Abstract
Background
Epidermoid cysts may remain asymptomatic for a long period of time due to their slowly growing pattern corresponding with the normal human skin turnover time and due to soft and light cyst content. They do not cause compression of neural and vascular structures initially that is why almost all of the cases in the literature are diagnosed during adulthood.
Methods
We report here an epidermoid cyst in childhood, which was located in the medulla oblongata, unusually and atypically with liquefied cyst content. The liquefaction may occur due to an intrauterine or early childhood infection. The reported case also suffered previously a severe respiratory infection. Although the cyst is located in and around a highly eloquent neural area, plasticity of the brain stem prevented neurological deterioration due to this very slow growing extraaxial mass lesion. The ordinary cyst content found in the center of the cyst cavity during the operation suggested that the same ordinary material, which was previously at the periphery, ran to get liquefied in time.
Conclusion
We suggest that the symptoms of this present case appeared very early due to liquefaction of the cyst content with compression and displacement of the brain stem caudally. The recent infection process may predispose the pathological condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Introduction
Years ago, epidermoid tumors were first called due to their “mother of pearl appearance” as the “tumeur perlee” by French pathologist Cruveilhier and were defined as the “most beautiful of all tumors” [9]. The incidence of these tumors is between 1 and 2% of all intracranial mass lesions [6, 7, 19, 22, 31, 39]. Most of the cases are symptomatic during adulthood, and the age of presentation usually is in the fourth decade of life [39]. Some of the cases had been asymptomatic and diagnosed incidentally [13, 19]. There are very few cases diagnosed during childhood [4, 6, 24, 25, 40].
The usual location is parasellar region and pontocerebellar angle [1, 3, 27, 39, 41]. Other common locations are sylvian fissure, suprasellar region, cerebral [18, 29, 43] and cerebellar hemispheres [18, 32, 43], and lateral and fourth ventricles [12, 14, 18, 32, 39, 42, 43]. Posterior fossa epidermoid cysts usually arise in the lateral subarachnoid cisterns [3, 28, 43], and those located in the brain stem are rare [6, 15, 22, 25, 28, 40]. Tumors located in the medulla are even more rare [15, 28, 29, 36, 37, 40, 44]. As far as we know, there are only 17 cases of epidermoid tumors reported in the literature [4, 15, 20–22, 24–29, 35, 37, 40, 44] (Table 1).
These maldevelopmental lesions contain classically waxy and pearly white material composed of desquamated keratin, cholesterol crystals, and cellular debris. The typical cyst content is cheesy and flaky white and soft and putty-like. As to our knowledge, there is no reported case of an epidermoid tumor with liquefied cyst content.
Pathogenesis
Epidermoid cysts are slow growing tumors and develop from inclusion of epidermal elements during closure of the neural groove between the third and fifth weeks of embryonic life. There is a lateral preference due to proliferation of multipotential embryonic or transplanted epithelial cell remnants moved with migration of otic vesicles or developing neurovasculature [12, 43]. Median location of the epidermoid tumors which is usually typical for dermoid tumors occurs with separation of neuroectoderm from the cutaneous counterpart. Iatrogenical occurrence following serial subdural taps was also reported [16]. Multiplicity is very rare [29].
Epidermoid tumor fills the subarachnoid space before displacing the neural and vascular structures. Enlargement of the tumor is due to accumulation of breakdown products of desquamated epithelial cells. Keratin and cholesterol accumulate in the subarachnoid space and give the milky-white or pearly appearance. Caldarelli et al. [6, 7] reported that the cyst content may appear as a milky fluid at the surgery, although no one in their reported cases showed this pattern.
The tumor extends from the cisternal space into the brain stem and is usually demonstrated as an exophytic lesion in the pons and medulla. The brain stem parenchyma may cover the cyst content in time and may give the appearance that the lesion is endophytic. Additionally, the intraaxial location of epidermoid cysts was denied by some authors. It was stated that the endophytic location should be attributed to the progressive invasion and splitting of the brain stem brought about by the growth of ectodermal remnants primarily developing around the basilar artery [6, 28].
Clinical presentation
These very slowly growing congenital lesions tend to be symptomatic in adulthood. They may be associated with cutaneous lesions such as dermal sinus [39]. The neurological symptoms depend on the location of the epidermoid tumor. Recurrent episodes of aseptic meningitis after spontaneous rupture or leakage of cyst content may be another type of clinical presentation [20, 25, 26, 35]. On the other hand, due to the plasticity of the neural architecture, these slowly growing lesions may stay silent or present with minor symptoms, although there may be severe radiological compression of important neural structures.
