Abstract
Introduction
We report three cases of brain malformation presenting with a midline mass of dysplastic cortex that we have termed “brain in brain” malformation.
Results
The three cases have holoprosencephalic features, including bilateral hemispheric continuity across the midline, single ventricle, midline facial defect and missing olfactory bulbs. All three cases have a midline conglomerate mass of deeply infolded, cortex-lined fissures with major arterial branches, heterotopia and large amount of white matter. The dysplastic mass of cortex and white matter extended into the third ventricle. The cortex and white matter of the dysplastic lesion was continuous with the cortex and white matter, respectively, of the cerebral hemispheres.
Conclusion
The midline “brain in brain” malformations have some similarities to subcortical heterotopia and extracerebral glioneuronal heterotopia. However, the continuity with the cerebral hemispheres and extension into the ventricle were not reported in subcortical or glioneuronal heterotopia. The common involvement of the midline cortex and extension into the third ventricle implied an anterior segmental prosencephalic abnormality (prosomeres 5/6). However, its pathogenesis remains to be explained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Various classification systems have been proposed to assist in our understanding of the multitude of brain malformations. Since the advent of magnetic resonance (MR) imaging, these classifications have relied heavily on the features depicted by neuroimaging. Recently, there is a trend towards incorporating this knowledge into comprehensively developing classification systems, which include knowledge on embryology, genetics and pathology. Despite the great strides made in our understanding, some malformation do not fall into any of the currently available classification systems, and therefore, raise new questions about their development. We describe three cases of such a gross malformation of the brain that does not fit into any of the currently proposed classification scheme. All these cases have a large conglomerate mass of abnormal convolutions consisting of gray and white matter, thereby, giving the appearance of “brain in brain” malformation.
Case presentation
Case 1
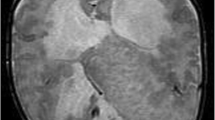
A 7-month-old girl presented with developmental delay and spasticity. The head circumference was within normal limits. MR brain demonstrated overconvoluted cortex lined with white matter in the midline, forming an interhemispheric conglomeration of abnormal brain parenchyma that was continuous with the cortex of both cerebral hemispheres, laterally, and bulged into a single ventricular cavity, posteriorly. It was also continuous with the left basal ganglia, anteriorly, and with the walls of the anterior third ventricle, inferiorly. There was incomplete cleavage of the thalami. The anterior interhemispheric fissure and falx were well formed, but behind the mass, there was a single ventricular cavity with a dorsal sac and no normal midline structures. The right olfactory nerve and olfactory sulcus were absent. Both hippocampi were small. The right hemispheric cortex was grossly distorted with loss of the normal gyral arrangement and lobar segmentation. The left hemispheric pattern was grossly normal. On MR angiography, the midline mass of gray and white matter was fed by an azygos anterior cerebral artery. Single voxel MR spectroscopy acquired from the parenchyma of the abnormal right hemisphere demonstrated normal N-acetyl group, choline and creatine spectrum. The brainstem and cerebellum were grossly normal (Fig. 1).
Case 1. Seven-month old, developmental delay, spasticity. a Axial T2. The large interhemispheric cortical dysplasia is continuous with both hemispheres, and the lesion bulges into a large dorsal cyst. There is extensive dysplasia of the right hemisphere as well. b Sagittal T1. The lesion is clearly demarcated from the frontal cortex and extends downward to the third ventricle, replacing its normal features. c Coronal T2. The midline dysplastic mass is continuous with both hemispheres as well as with the left lentiform nucleus. d Axial T1. There is failure of cleavage of the thalami
Case 2
A 6-day-old neonate presented with developmental delay, seizures and hypotonia. There was also cleft lip and palate and a blind ending left nare. The child had panhypopituitarism. MR brain showed a midline mass of dysplastic cortex with irregular convolutions extending from one hemisphere to the other across the anterior part of the fissure. Multiple islands of gray matter heterotopia occupy the white matter of the mass. The midline mass of dysplastic cortex was continuous with the hemispheric cortex, bilaterally. It extended down to the anterior aspect of the third ventricle and herniated partially through a large sphenoid defect. The basal ganglia and thalami were displaced laterally. On each side, the mass of abnormal cortex appeared clearly demarcated from the more normal-looking cortex of the lateral and posterior aspect of the hemispheres by a deep fissure. The falx was deficient, anteriorly, and there was failure of formation of interhemispheric commissuration, anteriorly. However, more posteriorly, the falx was intact, and the splenium of the corpus callosum was present. The anterior aspect of the lateral ventricles was either markedly hypoplastic or failed to form. Posteriorly, the body and trigones of the lateral ventricles were present and formed a single cavity with the posterior part of the third ventricle. Both olfactory nerves were absent. Both hippocampi were small and the left hippocampus was malrotated. The brainstem and cerebellum were grossly normal (Fig. 2).
