarding progression/recurrence of resection with iMRI was available for both Liverpool and Tuebingen. In the Liverpool data, CR was achieved at time of the first iMRI in 77 operations (47%), without need for further resection. In 73 operations (44.5%) we proceeded with second-look surgery, which led to CR in 59 operations (80.8% of second-look operations). In 5 operations, CR was achieved but the images of iMRI were not useful due to technical difficulties (CR was confirmed on postoperative MRI in the first 48 h). In the total cohort of Liverpool, CR was achieved in 141 patients (86%) confirmed by MRI. Incomplete resection was mainly due to invasion of eloquent areas (more specific brainstem or cerebellopontine angle in the posterior fossa, supratentorial in deep-seated cerebral hemisphere). Long-term follow-up was available in 144 operations (87.8%) with median follow-up of 46 months. In the CR group, there was recurrence of disease in 18 cases (14.2%). In the incomplete resection group, there was progression in 10 cases (58.8%), which is significantly more than the CR group (chi-square test, p < 0.01).
In the Tuebingen data, CR was achieved at the time of the first iMRI in 4 operations (22.2%). In 14 operations (77.8%), we proceeded with second-look surgery, which led to CR in 11 operations (78.6%). In the total cohort of Tuebingen, CR was achieved in 15 patients (83.3%) confirmed by MRI. Incomplete resection was mainly due to the invasion of eloquent areas. Long-term follow-up was available in 17 operations (94.4%) with a median follow-up of 61 months. In the CR group, there was a recurrence of the disease in 3 cases (21.4%). In the incomplete resection group, there was progression in one case (33.3%). These results are not significantly different (chi-square test, p 0.531). If we combine the long-term follow-up data of Liverpool and Tuebingen, in the group of CR, there is recurrence of disease in 21 out of 141 cases (14.9%). In the incomplete resection group, there is progression in 11 out of 20 cases (55%), which is significantly more than the CR group (chi-square test, p < 0.01).
Discussion
Optimizing the balance between the EOR and preservation of neurological function remains the goal of brain tumor surgery. The value of structural monitoring during brain tumor surgery to contribute to this has been established in numerous studies [20,21,22]. Our results present an overview of the use of perioperative imaging in all-day neurosurgery practice for pLGG, to optimize the extent of safe resection. There is a difference in the results of GTR in the iUS data from Tuebingen versus Roma/Pretoria. In the Tuebingen data, there were more posterior fossa medullar tumors included. Supratentorial, there were multiple optic pathway gliomas included, which was different from the other centers, and there was no aim for GTR in these cases. For iMRI, there is a significant reduction in recurrence of pLGG when CR is confirmed on imaging in the Liverpool dataset. This was not significant in the Tuebingen data, but this was probably due to the relatively small sample size.
The value of iUS for surgical image guidance has enjoyed a tremendous upsurge in recent years. The real-time, portable, cost-effective benefits combined with the improved quality of imaging provided by modern probes has contributed to its increased utility in neurosurgery. First, it can be used to delineate and optimize the approach to the lesion, even before the dura is opened. Combining real-time iUS with neuronavigation, the last becoming less reliant during surgery due to resection and loss of cerebrospinal fluid, has further addressed some of the challenges limiting its widespread use. With the possibility of Doppler images in iUS, also blood vessels and flow are pictured which will help in the decision-making during resection [10, 17, 23, 24]. iUS is considered an accessible first step to implement in a pediatric neurosurgery unit as an anatomical perioperative monitoring tool.
Furthermore, iUS may guide the surgical trajectory for deep-seated tumors and finally assess EOR. Assessment of EOR is affected by known limitations, that are largely described through literature. iUS features that define surgical margins as disease-free are unclear to date. Moreover, surgical artifacts may significantly hinder the assessment of surgical margins, in particular in large tumors with large cortical access and/or collapsed surgical cavity, as well as open ventricle. Several advances in the multiparametric application of ultrasound, which includes the use of ultrasound contrast agents (UCAs), ultrasound elastography, 3-dimensional navigated ultrasound and Doppler flow contribute significantly to improving the sensitivity of iUS for detecting residual tumor volume, though these modalities may not be available on many US systems and sufficient data on their use still does not exist in children. Despite these limitations, gaining experience with this technology may aid in defining surgical strategies or technical tricks to enhance the reliability of iUS [10].
