Abstract
Purpose
Iatrogenic aneurysms are very rare in children. Characteristic clinical manifestations are variable and asymptomatic course is possible especially for fusiform dilatation of internal carotid artery. Even though radiological diagnosis is easy, the management of iatrogenic intracranial aneurysm is still a subject for discussion.
Methods
Fusiform dilatations of internal carotid artery were diagnosed on three pediatric patients during follow-up imaging after primary surgery for suprasellar–parasellar tumor. All patients were asymptomatic. Conservative treatment was proposed because the lesion did not show any progression in subsequent examinations. Patients are stable under conservative treatment.
Conclusions
Iatrogenic aneurysm may have an unusual presentation and their therapy still remains unclear. Fusiform dilatation of internal carotid artery rarely causes symptoms and there is no published paper of subarachnoid bleeding. Treatment would be difficult, since the main arterial branches arise from the dilated carotid segment. Conservative treatment is a choice only if aneurysm has no progression or in case of spontaneous healing. Intervention should be performed only in case of progression or if the aneurysm becomes symptomatic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracranial aneurysms are extremely rare in the pediatric population in comparison with adults. Only 1–5 % of all intracranial aneurysms are shown by the pediatric population [7, 26]. Intracranial aneurysms affect more frequently male children than females (1.3–2.8:1) [5, 7, 26]. Infections and trauma are most common risk factors for childhood intracranial aneurysms [5, 7, 26, 28]. Approximately, 33 % of childhood aneurysms are traumatic [5, 16, 28]. Prompt treatment by surgery or endovascular techniques should be done because traumatic aneurysms may gradually expand and rupture in a few weeks or months [3, 24, 26]. Although urgent treatment is necessary to prevent intracranial hemorrhage, especially pseudoaneurysms are usually difficult to treat without sacrificing the parent artery [3, 24].
Iatrogenic aneurysms take a small part among traumatic aneurysms. To our knowledge, 37 cases of iatrogenic intracranial aneurysm in children have been reported to develop after surgical interventions, such as tumor removal [2, 5, 6, 10, 16, 21–23], endoscopic procedures [8, 14, 18, 26], ventricular puncture or shunting procedure [9, 19, 20, 27], aspiration of subdural hematoma [17], aspiration of abscess [12], myringotomy [25], temporary clip occlusion [28], and decompressive craniectomy for closed head injury [28]. The clinical data of those cases are displayed in Table 1. The best way to manage iatrogenic intracranial aneurysm is still a subject for discussion, although it was like that for posttraumatic aneurysms. Most authors are in favor of a prompt surgical or endoscopic treatment [3, 24, 26]. Fusiform aneurysmal dilatation of the internal carotid artery (FDCA) is another vascular complication that can occur following radical surgery for sellar–parasellar region tumors [2, 10, 16, 21–23]. The management of this vascular complication and its pathogenesis are still controversial [23].
Classification and pathology
Lasjaunias et al. classified childhood aneurysms in four groups: (1) dissecting aneurysms, (2) infectious aneurysms, (3) traumatic aneurysms, and (4) classic saccular aneurysms [11]. Group 1 and group 3 can be evaluated together. Traumatic intracranial aneurysms (TICA) can be classified according to their mechanism of injury or histopathological structure (Table 2).
FDCA is another entity. Focal arterial disruption while dissecting the tumor from arterial wall may lead to injury of the vasa vasorum [2, 22, 23]. This arterial wall weakening is thought to cause fusiform dilatation (Fig. 1). However, all wall layers are intact in FDCA; if the tumor itself invades the adventitia of internal carotid artery (ICA), it may cause disruption during surgery [10, 21]. Also, another mechanism which injury of the sympathetic plexus of the ICA was postulated to lead to neurogenic vasoparalysis and, thus, arterial dilatation may occur [2, 23]. Radiation therapy might develop FDCA [23].
