Abstract
Objective
Intracranial aneurysms (IA) in children are rare, accounting for less than 5% of all IA. Due to their scarcity, the epidemiology is poorly understood and differs from adults in term of clinical presentation, size, location, and origin. Consequently, the treatment strategies are specific and cannot be only based on data from adult series. The aim of our study was to report the characteristics, management, and outcomes of children treated for IA in two university hospitals located in Normandy (France) over the last 17 years and to perform a literature review of this rare pathology.
Methods
This retrospective study included 18 consecutive children (< 18 years old) admitted with cerebral aneurysm treated in two neurosurgery departments in Normandy, from 2001 to 2018. Computerized tomography and cerebral angiography established the diagnosis. Both endovascular and surgical procedures were discussed in all cases. Data focused on clinical condition at admission, characteristics of the IA, choice of the treatment modalities, and complications. The outcome at follow-up is based on Glasgow outcomes scale (GOS) at 1 year.
Results
During the study period, 18 children (mean age: 12.6 years; sex ratio male/female: 2.3) were admitted with 21 IA. Aneurysms had a mean size of 13.6 mm with 4 giant aneurysms and were mostly located in the anterior circulation (16/21). Clinical presentations at onset were sudden symptoms related to a subarachnoid hemorrhage in 13 patients, headaches in 4 patients with giant aneurysm, and asymptomatic in one patient. Among the 13 patients with ruptured IA, 6 presented in poor preoperative condition (Hunt and Hess Grade ≥ 4). Treatment modalities consisted in embolization in 9 patients and surgery in 9 patients including 2 by-pass surgeries in fusiform aneurysms. Complications were similar in the two groups, but two cases of recanalization were observed in the endovascular group. At 1 year of follow-up, 14 children were in good condition (GOS Score > 4) and one died. Three children presented associated IA treated by the same technique as initial aneurysm.
Conclusions
Pediatric aneurysm is a different pathology compared with adults, occurring more frequently in male population with a higher proportion of giant aneurysms and aneurysms located in the internal carotid bifurcation. The use of endovascular techniques has progressed in the last years, but surgery was proposed for half of our population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial aneurysm (IA) is a rare condition in the pediatric population accounting for less than 5% of all IA [3, 22]. The onset of ruptured IA is still responsible for a high mortality and morbidity rate up to 10 [26] and 17%, respectively [27]. IA in children are different in many ways compared with adults with a predominance of IA located on internal carotid artery [36] [48] and more frequent in male population [18]. Moreover, in children, IA tend to be giant, non-saccular and at higher risk of bleeding [27, 41]. Consequently, the treatment strategies are specific and cannot be based only on data from adult series. Due to the scarcity of pediatric IA and the growing place of endovascular techniques in the recent years, we are lacking evidence structuring clear recommendations in the management of pediatric IA, especially to guide our choice between endovascular and surgical treatment. The aim of our study was to report the characteristics, management, and outcomes of children treated for IA in two institutions over the last 17 years and to perform a literature review on this rare pathology.
Materials and methods
Reporting on this cohort study is in accordance with the STROBE statements. It was reviewed and approved by the Institutional Ethics Committee of Rouen University Hospital (Rouen, France).
Setting and study design
This study is based on data provided by two registries from two tertiary neurosurgical units located in Normandy (University Hospital of Rouen and University Hospital of Caen, France). Normandy covers an area of around 3.5 M inhabitants. These registries retrospectively included all children younger than 18 years who were treated in our institutions for intracranial aneurysm between 2001 and 2018. Medical records and available imaging and operative reports were retrospectively reviewed for each child. Collected data concerned general characteristics (age, sex, medical personal and family history), clinical presentation (subarachnoid hemorrhage, headaches, asymptomatic), morphology, etiology of aneurysm (fusiform, saccular, giant, mycotic), and location. Fusiform IA were defined as nonsaccular dilatations involving the entire vessel for a short distance [34]. Giant IA were defined as having a diameter ≥ 25 mm [49]. Modalities of treatment either surgical (simple clipping or bypass) or endovascular (coiling, stenting, vessel occlusion) and their complications (need for shunting, vasospasm, recanalization) were collected. Vasospasm was defined by a sudden onset of neurological deficit, transient or not, associated with angiographic confirmation of vessel narrowing in the corresponding territory [54].