Case 2. Six-day-old neonate, poor development, seizures, hypotonia, midline facial defects and panhypopituitarism. a Axial T2. The large frontal mass of dysplastic cortex has well defined fissures laterally and is clearly demarcated from the cortex posteriorly. The continuity of the cortex across the midline is evident. b Sagittal T2. Multiple islands of gray matter heterotopia are seen within the white matter of the mass. The lesion occupies a large part of the third ventricle and extends more inferiorly through the craniopharyngeal canal to form the basal sphenoidal encephalocele. c Coronal T2. The midline dysplastic cortex is continuous with the gray and white matter of the cerebral hemispheres. d Axial T1. The mass obliterates the single ventricular cavity; there is a splenium-like structure posteriorly
Case 3
A 2-day-old neonate was referred with hydrocephalus and bilateral cleft palate, frontonasal cutaneous angioma, hypertelorism, low implanted ears and right posterior plagiocephaly. On MR, there was excessive infolding of a midline mass of dysplastic cortex located between and continuous with the cortex of both cerebral hemispheres. The lesion displaced the deep gray matter, laterally, and the thalami could not be distinguished from the basal ganglia. The dysplastic mass extended into the third ventricle and was continuous anteriorly with the third ventricular walls. The interhemispheric fissure was recognized both anteriorly and posteriorly, and the fissure extended anteriorly to the dysplastic cortical mass. The markedly enlarged lateral ventricles were lined by a thin, malformed cerebral mantle; the cerebral aqueduct was dilated and deformed. The interhemispheric commissures were absent. The brainstem and cerebellum were grossly normal and the cerebellar tonsils were herniated inferiorly. MR performed 4 months later after shunting demonstrated no significant change in the brain morphology (Fig. 3).
Case 3. Two-day-old neonate with hydrocephalus, dysmorphic features and midline facial defects. a Axial T1. The large interhemispheric dysplastic mass is continuous with the cerebral hemispheres. There is severe bilateral hydrocephalus with thin, abnormal hemispheric cortex, especially posteriorly. b Sagittal T1. The large midline, clearly demarcated convoluted mass involves most of the third ventricle. c Coronal T1 post-contrast. The interhemispheric dysplastic mass does not enhance. d Sagittal T2 at 4 months of age. The involvement of the third ventricular structure is well demonstrated
Discussion
Rather than a longitudinal division in diencephalic and telencephalic vesicles, modern embryology proposes that the forebrain (prosencephalon) develops as a single structure with ventrodorsal patterning (ventral and dorsal forebrain) and longitudinal segmentation (prosomeres 1 to 6 from caudal to cranial) [1, 2]. Defective regionalization of the forebrain is thought to be due to an initial failure of the prechordal plate to induce the overlying neuro-ectoderm to become the ventral forebrain, in the first 4 weeks of gestation [3–5]. This failure of regionalization of the forebrain results in holoprosencephaly (HPE). In fish and chick, it has been found that the ventral forebrain is originally located caudal to the dorsal forebrain and that the secondary rostral movement of the ventral forebrain cells divides, and displaces laterally, the early optic and hemispheric primordia [6]. Dorsoventral patterning is regulated by a variety of molecules, mainly, sonic hedgehog (Shh) and bone morphogenetic proteins (BMP). Animals with mutations in Shh fail to induce ventral markers and cell types, and therefore, lack ventral forebrain development [7–9]. Shh also plays a major role in the early expansion of the midbrain and forebrain [10] and on the craniofacial neural crest [11] which will form the midline facial structures, and in turn, have a trophic effect on the forebrain [12]. BMP participates in dorsal patterning of the forebrain [2, 13, 14]; ectopic localization of BMP in chick embryo results in decreased expression of ventral forebrain [15].