Clinical presentation
Two thirds of pediatric patients harboring traumatic aneurysms will experience a symptomatic aneurysmal hemorrhage [5, 28]. However, asymptomatic patients were also reported whose iatrogenic aneurysm was diagnosed during follow-up [5, 26]. Clinic presentation varies from simple headache to loss of consciousness and even sudden worsening due to acute bleeding is possible [9]. Dysphasia, hemiparesis, visual impairment, and seizure have been reported associated with iatrogenic intracranial aneurysms in childhood [9, 20]. After aneurysm rupture, mortality rate is more than 30 % [5, 14, 26–28].
Diagnostic imaging and evaluation
Although digital subtraction angiography (DSA) is still the gold standard technique, the role of computed tomography angiography (CTA) and magnetic resonance angiography (MRA) is still under evaluation even though some authors propose them as alternative to invasive techniques. A few reports have presented that CTA and MRA can diagnose aneurysm larger than 5 mm with an accuracy of 90 % [29, 30].
Doppler ultrasound detects anterior cerebral artery (ACA) and middle cerebral artery (MCA) aneurysms larger than 5 mm with sensitivity of about 0.82 and 0.79, respectively. A significantly lower accuracy was reported for aneurysms of the cavernous and terminal internal carotid arteries and posterior communicating artery [31].
Both true and false aneurysm may be fusiform or saccular [9]. Most common localization for TICA is the ACA (38 %) [5, 28]. Thirty-seven children with iatrogenic aneurysms were reported in the literature [2–4, 6, 8–10, 12, 14, 16–23, 25–28]. The acquired malformation involved the ICA in 25 cases. Additionally, a few iatrogenic aneurysms were reported to involve the ACA, the MCA, the posterior inferior cerebellar artery, and the basilar tip (Table 1).
There is no particular indication universally shared on how to search for iatrogenic aneurysms in children who had undergone intracranial surgery. However, Tirakotai et al. advice pediatric neurosurgeons to consider FDCA within 2 years after surgery for craniopharyngioma or midline gliomas [23].
Management and treatment
Conservative treatment
Although TICA try to expand gradually and rupture in a few weeks or months, spontaneous regression was also reported [3, 15, 24]. Nevertheless, even after spontaneous healing, angiographic follow-up should be continued because of possible risk for re-expansion.
There is no mention in literature about the management protocol for iatrogenic aneurysm in childhood. In general, conservative treatment is a choice only if the aneurysm does not progress or in case of spontaneous healing. Dunn et al. followed pericallosal artery aneurysm for 6 months which was initially interpreted as residual tumor [5]. The patient underwent surgery because of progression.
In case of conservative treatment, the patients should undergo annual follow-up magnetic resonance (MR) imaging studies to evaluate aneurysmal growth and stability [7]. Sutton et al. first reported in 1991 and then updated in 1994 nine (15.7 %) supraclinoid carotid artery aneurysms among 57 operated on children for craniopharyngiomas between 1982 and 1993 [21, 22]. Only one patient underwent aneurysm clipping while operating for regrowth of tumor and died because of tumor recurrence. Eight of the nine patients remain alive at a mean of 6.6 years after diagnosis. Similarly, in 1998, Bendszus et al. reported FDCA in 7 children among 62 (11.3 %) operated cases because of suprasellar–parasellar tumor [2]. Three children had progression and one child had regression in 6 months follow-up. However, all of them were conservatively followed (Table 1).
FDCA rarely causes symptoms and there is no published paper of subarachnoid bleeding (Table 1). In any case, treatment of FDCA would be difficult, since the posterior communicating artery and anterior choroidal arteries (and in some cases the middle cerebral arteries) arise from the dilated carotid segment [21, 22]. Therefore, according to the conservative option, intervention should be considered only in case of progression or if the aneurysm becomes symptomatic [2, 16, 21, 23].
Surgery
Although successful surgical interventions such as clipping, trapping, wrapping, resection, primary reconstruction, or arterial graft interposition have been described [3, 4, 6, 9, 12, 14, 17, 19, 20, 23, 25, 27, 28], especially pseudoaneurysms are not always suitable for surgical occlusion because of absence of a true neck or an aneurysm wall [18, 26]. They may be difficult to treat without sacrificing parent vessels [1, 3, 16, 24]. Surgery for dissecting aneurysm may also be difficult especially for the ones in vertebrobasilar system due to the risk of incidental damage to the vertebral perforating arteries [13].