Thus, iUS is a versatile surgical adjunct with an important role during the different stages of surgery, though the present study, as most of the studies published in the literature so far, focuses on the role of iUS in aiding to achieve CR [25, 26]. The above-named advantages provided by using iUS, along with its ease-of-use and repeatability, are the main difference when compared to iMR. iUS may therefore significantly contribute to steepen the learning curve in tumor surgery of young surgeons and we are convinced it helps to perfect the CR rates of experienced surgeons in all superficial tumor locations not deeper than 5–6 cm from the brain surface.
iMRI has the advantages of higher resolution, less artifacts, and being less dependent on the experience of the user. Especially in the deeper-seated locations, visualization with iMRI is better compared to iUS [27]. The high initial cost of equipment and the time it adds to the operation with an extensive staff make it not widely available [28]. However, it has been proven that the use of iMRI reduces the need for early repeat surgery in pediatric brain tumors and that the perioperative images that confirm GTR pre-empt new postoperative MRI imaging [14, 29]. Also in MRI, there is a continuous evolution in advanced imaging, which contributes to safely maximize the level of resection [30].
Conclusion
Intraoperative ultrasound and MRI have specific pros and cons, but both have been proven to improve the extent of resection in pediatric low-grade gliomas. For iMRI, since this is a static tool, it can easily be proven on sequential imaging with the extent of resection and recurrence of disease as shown in our results. This is more difficult for iUS as it is a dynamic tool which is user dependent. Due to advances in image quality and cost- and time-efficiency, this is however the most accessible tool to date to aid in achieving the maximum possible tumor resection and should be combined whenever possible or meaningful with intraoperative neurophysiological monitoring.
Data availability
Data is available and anonymized at the participating centres.
References
Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP (2005) Years of life lost (YLL) from cancer is an important measure of population burden — and should be considered when allocating research funds. Br J Cancer 92(2):241–245
Fangusaro J, Jones DT, Packer RJ, Gutmann DH, Milde T, Witt O et al (2024) Pediatric low-grade glioma: state-of-the-art and ongoing challenges. Neuro Oncol 26(1):25–37
Collins KL, Pollack IF (2020) Pediatric low-grade gliomas. Cancers (Basel) 12(5):1152
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23(8):1231–1251
Bale TA, Rosenblum MK (2022) The 2021 WHO classification of tumors of the central nervous system: an update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol 32(4):e13060. https://doi.org/10.1111/bpa.13060
Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ et al (2014) Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 61(7):1173–9
Kelly PJ, Kall BA, Goerss S, Earnest F (1986) Computer-assisted stereotaxic laser resection of intra-axial brain neoplasms. J Neurosurg 64(3):427–439
D’Amico RS, Kennedy BC, Bruce JN (2014) Neurosurgical oncology: advances in operative technologies and adjuncts. J Neurooncol 119(3):451–463
Padayachy LC, Fieggen G (2014) Intraoperative ultrasound-guidance in neurosurgery. World Neurosurg 82(3–4):e409–e411
Frassanito P, Stifano V, Bianchi F, Tamburrini G, Massimi L (2023) Enhancing the reliability of intraoperative ultrasound in pediatric space-occupying brain lesions. Diagnostics 13(5):971
Singhal A, Ross Hengel A, Steinbok P, Doug CD (2015) Intraoperative ultrasound in pediatric brain tumors: does the surgeon get it right? Child’s Nerv Syst 31(12):2353–2357
Mattei L, Prada F, Legnani FG, Perin A, Olivi A, DiMeco F (2016) Neurosurgical tools to extend tumor resection in hemispheric low-grade gliomas: conventional and contrast enhanced ultrasonography. Child’s Nerv Syst 32(10):1907–1914