All treated IA were considered in this study. Aneurysms associated with arteriovenous malformations were excluded from the analysis. Aneurysms were diagnosed by contrast-enhanced imaging and/or angiography. Surgical or endovascular treatment was available, and the choice of treatment method was made by a multidisciplinary team including neurosurgeons and interventional neuroradiologists. Clinical signs of rupture, blood localisation and morphological characteristics were considered in decision-making.
After treatment, children were monitored in pediatric intensive care unit. Clinical evaluation and regular imaging were performed to detect complications like rebleeding, vasospasm, or hydrocephalus. Once clinical condition was stable, children were hospitalized in a conventional unit. Later, the follow-up consisted of regular consultation with a neurosurgeon or neuroradiologist to determine other complications, neurological deficits, discovery of new aneurysm, and final autonomy (Glasgow Outcome Scale).
Patients’ characteristics were compared using Fisher’s exact test for discrete variables and Wilcoxon-Mann Whitney test were used for continuous variables. Statistical significance was set at the 0.05 level.
Literature review
The PubMed/MEDLINE databases were used to perform an online search of reported studies using the following keywords and MeSH terms: “neonate,” “intracranial,” “aneurysm,” “cerebral,” and “pediatric.” Only full-text articles in English were chosen. Articles were selected from January 2000 to January 2020 to offer a recent up-to-date review of the literature. For each article, we collected the number of IA, the mean age of the cohort, the sex ratio, the rate of subarachnoid hemorrhage, the rate of good grade at presentation with a Hunt and Hess score ≤ 3, morphological data of IA, the rate of infectious, giant, traumatic and multiple IA, the location, the proportion of surgical and endovascular treatment, the rate of good outcome grade defined by a Glasgow Scale Outcome ≥ 4, and the mortality.
According to the best match and relevance, the search results were refined. Only cases of spontaneous IA in children < 18 years were included. Aneurysm associated with arteriovenous malformation was excluded from the review.
Results
Patient characteristics
During the study period, 22 pediatric patients with IA (mean age 12.6 years ± 5.3 years—range: from 0.1 to 18 years) were treated in our hospitals. We have excluded two cases of IA associated with arteriovenous malformation and two other cases for incomplete data. In total, we have analyzed eighteen children. The main results of our cohort are presented in Table 1. We observed a sex ratio male/female of 3.5 and only three patients (n°11, n°12, and n°15) were younger than 10 years. Three patients (n°2, n°10, and n°17) had a medical condition like Noonan syndrome, high blood pressure, and aortic valvulopathy. We have also noted a family history of IA in two children (n°5 and n°6). Intracranial aneurysms were multiple in three patients (cases n°2, 3 and 10). Thirteen children (72.2%) were admitted with subarachnoid hemorrhage (SAH). In these patients, clinical status was poor (Hunt and Hess (HH) grading ≥ IV) in 6 children. The remaining patients (7/13) with ruptured IA were in good clinical condition at admission with HH grading from I to III. For child n°5, IA was discovered during screening as regards family history.
Aneurysm characteristics
On initial computerized tomography (CT) imaging, 11/13 patients presented with a Fisher IV SAH (6 tetraventricular hemorrhage and 5 intracerebral hematoma). The remaining 2 others had a Fisher 3 SAH. In patients with unruptured IA, brain imaging was motivated by headaches in 4 and by family history of cerebral aneurysm for the rest (case n°5). The mean size of IA was 13.6 mm ± 15.1 mm (range: from 1.5 to 50 mm). Giant aneurysms were present in 4 male patients. With regard to aneurysm morphology, 12 IA were saccular (67%) and the other 4 were fusiform. The most common location was the internal carotid artery (38%,7/18). The characteristics and location of IA are listed in A 1.