Endovascular treatment
Endovascular techniques which is virtually less invasive than open microsurgery widely demonstrated to be associated with successful results. First, Horowitz et al. described endovascular treatment of a 30-month-old child with a traumatic basilar artery aneurysm after endoscopic third ventriculostomy [8]. The false aneurysm was managed with endovascular trapping without morbidity. However, endovascular trapping may be risky especially when the collateral blood circulation is not sufficient [8, 18]. Afterward, few selective endovascular coiling was reported for childhood iatrogenic aneurysm [18, 26]. Selective coiling should be done cautiously because traumatic aneurysms can be pseudoaneurysms. Fibrocollagenous capsule laceration may lead to rebleeding [1, 18, 24, 26].
Ogilvy et al. described stent-assisted coiling to FDCA which was operated for craniopharyngioma first [16]. The aneurysm was first diagnosed 3 months after surgery; stent-assisted coiling was performed 5 months later because of radiological progression. This technique suggests better vessel preservation [16].
Prognosis
There is no large series to determine prognosis of iatrogenic aneurysms but approximately two thirds of patients with traumatic aneurysm experience symptomatic hemorrhage, with an associated mortality rate of 30–41 % [5, 26, 28]. Surgical or endovascular obliteration of aneurysm reduces the overall risk of mortality [5, 26–28].
Conclusion
Iatrogenic intracranial aneurysms are very rare in childhood and hard to be recognized. These lesions may insidiously progress making their management a real challenge. Conservative treatment is a choice only if aneurysm has no progression or in case of spontaneous healing. Fusiform aneurysms should also be followed especially when involving the main arterial branches. Follow-up observation should be continued regarding the risk of expansion. Endovascular techniques are effective therapeutic options when the aneurysms are considered as unclippable. Like surgical treatment, lethal complications may occur after endovascular treatment due to the fragile fibrocollagenous wall of aneurysm or lack of collateral arterial branches.
In spite of the good outcomes of iatrogenic aneurysms reported by the only two papers dealing with clinical series [2, 21, 22], most of the anecdotal reported cases have been treated surgically. This attitude may be influenced by the evolutive character of congenital aneurysm and the progression of the lesion often observed in cases following traumatic vascular injuries associated with head injuries.
Exemplary cases
Case 1
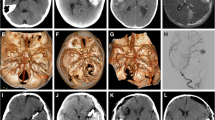
A 6-year-old boy, with a 3-month history of headache and transient dysarthria and progressing left hemiparesis, was admitted to Pediatric Neurosurgery Department of Rome Catholic University in 2008. His neurological condition improved with steroid administration. No history of minor or major cranial injuries was reported. Neuroimaging (MRI) revealed an extra-axial fatty mass at the left cerebellopontine angle extending to the prepontine cistern (Fig. 2). Auditory brain stem response and motor-evoked potentials were normal. He underwent surgery, and the lesion was gross totally removed by left pterional approach. After the operation, he experienced partial transitory deficit of the left third cranial nerve. Brain MR on the fifth postoperative day revealed no residual tumor (Fig. 3). Histopathological examination revealed an epidermoid cyst. A 6-mm-diameter fusiform aneurysm at terminal internal carotid artery with extension to M1 was diagnosed after 1 year of follow-up (Fig. 4). He was asymptomatic. Conservative treatment was proposed because of absent progression. After 4 years follow-up, he presents normal psychomotor development and his hemiparesis and third nerve palsy have totally recovered. Fusiform aneurysm is stable under conservative treatment.