Evaluation and management
Magnetic resonance imaging (MRI) scan is the best modality for the diagnosis of epidermoid cysts. On T1-weighted scans, the tumor usually is hypointense, an intensity that is intermediate between the brain and the cerebrospinal fluid (CSF), with no contrast enhancement [10, 22, 23, 38]. If contrast enhancement occurs, it is usually at the margins of the tumor [43]. On T2-weighted images, they are usually hyperintense without having peritumoral edema. If the MRI scan reveals CSF-like signal of the cyst, diffusion-weighted imaging studies will differentiate the epidermoid cyst with restricted diffusion from an arachnoid cyst [6]. On computed tomography (CT) scan, the tumor appears hypodense or isodense or sometimes spontaneous hyperdense due to protein, lipid, calcium, and hemosiderin content [5]. On operated patients, cholesterin crystals are clues of capsule remnant. Calcification may be present; however, it is more prevalant in dermoid tumors. Sometimes, the neuroradiological findings may be atypical and misleading [6]. The differential diagnosis should be performed with arachnoid cysts, dermoid tumors, lipomas, and cholesterol granulomas [10, 23, 39, 43]. Although teratomas are neoplastic lesions, they may also include epidermoid component, and both lesions may appear simultaneously in the same patient [39, 42]. There are cases in the literature where the diagnosis could be performed only after autopsy.
Some cases of epidermoid cyst may undergo spontaneous remission with two different mechanisms. The first one is tumor decompression due to the leakage of the cyst content into the subarachnoid space or CSF pathway. In time, occurrence of local arachnoiditis may prevent this drainage, and the neurological symptoms may reappear [39, 41]. The second mechanism is the spread of the cyst content to subarachnoid spaces with slow growth, which does not cause any significant compression of the neurovascular structures. On such cases, the CSF flows through perivascular interstices [14, 39].
These tumors should be removed as radical as possible without risking the patient’s neurological status. Although the cyst content can be removed easily [28], radical removal with the germinative layer is not always possible because it is usually adherent to important neural and vascular structures. Especially, for brain stem epidermoid cysts, simple aspiration or subtotal excision of the tumor may be performed. Of all reported 18 cases of brain stem epidermoid cysts in the literature, six patients died due to the postoperative progressive deterioration [4, 15, 25, 29, 30, 35].
It was reported that neither type of excision nor preoperative tumor size has significant incidence on prognosis [39]. Postoperative aseptic meningitis occurs due to incomplete excision of the cyst capsule and spillage of the cyst content to the subarachnoid space during the operation. The incidence of this complication in different series changes between 2 and 50% [14, 19, 33, 39, 41, 45]. Irrigation with hydrocortizon solution perioperatively and administration of dexametasone postoperatively may avoid occurrence of an aseptic meningitis [3, 31, 33, 39, 41, 43].
The recurrence rate is between 1 and 54% and may be avoided to devitalize the remnant of capsule fragments during the operation [3, 31, 33, 34, 41, 43]. The growth rate of an epidermoid tumor is one generation per month, which corresponds with the turnover time of normal human skin. For a single cell remnant, the time of recurrence is equal to the patient’s age at that time of the resection plus 9 months [2, 43]. Reoperation should be performed when the patient becomes symptomatic again, because no dissection plan between the capsule and the arachnoid may be present during the second operation. The reoperation is usually performed for decompression. Malignant degeneration for recurrent epidermoid tumors is also reported [8, 11, 17, 27, 33].
Illustrative case
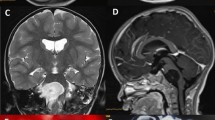
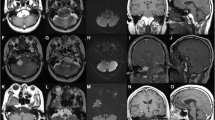
A 5-year-old female with progressive dysphagia, diminished gag reflex, and hoarseness was admitted. Three months ago, she suffered a severe respiratory system infection and sinusitis, and at that time, she was hospitalized for a month. Neurological examination revealed moderate IX, X, XI, and XII nerve palsies, and MRI scan demonstrated a round mass lesion in the medulla oblongata (Fig. 1). Actually, the tumor was insinuating itself from the premedullary cistern into the medulla. The patient was operated with a suboccipital craniotomy. Shortly following midline medullotomy, a white-milky fluid drained from the lesion cavity. There was also some ordinary epidermoid cyst content in the center of the lesion cavity. This material was also removed. Postoperative MRI scan demonstrated gross total removal of the mass lesion (Fig. 2a). Histopathological examination of the ordinary material removed from the center of the lesion revealed an epidermoid cyst (Fig. 2b). Several days after the operation, the patient’s lower cranial nerve palsies gradually improved.