Treatment modalities
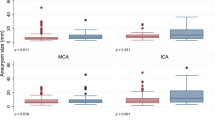
From all IA, 9 were treated by endovascular means and 9 with surgery. Concerning ruptured IA (n = 13), seven were treated by endovascular approach and six by surgical exclusion. The mean age was similar between the two groups of treatment, but when regarding the functional outcome criterion evaluated by the hunt and hess score, a non-statistically significant trend in favor of surgical treatment is recorded (2.1 versus 3.3 in embolization group; p = 0,47). With regard to unruptured IA treatment (n = 5), surgery was used in 3 cases (two clipping and one surgical bypass) and embolization for the two others (cases n°7 and 16). An endovascular approach was preferably used for all IA located in posterior circulation. Five out of 7 ICA aneurysms (71%) were treated by surgery and 3 out of 5 (60%) of MCA ones. The treatment of giant IA (n = 4) consisted in three endovascular treatments by coils (cases n°7, 8, and 16) and a surgical intracranial bypass for the biggest (case n°6). Compared with children with endovascular treatment, children in surgical group seemed older (13.8 versus 11.5 years old; p = 0,27) with smaller aneurysms (10.8 mm versus 16.3 mm; p = 0,65).
Three patients were treated for multiple IA (cases n°2, 3 and 10). In the case of n°2, the patient presented with bilateral IA on the posterior cerebellar arteries and with an aneurysm on the superior cerebellar artery. All these aneurysms were treated by simple coiling. In the case of n°3, the child had a mirror aneurysm on the right internal carotid artery, treated by a surgical approach. Finally, in case n°10 initially treated for a left ICA aneurysm, a middle cerebral artery was discovered and treated using surgical approach. In our institution, we have decided, if possible, to use the same treatment modalities in a context of multiple IA so as to simplify follow-up.
Outcomes
In the 13 children presenting with SAH, one died quickly after clipping and receiving medical care (case n°11). After IA treatment, six patients (6/13, 46%) developed hydrocephalus with the need for an external ventricular shunt. Two children required permanent shunt (cases n°1 and 6). Two children presented an early aneurysm recanalization after endovascular procedure (cases n°2 and 8) with rebleeding. In contrast to embolized aneurysms, we report complete exclusion without recanalization after surgical clipping at 1 year follow-up. We had no procedural morbidity in the endovascular group and one transient hemiparesis after surgical bypass for the treatment of giant IA (case n°6). Six children received calcium-blockers treatment for cerebral vasospasm (cases n°1 to 3 and 10 to 12) with good outcomes in all except one (case n°11). After a mean follow-up of 1 year, 2 patients were hemiplegic (cases n°8 and10), one presented a mutism (case n°2) and epilepsy (case n°3). Two children (cases n°1 and 7) had cognitive impairment. In total, the morbidity rate among survivors was of 35.3% (6/17). We did not note difference in morbidity with IA morphology and types of treatment. All children with unruptured IA had a GOS 5 except one (case n°6).
Discussion
Intracranial aneurysm occurring during childhood is very uncommon. Herein, we report the experience of two tertiary referral centers of this rare pathology and perform an updated literature review (Table 2). After applying the above-mentioned criteria, the literature review yielded 972 IA through 26 articles. The selected cases of pediatric IA and summarized results of these articles described in the methods section are presented in Table 2.
Demographic data
In our series, we observed a prevalence at onset of IA at early adolescence (mean age 12.6 years ± 5.3 years). These results are concordant with literature data in which the majority of the patients age range from 10 to 15 years old [22, 46]. The occurrence of IA in the neonatal period is exceptionally rare [32, 55] and the incidence of IA in children increases with age. In childhood, there is a male predominance in the onset of IA [28, 36] compared with adults’ series [22, 33]. The sex ratio is dependent of age: Lasjaunias et al. [28] have reported a sex ratio male/female of 3/2 in their entire cohort but below 2 years of age, this sex ratio female/male was of 5/1. In our series, 3 out of 18 patients presented with predisposing factors (Aortic valvulopathy, Noonan syndrome and high blood pressure). Unlike IA in adulthood, pediatric IA are more frequently related to infectious or traumatic context [22], collagen mutation syndrome [18, 35] or other vascular condition [1].
IA characteristics
Similarly to the recent literature [5, 27], the mean size of IA in our cohort was > 10 mm, and the incidence of giant IA is higher compared with adult series [25, 37] up to 30% of cases [29, 42]. We have observed in the literature [25, 46] a predominance of IA located in the anterior circulation, preferentially in the internal carotid artery (ICA) and the proportion of IA of the posterior circulation is higher compared with adult population (21% versus less than 5%) [33, 38]. The incidence of fusiform IA is higher compared with adult series (30% versus less than 15%) [5, 7]. The high prevalence of fusiform IA in children can be explained by the vascular conditions more frequently associated with pediatric IA [28].
