Abstract
A 28-day microcosm experiment was conducted using three paddy soils (an alluvial paddy soil, a loess-formed paddy soil, and a yellow clayey paddy soil) to investigate the impact of procyanidin on N2O emissions and associated microbial mechanisms. The efficacy of procyanidin on N2O emissions varied among the paddy soils tested, with an average inhibition rate ranging from 2.7% in the alluvial paddy soil to 57.1% in the loess-formed paddy soil. Furthermore, suppression of N2O emissions by procyanidin occurred alongside fluctuations in nitrate reductase activity and nirS- and nirK-type denitrifiers abundance. The correlation analysis indicates that nitrate reductase, clade I nirS-denitrifiers, clade I or II, and clade III nirK-denitrifiers were closely linked to N2O emissions. These findings provide evidence that procyanidin is capable of limiting N2O emissions in paddy soils by inhibiting nitrate reductase and different clades of nirS-/nirK-denitrifiers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) as a potent greenhouse gas that greatly contributes to global warming and stratospheric ozone depletion (Montzka et al. 2011; Ravishankara et al. 2009). Agricultural soils are a predominant source of anthropogenic N2O, which accounts for about 60% of the global emissions (Bhatia et al. 2010). Production of N2O in soils is principally associated with microbially-mediated nitrification and denitrification processes, occurring under aerobic and anaerobic conditions, respectively (Volpi et al. 2017; Zhang et al. 2015b). Paddy soils are submerged during most of the growing season; the submergence induces an anaerobic environment for microorganisms, resulting in denitrification as the dominant N2O-producing process (Mathieu et al. 2006). The denitrification-derived N2O emissions across paddy soils have caused massive losses of agricultural N (Lan et al. 2015; Wang et al. 2017) and exacerbated global warming. Therefore, developing optimal management strategies to curb N2O emissions from paddy soils has been extensively researched (Linquist et al. 2015; Shaukat et al. 2019; Song et al. 2017; Xia et al. 2020).
Application of inhibitors, including both synthetic and biological origin, has been recommended as a potential option to mitigate agricultural N2O emissions (IPCC 2014) and has been widely investigated across diverse agroecosystems (Akiyama et al. 2010; Qiao et al. 2015). The most regularly used and best understood are synthetic nitrification inhibitors to mitigate nitrification-induced N2O emissions (Li et al. 2021a; Rodrigues et al. 2018). In contrast, very few inhibitors have been proven to be effective in regulating denitrification-derived N2O emissions. Recently, a novel biological denitrification inhibitor (BDI) has been discovered from the roots of Fallopia spp. (Bardon et al. 2014). Bardon et al. (2016a) demonstrated that BDI from root extracts, a B-type procyanidin, can suppress soil microbial denitrification, by inhibiting denitrifiers (Galland et al. 2019). The addition of procyanidin to soil decreased N2O emissions from denitrification (Bardon et al. 2017), but its efficacy depended on soil properties (Bardon et al. 2016a). However, the inhibitory efficacy of procyanidin on N2O emissions has only been tested in a riparian soil (Bardon et al. 2017), but not in diverse agricultural soils, especially in paddy soils.