Preoperative sagittal (a), coronal (b) and axial (c) MRI scans of a 5-year-old female demonstrated a round mass lesion in medulla oblongata. Perioperatively, shortly after midline medullotomy, a white-milky fluid drained from the lesion cavity. There was also some ordinary epidermoid cyst content in the center of the lesion cavity which can also be recognized on the preoperative MRI scans
a Postoperative axial MRI scan of the patient demonstrated gross total removal of the mass lesion. b Histopathological examination of the material revealed the epidermoid cyst, including desquamated uniform superficial and intermediate squamous cells with abundant cytoplasm, demonstrating varying degrees of keratinization (May-Grunwald Giemsa, ×400)
Conclusion
Speculations may be obtained about the liquefaction mechanism of epidermoid cyst content. It might depend on severe infection process, which occured shortly before the clinical presentation. The absence of drainage in this liquefied cyst content may cause symptoms. The disturbance on the vascular supply of the cyst due to any reason may be another mechanism.
References
Altschuler EM, Jungreis CA, Sekhar LN, Jannetta PJ, Sheptak PE (1990) Operative treatment of intracranial epidermoid cysts and chlosterol granulomas: report of 21 cases. Neurosurgery 26:606–614
Alvord ED Jr (1977) Growth rates of epidermoid tumors. Ann Surg 2:267–370
Berger MS, Wilson CB (1985) Epidermoid cysts of the posterior fossa. J Neurosurg 62:214–219
Bhatia R, Shankar SK, Tandon PN (1978) Pre-pontine epidermoid traversing the brain stem: a case report. Neurol India 26:76–78
Braun IF, Naidich TP, Leeds NE, Koslow M, Zimmerman HM, Chase NE (1977) Dense intracranial epidermoid tumors: computed tomographic observations. Radiology 122:717–719
Caldarelli M, Colosimo C, Di Rocco C (2001) Intra-axial dermoid/epidermoid tumors of the brainstem in children. Surg Neurol 56:97–105
Caldarelli M, Massimi L, Kondageski C, Di Rocco C (2004) Intracranial midline dermoid and epidermoid cysts in children. J Neurosurg 100(5):473–480
Cobbs CS, Pitts LH, Wilson C (1996) Epidermoid and dermoid cysts of the posterior fossa. Clin Neurosurg 44:511–528
Cruveilhier J (1829) Anatomie pathologique du corps humain, vol 1, book 2. Baillere, Paris
Doll A, Abu Eid M, Kehrli P, Esposito P, Gillis C, Bogorin A, Jacques C, Dietemann JL (2000) Aspect of FLAIR sequences, 3D-CISS and diffusion-weight MR imaging of intracranial epidermoid cysts. J Neuroradiol 27(2):101–106
Dubois PJ, Sage M, Luther JS, Burger PC, Heinz ER, Drayer BP (1981) Malignant change in an epidrmoid intracranial epidermoid cyst. J Comput Assist Tomogr 5(3):433–435
Eekhof JLA, Thomeer RTWM, Bots GTAM (1985) Epidermoid tumor in the lateral ventricle. Surg Neurol 23:189–192
Emery E, Zerah M, Comoy J, Tardieu M, Husson B, Hurth B (1993) Kyste epidermoide du 4e ventricule. A propos d’un cas chez un enfant et revue de la litterature. Neurochirurgia 39:241–247
Fiume D, Gazzeri G, Spallone A, Santucci N (1988) Epidermoid cysts of the fourth ventricle. Surg Neurol 29:178–182
Fournier D, Mercier P, Menei P, Pouplard F, Rizk T, Guy G (1992) Recurrent intrinsic brain stem epidermoid cyst. Childs Nerv Syst 8:471–474
Gutin PH, Boehm J, Bank WO, Edwards MS, Rosegay H (1980) Cerebral convexity epidermoid tumor subsequent to multiple percutaneous subdural aspiration. Case report. J Neurosurg 52:574–577
Goldman SA, Gandy SE (1987) Squamous-cell carcinoma as a late complication of intracerebroventricular epidermoid cyst. J Neurosurg 66:618–620
Grant FC, Austin GM (1950) Epidermoids: clinical evaluation and surgical results. J Neurosurg 7:190–198
Guidetti B, Gagliardi FM (1977) Epidermoid and dermoid cysts: clinical evaluation and late surgical results. J Neurosurg 47:12–18