The three cases presented here have severe anomalies involving the prosencephalon. Undivided forebrain was present in all, with failure of cleavage of the thalami in case 1. Therefore, they can be best described as variants of HPE. Classically, HPE was classified into alobar, semilobar and lobar HPE depending on the degree of severity [16, 17]; midline facial defects ranging from cyclopia to minimal cleft or single incisor were associated with the most severe cases (“face predicts the brain”). In fact, causes for poor differentiation of the forebrain are multiple and diverse (chromosomal, genetic but also cholesterol metabolism or maternal diabetes), and various phenotypes can be encountered that do not really fit such a simple classification [18–20].
Our cases all present major features of HPE: cortical continuity across the midline for all (cases 1–3), azygos anterior cerebral artery (case 1), single ventricular cavity (case 1 and 2), undivided thalami (case 1), missing olfactory bulbs (cases 1 and 2), midline facial abnormalities (cases 2 and 3), panhypopituitarism (case 2) possibly explained by a basal encephalocele. But they all differ from the reported appearance of HPE by the presence of a midline conglomeration of overconvoluted cortex located at the midline continuity between the hemispheres. We have called this feature “brain in brain malformation” because of its appearance (Figs. 1, 2, and 3). In all patients, this cortical hyperplasia was not restricted to the hemispheres but extended to, and involved, most of the third ventricle.
In all three cases, the midline cortical mass demonstrated similar MR features: it was clearly demarcated by a fissure from the adjacent, more normal-looking hemispheric cortex (anterior medial cortex in cases 1 and 3, lateral cortex in case 2). It extended into the third ventricle, obliterating most of it in all three cases. Structurally, it contained both gray and white matter. The gray matter was either cortex-like or formed gray matter heterotopia. The cortex of the dysplastic lesion was continuous with the cortex of both hemispheres. The white matter also was continuous with the hemispheric white matter on both sides. The dysplasia was penetrated by well-defined, invaginated fissures that were lined with a cortical ribbon and contained major arterial branches. In case 1 and 2, the mass bulged posteriorly into a single prosencephalic ventricle and obliterated a significant proportion of the ventricular lumen; in case 3, it completely obliterated the ventricular space in the midline. This hyperplastic corticalization extended downward to involve the anterior third ventricle; it is, therefore, a truly prosencephalic abnormality. As it abutted the medial aspect of the basal ganglia and thalami, it was also strictly a midline malformation.
The “brain in brain” malformation demonstrated some similarities with the subcortical heterotopia. However, it fits none of the curvilinear, nodular or mixed types described [21]. Also, subcortical heterotopia are typically associated with expanded, not obliterated ventricles; they are within the cerebral mantle and do not present such a clear infolded, almost invaginated appearance. In HPE with undivided forebrain, midline location of the dysplasia is remarkable. Subcortical heterotopia have already been reported in HPE [22]; they were large, located across the midline anterior to the anterior termination of the interhemispheric fissure and were connected to the cortex lining the anteriormost interhemispheric fissure and to the frontal cortex [22]. However, these heterotopia were not described as masses obliterating the ventricles and did not involve the anterior third ventricle, as in our cases. In addition, in two of our cases, the mass was actually located behind the well developed anterior interhemispheric fissure.
Rare intracranial extracerebral glioneuronal heterotopia have been reported [23–27]. They were predominantly located in the anterior or middle cranial fossae. They were thought to arise from the leptomeninges, and about 62% of these develop in the base of the brain [28]. The pathogenesis was uncertain (for review, see [27]). Pathological studies have demonstrated mature but disorganized brain tissue composed of both gray and white matter [26]. Histologically, the gray matter presented features of cortical dysplasia and disruption of the normal laminar architecture. We do not have pathological correlation in our cases, but it is likely that the histological features would have been similar. MR spectroscopy acquired from the lesion in case 1 demonstrated normal spectrum; previous studies on MR spectroscopy of cortical malformations have found normal spectrum in gray matter heterotopia [29].
Hypothalamic hamartomas are non-evolving masses of heterotopic gray matter resembling the hypothalamus and developed from the tuber cinereum. They may extend dorsally beyond the chiasm, up to the lamina terminalis [30]. A “proliferative-type” dysplasia of the posterior cortex has been reported in association with the hamartoma in one case, but it was not midline, and not in continuity with the hamartoma [30]. Solid, or less frequently, cystic hypothalamic hamartomas contain no white matter; however one case of brain-looking mass in the hypothalamus, with mixed gray and white matter layerings, was operated in a neonate and pathologically identified as a hamartoma (unpublished, personal communication).