Denitrification is the fundamental biogeochemical pathway through which N2O is produced (Tao et al. 2018), and it involves the transformations of NO3¯ and NO2¯ into gaseous forms of N, such as NO, N2O, and N2 (Zumft 1997). A key step in the process is the reduction of NO3¯ to NO2¯, which is catalyzed by the nitrate reductase enzyme (NaR) (Enwall et al. 2005), and it is inhibited by procyanidin, likely by perturbing membrane stability (Bardon et al. 2016b). Among the other denitrification reactions, the reduction of NO2¯ to gaseous forms is catalyzed by the nitrite reductase enzyme (NiR). Functional genes encoding NiR (i.e., nirS and nirK) are commonly used as biomarkers in the analysis of the denitrifier population (Kuypers et al. 2018). Denitrifying organisms harboring the nirS or nirK gene are closely connected to N2O emissions in soils (Cui et al. 2016; Fan et al. 2019; Qiu et al. 2018; Yin et al. 2017). Procyanidin inhibiting denitrification also affects the abundance of nirS- and nirK-containing denitrifiers (Galland et al. 2019). Therefore, procyanidin can regulate N2O emissions during the denitrification process, both at the enzyme and soil population levels. However, the traditional primer pairs targeting nirS and nirK poorly cover some potentially abundant denitrifiers such as Burkholderiales and Rhizobiales bacteria in rice paddies (Yoshida et al. 2009), but not all functional clades involved in N2O production (Jones et al. 2008; Saito et al. 2008; Wei et al. 2015).
This study dealt with the effect of procyanidin on N2O emissions and associated microbial mechanisms in paddy soils. The main objectives of this study were to (i) assess the effect of procyanidin on N2O emissions in different paddy soils; (ii) evaluate the inhibition of denitrifying enzymes (NaR and NiR) by procyanidin; and (iii) understand the relationship between N2O emissions and the nirS- and nirK-type denitrifiers in the paddy soils amended with procyanidin.
Materials and methods
Site description and sample collection
The soil samples used for the experiment were collected from three geographically different sites. The three soils studied include an alluvial paddy soil, a loess-formed paddy soil, and a yellow clayey paddy soil; those soils were developed in contrasting environments and have different properties. The yellow clayey paddy soil was collected from Jinhua (JH, 29° 01′ N, 119° 28′ E), Zhejiang province; the loess-formed paddy soil was sampled from Jintan (JT, 31° 39′ N, 119° 28 ′E), Jiangsu province; and the alluvial paddy soil was collected from Qinhuangdao (QHD, 40° 7′ 34″ N, 119° 11′ 27″ E) in Hebei province. The three sites are located in typical rice-growing areas in China, with average annual precipitations of 1424 mm (JH), 1063 mm (JT), and 551 mm (QHD), and mean annual temperatures of 17.5 °C (JH), 15.3 °C (JT), and 11.2 °C (QHD). The yellow clayey paddy soil at JH was derived from Quaternary red earth, the loess-formed paddy soil at JT from Xiashu loess earth, and the alluvial paddy soil at QHD from an alluvial deposit. All three paddy soils were slightly acidic to neutral. At each sampling site, twelve cores (top 0–20 cm) were collected, mixed into a composite sample, and immediately transported to the laboratory. The soils were sieved (< 2 mm) to remove visible plant debris and coarse fragments, homogenized, then air-dried, and stored at room temperature before the construction of microcosms.
Determination of soil properties
Soil pH was determined using a glass electrode in 1:2.5 soil:water solution (w:v). Soil moisture content was measured gravimetrically after drying at 105 °C for 24 h. Soil organic matter (SOM) content was determined using the K2Cr2O7 wet oxidation method (Kalembasa and Jenkinson 1973). Total N (TN) of soil was measured by Kjeldahl method using a Lachat flow-injection autoanalyzer (Lachat Instruments, WI, USA) (Bremner and Mulvaney 1982). Soil exchangeable NH4+-N and NO3¯-N were extracted by 0.01 M CaCl2 and analyzed colorimetrically using an ultraviolet spectrophotometer (ThermoFisher Scientific, Massachusetts, USA) (Denmead et al. 1976; Norman et al. 1985). Total P (TP) was determined by molybdenum antimony blue colorimetry after digestion with HClO4-H2SO4 (Thomas et al. 1967). Soil available P (Olsen P) was extracted with 0.5 M NaHCO3 (Olsen et al. 1954) and measured colorimetrically (Murphy and Riley 1962). Available K (AK) was extracted by 1 M CH3COONH4 and quantified with a flame photometer (Inesa Instrument, Shanghai, China) (Standford and English 1949). Details of the soil physicochemical properties prior to the incubation experiment are presented in Table 1.