Clinical presentation
Subarachnoid hemorrhage is the main presentation of pediatric IA accounting for more than 70% of clinical presentation, followed by headaches, seizures, and focal deficit [5, 23, 28]. The proportion of patients presenting with SAH is lower compared with adult series in which around 80–90% of IA are presenting with SAH [33]. Giant IA in pediatric population is responsible for mass effect and diagnosed with headaches [37]. Epileptic seizures in patients with IA are also more frequent in childhood compared with adulthood [15]. Two explanations are suggested: the immaturity of the cortex in children makes it prone to seizures and the larger proportion of IA in children, related to infectious processes [8, 13].
Treatment modalities
When pooling the results of our literature review concerning treatment modalities, it appeared that the management of saccular pediatric IA was divided between endovascular and surgical procedures in a balanced way but with huge discrepancies between centers [27, 28]. There is no recommendation concerning the management of pediatric IA between both surgical or endovascular procedures. Nevertheless, we have observed an increased incidence of endovascular procedures in our review compared with former studies performed before 2000 [47].
We believe that the factors influencing the choice of treatment should be the personal and team expertise, the age of the child, the location of IA, and clinical context. In the literature [20, 57], surgery is preferentially proposed for IA from the anterior circulation with wide necks. On the contrary, endovascular procedures are preferred in the case of narrow neck’ IA from the posterior circulation. In pediatric patients, IA are more frequently giants. Giants IA are prone to present residual circulation or recanalization with endovascular procedures. As a consequence, younger children present a higher risk of long-term recanalization. In the work from Amelot et al. [5], the risk of annual IA recurrence is estimated around 2.6%. By definition, due to their age, children experience a higher risk of recanalization compared with adults.
We also note specificities in children cerebral circulation. The total cerebral blood-flow and vascular velocities are increased compared with adults which could lead to IA genesis. This is particularly true before 7 years of age and decline after [52, 57]. It has been shown [11, 12] that flow-diverter may increase the pressure in IA, particularly in the case of giants IA and could lead to IA bleeding. As children are prone to present with giants IA, and due to small caliber of the vessels, the use of flow-diverter is at higher risk in children. Moreover, in neonates, most IA concern the distal circulation, mainly the MCA artery, and are responsible for a higher rate of ICH compared with adults. This could emphasize the role of surgical treatment in this population [32].
Outcomes
In our series, 6 children (46%) developed a cerebral vasospasm. Evolution was good for 5 of them (83%) after medical treatment. The reported incidence of vasospasm following SAH in children is around 25% [43, 44] but the impact of vasospasm is minor in children compared with adult population [4] with good clinical outcomes even in the absence of medical treatment. This is due to the existence of peripheral recirculation in children. As regards vasospasm treatment, the use of nimodipine following SAH is based on adult’s series. Few series [21, 45] have reported their experience with discordant results and risk of hypotension. As a consequence, nimodipine in child can be discussed as a case by case situation and is not a standard of care. Another management of vasospasm is the use of mechanic balloon angioplasty [43]. In our study, no patient received this treatment.
Outcomes appeared favorable in the literature in up to two thirds of the patients [5, 27, 36]. Outcome was determined by the clinical status at admission, severity of the hemorrhage, age of the patients, and type of IA [22, 36]. The mortality ranges from 1.3 to 28% [22, 23] reflecting the large heterogeneity between cohorts and pathologies. Unfavorable outcomes were mostly in neonates and patients with giant or dissecting IA [28, 32, 37]. On the contrary, when matched with clinical status at admission, outcomes in pediatric patients appeared better compared with adult population [25, 27, 35].
Pediatric specificities
The characteristics of our cohort are similar to data from the literature but differ from IA in adult population. Although women are at higher risk of aneurysm formation in adult population [12], pediatric IA are more often found in boys with an average sex ratio M/F of 1.4:1. One explanation is the role of female hormone in IA genesis which appears to be a risk factor of IA [12]. Then, this could explain why, before puberty, females are not at risk of IA compared with boys. On the contrary, some conditions predisposing to IA in children may be overrepresented in male populations.