Case 2
A 11-year-old girl, with a 5-month history of anorexia, was admitted to Pediatric Neurosurgery Department of Rome Catholic University in 1988 because of severe weight loss. Neurological examination was normal. No history of minor or major cranial injuries was reported. Neuroimaging (CT scan) revealed 2.5-cm-diameter sellar–suprasellar and solid-cystic mass with gross calcifications. This mass had prechiasmatic extension. She underwent surgery, and the lesion was gross totally removed by right pterional approach. External wall damage on the right ICA was seen at the end of the procedure without, however, no aneurysmatic dilatation (Fig. 5). After the operation, she experienced insipid diabetes, nystagmus, and hemianopsia. Histopathological examination diagnosed adamantinomatous craniopharyngioma and complete recovery of her postoperative neurologic deficits. Patient was discharged with hormone replacement therapy because of hypopituitarism. A fusiform aneurysm at supraclinoid segment of the right internal carotid artery was diagnosed during follow-up (Fig. 6). The lesion was not association in any sign or symptom. Conservative treatment was proposed and the lesion did not show any progression in subsequent examinations (Fig. 7). At last follow-up examination, 24 years after first surgery, no changes on size or shape of the aneurysm were noticed on the MR control. She works as a nurse under hormone replacement therapy.
References
Amirjamshidi A, Rahmat H, Abbassioun K (1996) Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management. A survey of 31 cases and review of the literature. J Neurosurg 84(5):769–780
Bendszus M, Sörensen N, Hofmann E, Röll E, Solymosi L (1998) Fusiform dilatations of the internal carotid artery following surgery for pediatric suprasellar tumors. Pediatr Neurosurg 29(6):304–308
Dario A, Dorizzi A, Scamoni C, Cerati M, Balcone Grimaldi G (1997) Iatrogenic intracranial aneurysm. Case report and review of the literature. J Neurosurg 41(2):195–202
Dubey A, Sung WS, Chen YY, Amato D, Mujic A, Waites P, Erasmus A, Hunn A (2008) Traumatic intracranial aneurysm: a brief review. J Clin Neurosci 15(6):609–612
Dunn IF, Woodworth GF, Siddiqui AH, Smith ER, Vates GE, Day AL, Goumnerova LC (2007) Traumatic pericallosal artery aneurysm: a rare complication of transcallosal surgery. Case report. J Neurosurg 106(2 Suppl):153–157
Gutierrez FA, Bailes J, McLone DG (1987) Intracranial aneurysm and pseudo-aneurysm occurring during infancy and childhood: diagnosis and surgical results. Concepts Pediatr Neurosurg 7:153–168
Hetts SW, Narvid J, Sanai N, Lawton MT, Gupta N, Fullerton HJ, Dowd CF, Higashida RT, Halbach VV (2009) Intracranial aneurysms in childhood: 27-year single-institution experience. AJNR Am J Neuroradiol 30(7):1315–1324
Horowitz M, Albright LA, Jungreis C, Levy EI, Stevenson K (2001) Endovascular management of a basilar artery false aneurysm secondary to endoscopic third ventriculostomy: case report. Neurosurgery 49(6):1461–1464, discussion 1464 – 5
Jenkinson MD, Basu S, Broome JC, Eldridge PR, Buxton N (2006) Traumatic cerebral aneurysm formation following ventriculoperitoneal shunt insertion. Childs Nerv Syst 22(2):193–196
Lakhanpal SK, Glasier CM, James CA, Angtuaco EJ (1995) MR and CT diagnosis of carotid pesudoaneurysm in children following surgical resection of craniopharyngioma. PediatrRadiol 25(4):249–251
Lasjaunias P, Wuppalapati S, Alvarez H, Rodesch G, Ozanne A (2005) Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst 21(6):437–450
Lassman LP, Ramani PS, Sengupta RP (1974) Aneurysms of peripheral cerebral arteries due to surgical trauma. VascSurg 8(1):1–5