Soil microcosm experiment
A microcosm experiment, comprising the control (Control) and procyanidin application (BDI) treatments, was established to investigate the effect of procyanidin on N2O emissions in paddy soils. All treatments received ammonium sulfate ((NH4)2SO4) and potassium nitrate (KNO3) as N substrate and were replicated three times for each sampling point (see below). Soil microcosms consisted of 125-mL glass bottles, each containing 20 g of soil (oven-dried weight basis), were pre-incubated around 30% water-filled pore space (WFPS) for 7 days at 25 °C. We used a commercial procyanidin (Macklin, Shanghai, China) with a composition of 99.06% procyanidin (C30H26O12) and 0.87% water. The solution with or without procyanidin was added evenly over the soil surface, and finally we adjusted soil moisture at 80% WFPS to provide anaerobic conditions favorable for denitrification (Bateman and Baggs 2005; Hu et al. 2015; Huang et al. 2014). Nitrogen was added at a rate of 50 mg (NH4)2SO4-N kg−1 and 50 mg KNO3-N kg−1 soil. The procyanidin-amended treatment received 20 mg procyanidin, amounting to 1 mg g−1 of dry soil (Bardon et al. 2017). All glass bottles were sealed with plastic bottle caps fitted with butyl rubber stoppers, and then incubated at 25 °C in the dark for 28 days. The water loss during the incubation was determined by periodically weighing the bottles and deionized water was added to replenish the lost soil moisture.
Gas samples (10 mL) were taken from the headspace by a gas-tight syringe at 1, 2, 4, 7, 10, 13, 19, and 28 days after incubation. The concentration of N2O was determined by gas chromatograph (GC-2010 Plus Shimadzu, Japan). After each sampling, all bottles remaining were ventilated for 20 min and then sealed again. Destructive soil samples were collected on days 0, 4, 7, 13, and 28 and monitored for exchangeable NH4+-N and NO3¯-N. Molecular analysis and enzyme activity assays were conducted on samples collected on days 0, 4, 13, and 28. The soil samples for molecular analysis were stored at − 80 °C until DNA extraction.
Denitrification enzyme activity
Denitrification enzyme activity (DEA) was measured using a method modified from Dassonville et al. (2011). Briefly, fresh soil (5 g oven-dry weight equivalent) was placed in 125-mL glass bottles and sealed with rubber stoppers. Then the air in each bottle was removed and replaced by a He/C2H2 mixture (90:10, v:v) to provide anoxic conditions and inhibit the N2O-reductase. A nutritive solution (5 mL) containing KNO3 (50 μg N g−1 dry soil), glucose (0.5 mg C g−1 dry soil) and glutamic acid (0.5 mg C g−1 dry soil) was added to the soil, followed by 5 h incubation at 25 °C with agitation (180 rpm). During the incubation, gas samples were taken at 1, 3, and 5 h and analyzed for N2O using a gas chromatograph (GC-2010 Plus Shimadzu, Japan) equipped with an ECD detector. The slope of the linear regression was used to estimate DEA as the N2O produced (g−1 h−1).
Enzyme activity assays
The NaR activity was determined with α-naphthylamine-sulfanilic acid using KNO3 as a substrate, and incubating the soil slurry for 25 °C in a gyratory shaker (180 rpm) (Abdelmagid and Tabatabai 1987). The NiR activity was measured with α-naphthylamine-sulfanilic acid using NaNO2 as a substrate, and incubating the soil slurry anaerobically for 24 h at 30 °C in a gyratory shaker (180 rpm) (Pu et al. 2019). After incubation, the produced or reduced nitrite was determined at λ = 520 nm using an ultraviolet spectrophotometer (ThermoFisher Scientific, Massachusetts, USA) to estimate NaR or NiR activity. The NaR and NiR activities were expressed as mg \(\mathrm{NO}_2^--\mathrm N\;\mathrm g^{-1}\) dry soil 24 h.