The mechanisms leading to aneurism development in adults were not very well known, but we can expose hypothesis to explain differences between IA in children and adults [12]. First, risk factors in adults are mainly atherosclerosis due to high blood pressure, smoking, or alcohol exposure. In children, IA are more frequently due to dissection or predisposing condition such as connective tissue mutations, explaining the higher prevalence of fusiform or giant IA [9]. Another condition predisposing to IA are mycotic IA which are more frequently found in pediatric population. The treatment and presentation of these IA differ from classical IA.
Concerning clinical features, a significant part of pediatric IA are diagnosed in the presence of headaches, most often in a context of giant IA. We also note that, in Krishna et al. [25], epileptic seizure is significantly more frequent in childhood compared with adulthood, maybe as neural networks are not totally mature. In the same way, children experience better outcomes compared with adults with the same initial status, certainly due to brain plasticity in children.
Regarding location [17, 25], IA are mostly on ICA in children whereas the main location is ACoA in adults. We also find more IA on posterior circulation in pediatric population compared with adults, and the same observation goes for fusiform aspect. Although there are more fusiform IA and more posterior location in childhood, treatment is divided between surgical clipping and embolization. Yet, endovascular treatment is, today, predominant in most centers in adult population. For example, Gawlitza et al. [17] have reported a 77.2% embolization rate. Nevertheless, pediatric IA is a separate entity and treatment modalities may differ from adults. Firstly, the development of endovascular techniques is relatively recent and therefore, due to the low incidence of pediatric IA, large series are often surgical. Secondly, due to the small size of the vessels, there is an increased risk of perforation or thrombosis in pediatric patients. Then, the risk of recanalization is higher in pediatric patients. Childhood is a population particularly vulnerable to recanalization due to an early treatment and a higher life expectancy [58]. In our study, 2 of the 9 children (22%) treated by embolization had an IA recanalization. The risk of recanalization is lower for surgery compared with endovascular techniques as reported by Costa et al. [14] (2 vs. 27%; P = 0.0008). IA recanalization requires new treatment in order to avoid a rebleeding. All these reasons could explain the lower use of endovascular treatment in pediatric population compared with adult population.
Conclusion
Intracranial aneurysm in pediatric patients remains a rare condition distinct from those in adults. Population characteristics are age related with a majority of male patients with IA after the age of 3 years old. The clinical presentation is mostly intracranial hemorrhage and headaches. Aneurysms are more frequently large or giant and located in the internal carotid artery. As regards treatment, we may encounter age-related difficulties in this very diverse population. Surgical procedure remains a therapeutic option with good outcomes in two-third of surgically treated pediatric aneurysm patients. Due to its scarcity, a multidisciplinary discussion is the cornerstone of the management of pediatric IA.
Abbreviations
- AC:
-
anterior circulation
- ACA:
-
Anterior cerebral artery
- ACoA:
-
anterior communicating artery
- CT:
-
Computerized tomography
- F:
-
female
- GO:
-
good outcomes (Glasgow Outcomes Scale ≥ 4)
- GOS:
-
Glasgow Outcomes Scale
- HH grading:
-
Hunt and Hess grading
- IA:
-
Intracerebral aneurysm
- ICA:
-
Internal carotid artery
- ICH:
-
intracerebral hematoma
- IVH:
-
intraventricular hemorrhage
- M:
-
male
- MCA:
-
Middle cerebral artery
- mm:
-
millimeters
- PO:
-
posterior circulation
- PCA:
-
posterior cerebral artery
- PICA:
-
Postero-inferior cerebelar artery
- SAH:
-
Subarachnoid hemorrhage
- y:
-
years
References
Agid R, Jonas Kimchi T, Lee S-K, Ter Brugge KG (2007) Diagnostic characteristics and Management of Intracranial Aneurysms in children. Neuroimaging Clin N Am 17(2):153–163
Agid R, Souza MPS, Armstrong D, Dirks P (2005) The Role of Endovascular Treatment for Pediatric Aneurysms. Childs Nerv Syst 21(12):1030–1036
Alawi A, Edgell RC, Elbabaa SK, Callison RC, Khalili YA, Allam H, Alshekhlee A (2014) Treatment of cerebral aneurysms in children: analysis of the kids’ inpatient database. J Neurosurg Pediatr 14(1):23–30
Amacher AL, Drake CG, Ferguson GG (1981) Posterior circulation aneurysms in young people. Neurosurgery 8:315–320
Amelot A, Saliou G, Benichi S, Alias Q, Boulouis G, Zerah M, Aghakhani N, Ozanne A, Blauwblomme T, Naggara O (2019) Long-term outcomes of cerebral aneurysms in children. Pediatrics 143(6):e20183036
Barburoglu XM, Arat XA (2017) Flow Diverters in the Treatment of Pediatric Cerebrovascular Diseases. Am J Neuroradiol 38(1):113–118
Barletta E, Ricci R, Silva RG, Gaspar RML, Araújo JM, Neves MF, de Aquino JB, Barba Belsuzarri T (2018) Fusiform aneurysms: a review from its pathogenesis to treatment options. Surg Neurol Int 9(1):189
Bell AH, Miller SL, Castillo-Melendez M, Malhotra A (2020) The neurovascular unit: effects of brain insults during the perinatal period. Front Neurosci 13:1452
Boulouis G, Blauwblomme T, Hak JF, Benichi S, Kirton A, Meyer P, Chevignard M, Tournier-Lasserve E, Mackay MT, Chabrier S, Cordonnier C, Kossorotoff M, Naggara O (2019) Nontraumatic pediatric Intracerebral hemorrhage. Stroke 50(12):3654–3661
Bowers C, Riva-Cambrin J, Couldwell WT (2012) Efficacy of Clip-Wrapping in Treatment of Complex Pediatric Aneurysms. Childs Nerv Syst 28(12):2121–2127
Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, Putman CM (2011) Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. Am J Neuroradiol 32(1):27–33
Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS (2012) Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 32(9):1659–1676
Chen R, Ren Y, Zhang S, You C, Liu Y (2018) Radiologic characteristics and high risk of seizures in infants with ruptured intracranial aneurysms: case report and review of the literature. World Neurosurg 118:e772–e777
Costa E, Vaz G, Finet P, Goffette P, Docquier M, Raftopoulos C (2019) Recanalization and rupture after intracranial aneurysm treatment. J Neurosug Sci 63(5):518–524
Deora H, Rao KVLN, Somanna S, Srinivas D, Shukla DP, Bhat DI (2017) Surgically managed pediatric intracranial aneurysms: how different are they from adult intracranial aneurysms? Pediatr Neurosurg 52(5):313–317
Fulkerson DH, Voorhies JM, Payner TD, Leipzig TJ, Horner TG, Redelman K, Cohen-Gadol AA (2011) Middle Cerebral Artery Aneurysms in Children: Case Series and Review: Clinical Article.J Neurosurg Pediatr 8(1):79–89
Gawlitza M, Soize S, Barbe C, le Clainche A, White P, Spelle L, et al (2019) Aneurysm characteristics, study population, and endovascular techniques for the treatment of intracranial aneurysms in a large, prospective, multicenter cohort: results of the analysis of recanalization after endovascular treatment of intracranial aneurysm study. Am J Neuroradio l40(3):517–523
Gemmete JJ, Toma AK, Davagnanam I, Robertson F, Brew S (2013) Pediatric cerebral aneurysms. Neuroimaging Clin N Am 23(4):771–779
Goia A, Garrido E, Lefebvre M, Langlois O, Derrey S, Papagiannaki C, Gilard V (2020). Ruptured intracranial aneurysm in a neonate: case report and review of the literature. World Neurosurg 140:219–223