Finally, there was an autopsy report of a neonate with HPE and hamartomatous growth of the cerebrum [31]. The authors described fusion of the interhemispheric fissure and partial fusion of the left frontal and temporal lobes, a wide fissure distorting the temporal lobe, absence of the ventricles and lack of clear distinction between the cortical gray and white matter. Histopathology showed ectopic and hypoplastic ependymal cells arranged as tubules or rosettes within the temporo-occipital cortex and disorganization of the cellular layer. This neonate also had dysplastic gangliocytoma of the cerebellum. The description of the macroscopic appearance of the autopsy findings has similarities to case 1, with failure to visualize the right lateral ventricle and abnormal fissuration of the right hemisphere. However, there was no extension of the abnormality to the third ventricle, and the gray matter and white matter were not as well segregated as they were in our cases.
The pathogenesis of subcortical heterotopia is not clear. They are assumed to result from the arrested migration of normal pyramidal neurons along their migration path between the germinal matrix of the mantle and the cortical plate. A defect of FLN1 gene on Xq28 (coding for Filamin A) has been demonstrated in some [32, 33], but not in all cases of periventricular heterotopia, with some phenotypic specificity [33]. In our cases, the location of the “brain in brain” malformation associated with HPE is in the anterior midline, even if the continuous growth of the expanding hemispheres has displaced the frontal lobes anterior to it; this location is intriguing. The downward extension of the lesion to the anterior third ventricle down to the chiasm implies “corticalization” of the basal forebrain. This extension is particularly surprising as the lamina terminalis, the site of the anterior neuropore closure, is not expected to behave as the cortical mantle. However, as the basal forebrain has not migrated forward to separate the hemispheres, the anterior neuropore might be more posterior than expected. The anterior midline location of the malformation encompassing both basal and dorsal forebrain matches the topography of the most cranial prosomeres 5/6; the “brain in brain” malformation could, therefore, represent a segmental prosencephalic cortical malformation associated with holoprosencephaly.
References
Rubenstein JLR, Martinez S, Shimamura K, Puelles L (1994) The embryonic vertebrate forebrain: the prosomeric model. Science 266:578–580
Rubenstein JLR, Shimamura K (1998) Regionalization of the prosencephalic neural plate. Annu Rev Neurosci 21:445–477
Li H, Tierney C, Wen L, Wu JY, Rao Y (1997) A single morphogenetic field gives rise to two retinal primordia under the influence of the prechordal plate. Development 124:603–615
Dale JK, Vesque C, Lints TJ, Sampath TK, Furley A, Dodd J et al (1997) Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90:257–269
Muller F, O’Rahilly R (1989) Mediobasal prosencephalic defects, including holoprosencephaly and cyclopia, in relation to the development of the human forebrain. Am J Anat 185:391–414
Wilson SW, Houart C (2004) Early steps in the development of the forebrain. Development 6:167–181
Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T (1995) Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell 81:747–756
MacDonald R, Barth K, Xu Q, Holder N, Mikkola I, Wilson S (1995) Midline signaling is required for Pax gene regulation and patterning of the eyes. Development 121:3267–3278
Chiang C, Litingtung Y, Lee E, Young K, Corden J, Westphal H et al (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413
Britto J, Tannahill D, Keynes R (2002) A critical role for sonic hedgehog signaling in the early expansion of the developing brain. Nat Neurosci 5:103–110
Ahlgren SC, Bonner-Fraser M (1999) Inhibition of Sonic hedgehog signaling in vivo results in craniofacial neural crest death. Curr Biol 9:1304–1314
Etchevers HC, Couly G, Vincent C, Le Douarin NM (1999) Anterior cephalic neural crest is required for forebrain viability. Development 126:3533–3543