DNA extraction and quantitative PCR of nirS and nirK genes
Total DNA was extracted from a 0.5 g of soil sample, collected at 0, 4, 13, and 28 days, using the FastDNA SPIN Kit for soils (MP Biomedicals, Cleveland, OH, USA) according to the manufacturer’s protocol. The DNA concentration and quality were checked by a Colibri Microvolume Spectrometer (Titertek Berthold, Germany).
Abundances of functional marker genes for microbial denitrification (nirS clade I, nirS clade II, and nirK clade I, nirK clade II, nirK clade III) were determined by quantitative real-time PCR in a LightCycler® 480II System (Roche Diagnostics, Switzerland). Particularly, the newly updated primers were adopted to achieve a more precise determination of nirS- and nirK-type denitrifiers (Wei et al. 2015). Dilutions (10-fold) of the soil DNA sample were used as the template for qPCR. Standard curves were generated using 10-fold serial dilutions of plasmids with insertions of target gene fragments. More details of gene-specific primers, reaction mixture compositions, and thermal cycling conditions are listed in Table S1.
Statistical analyses
Data were analyzed by one-way ANOVA followed by an LSD post hoc test to determine the significance of treatments at each incubation time (SPSS version 20). Statistically significant differences were tested at P < 0.05. Linear and polynomial regressions were used to explore the relationship between cumulative N2O emissions and exchangeable NH4+-N and NO3¯-N concentrations, DEA, or NaR activity, as well as the abundance of functional genes within each soil. Data analyses were performed using the R software (R Core Team 2021). Only correlations with a significance of P < 0.05 were presented on the scatter-plot diagrams.
Results
N2O emissions
The cumulative N2O emissions substantially increased over the incubation, but varied significantly among the three paddy soils (P < 0.05), with an order of JT > JH > QHD (Fig. 1). The addition of procyanidin greatly reduced cumulative N2O emissions in JH and JT soils by 47.3% and 57.1%, respectively (Fig. 1a and b) (P < 0.05). However, no significant inhibition was observed between the control and procyanidin-treated soils with respect to cumulative N2O emissions in the QHD soil (Fig. 1c).
Exchangeable \(\mathrm{NH}_4^+-\mathrm N\) and \(\mathrm{NO}_3^--\mathrm N\) dynamics during incubation
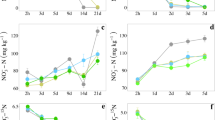
The change over time of exchangeable \(\mathrm{NH}_4^+-\mathrm N\) concentrations differed between paddy soils. The exchangeable \(\mathrm{NH}_4^+-\mathrm N\) concentration increased gradually in the JH soil, while it declined rapidly in the JT and QHD soils over the incubation (Fig. 2a–c). When compared with the control treatment, procyanidin application did not affect exchangeable \(\mathrm{NH}_4^+-\mathrm N\) concentrations throughout the incubation in the three paddy soils tested.
The dynamics of \(\mathrm{NO}_3^--\mathrm N\) concentrations in the incubation varied among the three paddy soils. The \(\mathrm{NO}_3^--\mathrm N\) concentration peaked on day 4 in the JH soil, and then declined gradually over time (Fig. 2d), but increased throughout the incubations in the JT and QHD soils (Fig. 2e and f). Procyanidin amendment significantly decreased the \(\mathrm{NO}_3^--\mathrm N\) concentrations in the JH and QHD soils after 7 days of incubation, compared with the control treatment (Fig. 2d and f) (P < 0.05). However, in the JT soil, procyanidin did not affect \(\mathrm{NO}_3^--\mathrm N\) concentration (Fig. 2e).
Denitrification enzyme activity
The denitrification enzyme activity (DEA) was higher in the JH and JT soils than in the QHD soil (Fig. 3) (P < 0.05). When compared with the control, the addition of procyanidin decreased the DEA in all three paddy soils; particularly in the JH and JT soils (P < 0.05), which had high denitrification activities.