Hayakawa M, Murayama Y, Duckwiler GR, Pierre Gobin Y, Guglielmi G, Viñuela F (2000) Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg 93(4):561–568
Heffren J, McIntosh AM, Reiter PD (2015) Nimodipine for the prevention of cerebral vasospasm after subarachnoid hemorrhage in 12 children. Pediatr Neurol 52(3):356–360
Hetts SW, Narvid J, Sanai N, Lawton MT, Gupta N, Fullerton HJ, Dowd CF, Higashida RT, Halbach VV (2009) Intracranial aneurysms in childhood: 27-year single-institution experience. Am J Neuroradiol 30(7):1315–1324
Huang J, McGirt MJ, Gailloud P, Tamargo RJ (2005) Intracranial aneurysms in the pediatric population: case series and literature review. Surg Neurol 63(5):424–432
Kakarla UK, Beres EJ, Ponce FA, Chang SW, Deshmukh VR, Bambakidis NC, Zabramski JM, Spetzler RF (2010) Microsurgical treatment of pediatric intracranial aneurysms: long-term angiographic and clinical outcomes. Neurosurgery 67(2):237249
Krishna H, Wani AA, Behari S, Banerjee D, Chhabra DK, Jain VK (2005) Intracranial aneurysms in patients less than or equal to 18 years:are they different from aneurysms in adult population? Acta Neurochirurgica (Wein) 147:469–476
Koroknay-Pál P, Laakso A, Lehto H, Seppä K, Kivisaari R, Hernesniemi J, Niemelä M (2012) Long-term excess mortality in pediatric patients with cerebral aneurysms. Stroke 43(8):2091–2096
Koroknay-Pál P, Lehto H, Niemelä M, Kivisaari R, Hernesniemi J (2012) Long-term outcome of 114 children with cerebral aneurysms. J Neurosurg Pediatr 9(6):636–645
Lasjaunias P, Wuppalapati S, Alvarez H, Rodesch G, Ozanne A (2005) Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst 21(6):437–450
Liang J, Bao Y, Zhang H, Wrede KH, Zhi X, Li M, Ling F (2009) The clinical features and treatment of pediatric intracranial aneurysm. Childs Nerv Syst 25(3):317–324
Lv X, Jiang C, Li Y, Yang X, Wu Z (2009) Endovascular Treatment for Pediatric Intracranial Aneurysms. Neuroradiology 51(11):749–754
Mehrotra A, Nair AP, Das KK, Srivastava S, et al. (2012) Clinical and Radiological Profiles and Outcomes in Pediatric Patients with Intracranial Aneurysms. J Neurosurg Pediatr 10(4):340–346
Mohotti JE, Carter NS, Zhang VJW, Lai LT, Xenos C, Asadi H, Chandra RV (2018) Neonatal intracranial aneurysms: case report and review of the literature. J Neurosurg Pediatr 21(5):471–477
Molyneux A, Group ISAT (ISAT) C et al (2002) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360(9342):1267–1274
Park S-H, Yim M-B, Lee C-Y, Kim E, Son E-I (2008) Intracranial fusiform aneurysms: It’s pathogenesis, clinical characteristics and managements. J Korean Neurosurg Soc 44(3):116–123
Pope FM, Kendall BE, Slapak GI, Kapoor R, McDonald WI, Compston DAS, Mitchell R, Hope DT, Millar-Craig MW, Dean JCS, Johnston AW, Lynch PG, Sarathchandra P, Narcisi P, Nicholls AC, Richards AJ, Mackenzie JL (1991) Type III collagen mutations cause fragile cerebral arteries. Br J Neurosurg 5(6):551–574
Proust F, Toussaint P, Garniéri J, Hannequin D, Legars D, Houtteville J-P, Fréger P (2001) Pediatric cerebral aneurysms. J Neurosurg 94(5):733–739
Pruvot A-S, Curey S, Derrey S, Castel H, Proust F (2016) Giant intracranial aneurysms in the paediatric population: suggested management and a review of the literature. Neurochirurgie 62(1):20–24
Regelsberger J, Heese O, Martens T, Ries T, Kunkel P, Westphal M (2009) Intracranial aneurysms in childhood: report of 8 cases and review of the literature. Cent Eur Neurosurg 70(02):79–85
Requejo F, Ceciliano A, Cardenas R, Villasante F, Jaimovich R,Zuccaro G (2010) Cerebral Aneurysms in Children: Are We Talking about a Single Pathological Entity? Childs Nerv Syst 26(10):1329–1335
Saleh E, Dawson RC 3rd (2011). Endovascular Management of Pediatric Aneurysms. Neuroradiol J 24(5):693–698