Furuta Y, Piston DW, Hogan BL (1997) Bone morphogenetic proteins (BMP) as regulators of dorsal forebrain development. Development 124:2203–2212
Shimamura K, Rubenstein JLR (1997) Inductive interactions direct early regionalization of the mouse forebrain. Development 124:2709–2718
Golden J, Bracilovic A, McFadden K, Beesley J, Rubenstein J, Grinspan J (1999) Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain leads to cyclopia and holoprosencephaly. Proc Natl Acad Sci USA 96:2439–2444
DeMyer W, Zeman W (1963) Alobar holoprosencephaly (arhinencephaly) with median cleft lip and palate: clinical, nosologic and electroencephalographic considerations. Confin Neurol 23:1–36
DeMyer W, Zeman W, Palmer CG (1964) The face predicts the brain: diagnostic significance of median facial anomalies for holoprosencephaly (arhinencephaly). Pediatrics 34:256–23
Roessler E, Muenke M (1999) The molecular genetics of holoprosencephaly: a model of development for the next century. Childs Nerv Syst 15:646–651
Cohen MM (2001) Problems in the definition of holoprosencephaly. Am J Med Genet 103:183–187
Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA (2004) Temporal perturbations on sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest 114:485–494
Barkovich AJ, Simon EM, Clegg NJ, Kinsman SL, Hahn JS (2002) Analysis of the cerebral cortex in holoprosencephaly with attention to the sylvian fissures. AJNR Am J Neuroradiol 23:143–150
Barkovich AJ (2000) Morphologic characteristics of subcortical heterotopia: MR imaging study. AJNR Am J Neuroradiol 21:290–295
Marubayashi T, Matsukado Y (1978) Intracranial extracerebral brain heterotopia. Case report. J Neurosurg 48:470–474
Nishio S, Mizuno J, Barrow DL, Takei Y, O’Brien MS (1988) Intracranial extracerebral glioneural heterotopia. Childs Nerv Syst 4:244–248
Harris CP, Townsend JJ, Klatt EC (1994) Accessory brains (extracerebral heterotopias): unusual prenatal intracranial mass lesions. J Child Neurol 9:386–389
Gyure KA, Morrison AL, Jones RV (1999) Intracranial extracerebral neuroglial heterotopia: a case report and review of the literature. Ann Diagn Pathol 3:182–186
Muzumdar D, Michaud J, Ventureyra ECG (2006) Anterior cranial base glioneuronal heterotopia. Childs Nerv Syst 22:227–233
Hirano S, Houdou S, Hasegawa M, Kamei A, Takashima S (1992) Clinicopathologic studies on leptomeningeal glioneuronal heterotopia congenital anomalies. Pediatr Neurol 8(6):441–444
Widjaja E, Griffiths PD, Wilkinson ID (2003) Proton MR spectroscopy of polymicrogyria and heterotopia. AJNR Am J Neuroradiol 24(10):2077–2081
Freeman JL, Coleman LT, Wellard RM, Kean MJ, Rosenfeld JV, Jackson GD, Berkovic SF, Harvey AS (2004) MR imaging and spectroscopic study of epileptogenic hypothalamic hamartomas: analysis of 72 cases. AJNR Am J Neuroradiol 25:450–462
Richieri-Costa A, Frederigue Junior U, Guinon-Almeida ML (1993) Holoprosencephaly, hamartomatous growth of the cerebrum, dysplastic gangliocytoma of the cerebellum, unique brain anomalies, and renal agenesis in a Brazilian infant born to a diabetic mother: a clinical and pathologic study. Birth Defects Orig Artic Ser 29(1):389–394
Eksioglu YZ, Scheffer IE, Cardenas P, Knoll J, DiMario F, Ramsby G et al (1996) Periventricular heterotopia: an X-linked dominant epilepsy locus causing aberrant cortical development. Neuron 16:77–87
Parrini E, Ramazzotti A, Dobyns WB, Mei D, Moro F, Veggiotti P, Marini C, Brilstra EH, Dalla Bernardina B, Goodwin L, Bodell A, Jones MC, Nangeroni M, Palmer S, Said E, Sander JW, Striano P, Takahashi Y, Van Maldergem L, Leonardi G, Wright M, Walsh CA, Guerrini R (2006) Periventricular heterotopia: phenotypic heterogeneity and correlation with Filamin A mutations. Brain 129(Pt 7):1892–1906
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Widjaja, E., Massimi, L., Blaser, S. et al. Midline “brain in brain”: an unusual variant of holoprosencephaly with anterior prosomeric cortical dysplasia. Childs Nerv Syst 23, 437–442 (2007). https://doi.org/10.1007/s00381-006-0233-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-006-0233-1