The changes of denitrification enzyme activity (DEA) in JH (a), JT (b), and QHD (c) paddy soils with N fertilizer addition (Control) and with N fertilizer plus procyanidin addition (BDI). Data are presented as mean values with standard errors (n = 3). Different letters indicate significant differences between Control and BDI treatments at P < 0.05 at each incubation time
Soil denitrifying enzyme activities
The addition of procyanidin significantly inhibited the NaR activity in the JH and JT soils (Fig. 4a and b) (P < 0.05), but not in the QHD soil (Fig. 4c). Furthermore, procyanidin did not affect the NiR activity compared with the control (Fig. S1).
The changes of nitrate reductase (NaR) activity in JH (a), JT (b), and QHD (c) paddy soils with N fertilizer addition (Control) and with N fertilizer plus procyanidin addition (BDI). Data are presented as mean values with standard errors (n = 3). Different letters indicate significant differences between Control and BDI treatments at P < 0.05 at each incubation time
Abundances of nirS and nirK genes
The abundance of the clades of nirS- and nirK-harboring denitrifiers varied among the soils (Table 2), and showed contrasting responses to the procyanidin amendment (Figs. 5 and 6). The abundance of nirS- and nirK-denitrifiers was higher in the JT soil than in the other two soils (Table 2) (P < 0.05). In addition, the procyanidin application significantly decreased the abundance of nirS clade I and II in the JH and JT soils (P < 0.05), while no differences between treatments were found in the QHD soil (Fig. 5). Procyanidin significantly reduced the abundance of nirK clade I in the JH soil, but increased their abundance in the JT and QHD soils (Fig. 6a–c) (P < 0.05). In contrast, procyanidin decreased nirK clade II gene copy numbers in the JH and JT soils (P < 0.05), but not in the QHD soil (Fig. 6d–f). Finally, the procyanidin application reduced the population size of nirK clade III in all three soils (Fig. 6g–i) (P < 0.05).
The abundance of the nirS Cluster I and II in JH (a and d), JT (b and e), and QHD (c and f) paddy soils with N fertilizer addition (Control) and with N fertilizer plus procyanidin addition (BDI). Data are presented as mean values with standard errors (n = 3). Different letters indicate significant differences between Control and BDI treatments at P < 0.05 at each incubation time
The abundance of the nirK Cluster I, II, and III in JH (a, d, and g), JT (b, e, and h), and QHD (c, f, and i) paddy soils with N fertilizer addition (Control) and with N fertilizer plus procyanidin addition (BDI). Data are presented as mean values with standard errors (n = 3). Different letters indicate significant differences between Control and BDI treatments at P < 0.05 at each incubation time
Relationship between cumulative N2O emissions and soil properties and microbial dynamics
The linear and polynomial regressions were analyzed to explore potential relationships between cumulative N2O emissions, soil properties, soil denitrifying activity, and the abundance of the functional genes (Fig. 7). The cumulative N2O emissions (y) of the JH soil was related positively to exchangeable ammonium concentration (x) (y = 0.081x – 13.611), and negatively to DEA (x) (y = 0.3233x2 – 1.8236x + 2.9074). The cumulative N2O emissions (y) from the JT soil was related negatively to exchangeable ammonium concentration (x) (y = – 0.0197x2 + 0.245x + 33.258), and positively to nitrate concentration (x) (y = 0.2635x – 21.067) and DEA (x) (y = 11.624x – 0.547). The cumulative N2O emissions (y) of the QHD soil was related negatively to exchangeable ammonium concentration (x) (y = – 0.0618x + 2.7192), and positively to nitrate concentration (x) (y = 0.0442x – 6.7734).