Saraf R, Shrivastava M, Siddhartha W, Limaye U (2012) Intracranial pediatric aneurysms: endovascular treatment and its outcome. J Neurosurg Pediatr 10(3):230–240
Sharma BS, Sinha S, Mehta VS, Suri A, Gupta A, Mahapatra AK (2007) Pediatric intracranial aneurysms—clinical characteristics and outcome of surgical treatment. Childs Nerv Syst 23(3):327–333
Snelling BM, Sur S, Shah SS, Peterson EC (2017) Treatment of Cerebral Vasospasm in an Infant Using a Modified Dotter Technique. J Cerebrovasc Endovasc Neurosurg 19(1):48
Skoch J, Tahir R, Abruzzo T, Taylor JM, Zuccarello M, Vadivelu S (2017) Predicting symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage with an artificial neural network in a pediatric population. Childs Nerv Syst 33(12):2153–2157
Song Y, Qian SY, Li Y, Liu J, Li Z, Jia XL, Gao HM, Zeng JS (2019) Effectiveness and safety of nimodipine in preventing cerebral vasospasm after subarachnoid hemorrhage in children. Zhonghua Er Ke Za Zhi 57(5):338–343
Sorteberg A, Dahlberg D (2013) Intracranial non-traumatic aneurysms in children and adolescents. Curr Pediatr Rev 9(4):343–352
Stergaard JR (1983) Intracranial arterial aneurysms in children and adolescents. J Neurosurg 58:6
Stiefel MF, Heuer GG, Basil AK, Weigele JB, Sutton LN, Hurst RW, Storm PB (2008) Endovascular and surgical treatment of ruptured cerebral aneurysms in pediatric patients. Neurosurgery 63(5):859–866
Sughrue ME, Saloner D, Rayz VL, Lawton MT (2011) Giant intracranial aneurysms: evolution of Management in a Contemporary Surgical Series. Neurosurgery 69(6):1261–1271
Takemoto K, Tateshima S, Golshan A, Gonzalez N, Jahan R, Duckwiler G, Vinuela F (2014) Endovascular Treatment of Pediatric Intracranial Aneurysms: A Retrospective Study of 35 Aneurysms. J Neurointerv Surg 6(6):432–438
Tatli M, Guzel A, Kilincer C, Goksel HM (2008) Pediatric cerebral aneurysms: A report of 9 cases. Cerebral vasospasm 104:411–414
Udomphorn Y, Armstead WM, Vavilala MS (2008) Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol 38(4):225–234
Vargas SA, Diaz C, Herrera DA, Dublin AB (2016) Intracranial Aneurysms in Children: The Role of Stenting and Flow-Diversion. J Neuroimaging 26(1):41–45
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YBWEM (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41(10):2391–2395
Vizcaíno-Díaz C, Sánchez-Zaplana H, Carneado Ruiz J, Jiménez-Cobo B (2009) Rupture of intracranial arterial aneurysms in neonates: case report and review of the literature. J Child Neurol 24(2):208–214
Wojtacha M, Bażowski P, Mandera M, Krawczyk I, Rudnik A (2001) Cerebral Aneurysms in Childhood. Childs Nerv Syst 17(1–2):37–41
Wu C, Honarmand AR, Schnell S, Kuhn R, Schoeneman SE, Ansari SA, Carr J, Markl M, Shaibani A (2016) Age-Related Changes of Normal Cerebral and Cardiac Blood Flow in Children and Adults Aged 7 Months to 61 Years. J Am Heart Assoc 5(1):e002657
Yang M, Wang S, Zhao Y, Zhao J (2008) Management of Intracranial Aneurysm in children: clipped and coiled. Childs Nerv Syst 24(9):1005–1012
Yasin JT, Wallace AN, Madaelil TP, Osbun JW, Moran CJ, Cross DT, Limbrick DD, Zipfel GJ, Dacey RG, Kansagra AP (2018) Treatment of Pediatric Intracranial Aneurysms: Case Series and Meta-Analysis. J Neurointerv Surg 11(3):257–264
Zhang Y-S, Wang S, Wang Y, Tian Z-B, Liu J, Wang K, Chen J-F, Yang X-J (2016) Treatment for Spontaneous Intracranial Dissecting Aneurysms in Childhood: A Retrospective Study of 26 Cases. Front Neurol 7:224
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garrido, E., Metayer, T., Borha, A. et al. Intracranial aneurysms in pediatric population: a two-center audit. Childs Nerv Syst 37, 2567–2575 (2021). https://doi.org/10.1007/s00381-021-05151-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05151-6