Massimi L, Moret J, Tamburrini G, Di Rocco C (2003) Dissecting giant vertebro-basilar aneurysms. Childs Nerv Syst 19(4):204–210
McLaughlin MR, Wahlig JB, Kaufmann AM, Albright AL (1997) Traumatic basilar aneurysm after endoscopic third ventriculostomy: case report. Neurosurgery 41(6):1400–1403, discussion 1403 – 4
Miyazaki S, Ohmori H, Munekata K, Fukushima H, Kamata K (1981) Spontaneous healing of traumatic aneurysm occurring after brain tumor removal. No Shinkei Geka 9(4):531–537, (author's transl) [in Japanese]
Ogilvy CS, Tawk RG, Mokin M, Yang X, Levy EI, Hopkins LN, Siddiqui AH (2011) Stent-assisted coiling treatment of pediatric traumatic pseudoaneurysm resulting from tumor surgery. Pediatr Neurosurg 47(6):442–448
Overton MC 3rd, Calvin TH Jr (1966) Iatrogenic cerebral cortical aneurysm. Case report. J Neurosurg 24(3):672–675
Rezende MT, Spelle L, Piotin M, Mounayer C, Lucas Cde P, Abud DG, Moret J (2008) Selective endovascular treatment of a traumatic basilar aneurysm after endoscopic third ventriculostomy. Neuroradiology 50(5):443–446
Scharfetter F, Fodisch HJ, Menardi G, Twerdy K (1976) [False aneurysm of the artery of the angular gyrus caused by vascular injury during ventricular puncture]. Acta Neurochir (Wien) 33(1–2):123–132 [Article in German]
Shirane R, Kondo T, Yoshida YK, Furuta S, Yoshimoto T (1999) Ruptured cerebral pseudoaneurysm caused by the removal of a ventricular catheter. Case report. J Neurosurg 91(6):1031–1033
Sutton LN (1994) Vascular complications of surgery for craniopharyngioma and hypothalamic glioma. J Neurosurg 21(Suppl 1):124–128
Sutton LN, Gusnard D, Bruce DA, Fried A, Packer RJ, Zimmerman RA (1991) Fusiform dilatation of the carotid artery following radical surgery of childhood craniopharyngioma. J Neurosurg 74(5):695–700
Tirakotai W, Sure U, Benes L, Aboul-Enein H, Schulte DM, Riegel T, Bertalanffy H (2002) Successful management of a symptomatic fusiform dilatation of the internal carotid artery following surgery of childhood craniopharyngioma. Childs Nerv Syst 18(12):717–721
Tokunaga K, Kusaka N, Nakashima H, Date I, Ohmoto T (2001) Coil embolization of intradural pseudoaneurysms caused by arterial injury during surgery: report of two cases. Am J Neuroradiol 22(1):35–39
Tranmer BI, Humphreys RP, Chuang SH (1985) Microsurgical recovery of a migrated balloon from the internal carotid artery of a child. Neurosurgery 16(3):381–386
Trivelato FP, Rezende MT, Ulhôa AC, Giannetti AV (2011) Endovascular treatment of a traumatic carotid artery aneurysm after endoscopic arachnoid cyst fenestration. Childs Nerv Syst 27(8):1329–1332
Tubbs RS, Acakpo-Satchivi L, Blount JP, Oakes WJ, Wellons JC 3rd (2006) Pericallosal artery pseudoaneurysm secondary to endoscopic-assisted ventriculoperitoneal shunt placement. Case report. J Neurosurg 105(2 Suppl):140–142
Ventureyra EC, Higgins MJ (1994) Traumatic intracranial aneurysms in childhood and adolescence. Case reports and review of the literature. Childs Nerv Syst 10(6):361–379
White PM, Teasdale E, Wardlaw JM, Easton V (2001) What is the most sensitive non-invasive strategy for the diagnosis of intracranial aneurysms? J Neurol Neurosurg Psychiatry 71(3):322–328
White PM, Wardlaw JM, Easton V (2000) Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology 217(2):361–370
White PM, Wardlaw JM, Teasdale E, Sloss S, Cannon J, Easton V (2001) Power transcranial Doppler ultrasound in the detection of intracranial aneurysms. Stroke 32(6):1291–1297
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egemen, E., Massimi, L. & Di Rocco, C. Iatrogenic intracranial aneurysms in childhood: case-based update. Childs Nerv Syst 28, 1997–2004 (2012). https://doi.org/10.1007/s00381-012-1907-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-012-1907-5