Guy G, Jan M, Guegan Y (1989) Les lesions chirurgicales du tronc cerebral. Neurochirurgia 35(Suppl 1):99–101
Iihara K, Kikuchi H, Ishikawa M, Nagasawa S (1989) Epidermoid cyst traversing the pons into the fourth ventricle. Case Report. Surg Neurol 32:377–381
Kachhara R, Bhattacharya RN, Radhakrishnan VV (2000) Epidermoid cyst involving the brain stem. Acta Neurochir (Wien) 142:97–100
Karantanas AH (2001) MR imaging of intracranial epidermoid tumors: specific diagnosis with turbo-FLAIR pulse sequence. Comput Med Imaging Graph 25(3):249–255
Kuzeyli K, Duru S, Cakir E, Pekince A, Ceylan S, Aktürk F (1996) Epidermoid cyst of the brain stem. Case report. Neurosurg Rev 19:179–181
Leal O, Miles J (1978) Epidermoid cyst in the brain stem. Case report. J Neurosurg 48:811–813
Malcolm GP, Gibson R, Ironside JW, Whittle IR (1996) Microsurgical excision of a pontomedullary epidermoid cyst with prepontine extension. Case report. Neurosurgery 38(3):579–583
Netsky MG (1988) Epidermoid tumors. Review of the literature. Surg Neurol 29:477–483
Obana WG, Wilson CB (1991) Epidermoid cyst of the brain stem. Report of three cases. J Neurosurg 74:123–128
Ogawa T, Sekino H, Fuse T, Nakamura N (1985) Multiple intracranial epidermoids located in the brain stem and the middle cranial fossa: case report (in Japanese). Neurol Med Chir (Tokyo) 25:393–397
Radhakrishnan VV, Saraswathi A, Rout D (1992) Epidermoid cyst of the brain stem: a case report. Indian J Cancer 29:215–217
Rubin G, Scienza R, Pasqualin A, Rotsa L, Da Pian R (1989) Craniocerebral epidermoids and dermoids. Acta Neurochir (Wien) 97:1–6
Sabin HI, Bordi LT, Symon L (1987) Epidermoid cysts and cholesterol granulomas centered on the posterior fossa: 20 years of diagnosis and management. Neurosurgery 21:789–805
Salazar J, Vaquero J, Saucedo G, Bravo G (1987) Posterior fossa epidermoid cysts. Acta Neurochir (Wien) 85:34–39
Samii M, Tatagiba M, Piquer J, Carvalho GA (1996) Surgical treatment of epidermoid cysts of the cerebellopontine angle. J Neurosurg 84:14–19
Schwartz JF, Balentine JD (1978) Recurrent meningitis due to an intracranial epidermoid. Neurology 28:124–129
Sinha AK, Panigrahi M, Billadvalla D, Reddy AK (1998) Epidermoid cyst of the brain stem: a case report. Neurol India 46:333–335
Sinha AK (1999) Brain stem epidermoid cyst. Surg Neurol 51:687–688
Tampieri D, Melanson D, Ethier R (1989) MR imaging of epidermoid cysts. Am J Neuroradiol 10:351–356
Tancredi A, Fiume D, Gazzeri G (2003) Epidermoid cysts of the fourth ventricle: very long follow up in 9 cases and review of the literature. Acta Neurochir (Wien) 145:905–911
Weawer EN Jr, Coulon RA Jr (1979) Excision of a brain stem epidermoid cyst. Case report. J Neurosurg 51:254–257
Yamakawa K, Shitara N, Genka N, Kanaka S, Takakura K (1989) Clinical course and surgical prognosis of 33 cases of intracranial epidermoid tumors. Neurosurgery 24:568–573
Yamaki T, Takeda M, Takayama H, Nakagaki Y (1990) Double intracranial tumours of maldevelopmental origin-teratoma at the pineal region and an epidermoid cyst in the fourth ventricle. Neurochirurgia (Stuttg) 33(3):88–90
Yasargil MG, Abernathey CD, Sarioglu AÇ (1989) Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery 24:561–567
Yoshizato K, Kai Y, Kuratsu J (1996) Intramedullary epidermoid cyst in the brain stem. Case report. Surg Neurol 45:537–540
Zhou LF (1990) Intracranial epidermoid tumours: thirty-seven years of diagnosis and treatment. Br J Neurosurg 4:211–216
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziyal, I.M., Bilginer, B., Bozkurt, G. et al. Epidermoid cyst of the brain stem symptomatic in childhood. Childs Nerv Syst 21, 1025–1029 (2005). https://doi.org/10.1007/s00381-005-1172-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-005-1172-y