Carai A, De Benedictis A, Calloni T, Onorini N, Paternò G, Randi F et al (2021) Intraoperative ultrasound-assisted extent of resection assessment in pediatric neurosurgical oncology. Front Oncol 11:660805
Avula S, Pettorini B, Abernethy L, Pizer B, Williams D, Mallucci C (2013) High field strength magnetic resonance imaging in paediatric brain tumour surgery—its role in prevention of early repeat resections. Child’s Nerv Syst 29(10):1843–1850
Roder C, Breitkopf M, Bisdas S, da Silva Freitas R, Dimostheni A et al (2016) Beneficial impact of high-field intraoperative magnetic resonance imaging on the efficacy of pediatric low-grade glioma surgery. Neurosurg Focus 40(3):13
Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA et al (2018) Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev 1(1):CD012788. https://doi.org/10.1002/14651858.CD012788.pub2
Aibar-Duran JA, Salgado-López L, Anka-Tugbiyele MO, Mirapeix RM, Gallardo Alcañiz A, Patino Alvarado JD et al (2024) Navigated intraoperative ultrasound in neuro-oncology: volumetric accuracy and correlation with high-field MRI. J Neurosurg 1:1–10
Craig E, Connolly DJA, Griffiths PD, Raghavan A, Lee V, Batty R (2012) MRI protocols for imaging paediatric brain tumours. Clin Radiol 67(9):829–832
Avula S, Peet A, Morana G, Morgan P, Warmuth-Metz M, Jaspan T (2021) European Society for Paediatric Oncology (SIOPE) MRI guidelines for imaging patients with central nervous system tumours. Child’s Nervous System 37(8):2497–2508
Nuwer MR, Dawson EG, Carlson LG, Kanim LEA, Sherman JE (1995) Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol/Evoked Potentials Sect 96(1):6–11
You H, Qiao H (2021) Intraoperative neuromonitoring during resection of gliomas involving eloquent areas. Front Neurol 23:12
Radtke RA, Erwin CW, Wilkins RH (1989) Intraoperative brainstem auditory evoked potentials. Neurology 39(2):187–187
Bastos DCDA, Juvekar P, Tie Y, Jowkar N, Pieper S, Wells WM et al (2021) Challenges and opportunities of intraoperative 3D ultrasound with neuronavigation in relation to intraoperative MRI. Front Oncol 3:11
Shi J, Zhang Y, Yao B, Sun P, Hao Y, Piao H et al (2021) Application of multiparametric intraoperative ultrasound in glioma surgery. Biomed Res Int 16(2021):1–18
Giussani C, Trezza A, Ricciuti V, Di Cristofori A, Held A, Isella V et al (2022) Intraoperative MRI versus intraoperative ultrasound in pediatric brain tumor surgery: is expensive better than cheap? A review of the literature. Child’s Nerv Syst 38(8):1445–1454
Moiyadi AV (2016) Intraoperative ultrasound technology in neuro-oncology practice—current role and future applications. World Neurosurg 93:81–93
Munkvold BKR, Jakola AS, Reinertsen I, Sagberg LM, Unsgård G, Solheim O (2018) The diagnostic properties of intraoperative ultrasound in glioma surgery and factors associated with gross total tumor resection. World Neurosurg 115:e129–e136
Abernethy LJ, Avula S, Hughes GM, Wright EJ, Mallucci CL (2012) Intra-operative 3-T MRI for paediatric brain tumours: challenges and perspectives. Pediatr Radiol 42(2):147–157
Avula S, Jaspan T, Pizer B, Pettorini B, Garlick D, Hennigan D et al (2021) Comparison of intraoperative and post-operative 3-T MRI performed at 24–72 h following brain tumour resection in children. Neuroradiology 63(8):1367–1376
Mato D, Velasquez C, Gómez E, Marco de Lucas E, Martino J (2021) Predicting the extent of resection in low-grade glioma by using intratumoral tractography to detect eloquent fascicles within the tumor. Neurosurgery 88(2):E190-202
Ethics declarations
Conflict of interest
Llewellyn Padayachy – Medical advisor for research commercial spinoff company Nisonic. Martin Ulrich Schuhmann – editor of the special Annual Issue Collection of CNS 2024. There is no conflict of interest for any of the remaining authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dietvorst, S., Narayan, A., Agbor, C. et al. Role of intraoperative ultrasound and MRI to aid grade of resection of pediatric low-grade gliomas: accumulated experience from 4 centers. Childs Nerv Syst (2024). https://doi.org/10.1007/s00381-024-06532-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00381-024-06532-3