Linear and polynomial regression relationships either between the cumulative N2O emissions and ammonium, nitrate, DEA, and NaR activity or between the cumulative N2O emissions and the abundance of N2O-emission-related functional guilds. Only correlations that are significant at P < 0.05 are given. The shaded bands show 95% confidence intervals
The correlation analysis of cumulative N2O emissions (y) with microbial abundance, shows that x is the log10-transformed gene copy number. The cumulative N2O emissions (y) were positively related to the NaR activity (x) (y = 58.273x – 0.0937, JH; y = 51607x2 – 6771x + 235.82, JT) and the population size of nirS clade I (x) (y = 33.978x2 – 469.21x + 1620.2, JH; y = 197.24x – 1564, JT) in JH and JT soils, but negatively to the NaR activity (x) (y = 383.19x2 – 131.1x + 12.29) and the population size of nirS clade I (x) (y = 90.707x2 – 1319.3x + 4797.3) in the QHD soil. There was no significant relationship between cumulative N2O emissions and the abundance of nirS clade II in any soil. Among the three soils, only the abundance of nirK clade I of the JH soil was positively related to cumulative N2O emissions (y = 5.4678x – 30.536). However, the cumulative N2O emissions were positively related to the population size of nirK clade II in the JT and QHD soils (y = 112.33x2 – 1628.5x + 5911.7, JT; y = 9.5575x – 63.978, QHD). Furthermore, the abundance of nirK clade III was positively related to cumulative N2O emissions in all three soils (y = 4.2846x – 13.18, JH; y = 80.087x – 372.11, JT; and y = 2.2941x – 7.611, QHD).
Discussion
Effects of procyanidin on soil mineral N
Our results consistently demonstrate that the application of procyanidin had little impact on the turnover of exchangeable \(\mathrm{NH}_4^+-\mathrm N\) in all tested soils (Fig. 2a–c), indicating that procyanidin most likely did not affect nitrification, but inhibited denitrification (Bardon et al. 2016a, 2017). The variable effect of procyanidin on soil \(\mathrm{NO}_3^--\mathrm N\) concentrations (Fig. 2d–f), as it decreased the \(\mathrm{NO}_3^--\mathrm N\) in the JH soil (Fig. 2d), was inconsistent with previous findings (Bardon et al. 2017; Galland et al. 2019, 2021). Microbial \(\mathrm{NO}_3^--\mathrm N\) immobilization and dissimilatory \(\mathrm{NO}_3^--\mathrm N\) reduction to NH4+-N (DNRA) compete with denitrification for NO3¯-N and can result in variable effects on soil NO3¯-N concentrations (Pandey et al. 2021; Zhang et al. 2015a). Procyanidin application might simultaneously enhance the microbial NO3¯-N immobilization and DNRA processes by inhibiting denitrification, with more NO3¯-N being converted into the microbial biomass N pool (Cheng et al. 2017) and reduced to soil-retainable NH4+-N (Silver et al. 2001), and could therefore increase soil N retention. The JH soil in our study might possess high microbial NO3¯-N immobilization and DNRA rates due to the high SOM content in relation to NO3¯-N (Table 1) (Li et al. 2021b; Putz et al. 2018; Yoon et al. 2015). These suggest that the conservation of N might be responsible for the declined NO3¯-N concentration in the JH soil. However, further research employing stable isotope techniques is necessary to elucidate the mechanism for N conservation.
Effects of procyanidin on N2O emissions in three paddy soils
The mitigatory effect of procyanidin on N2O emissions varied dramatically among soils (Fig. 1), as the denitrification inhibition efficacy of procyanidin is highly dependent on soil properties (Bardon et al. 2016a). In addition, the different dominant denitrifiers among different paddy soils (Figs. 5 and 6) made it difficult for procyanidin to have consistent effects in different soils. Therefore, the contrasting effects of procyanidin on N2O emissions were likely due to the variations in both soil properties and soil microbial community compositions.
The denitrification activity is an important factor affecting N2O emissions generated from denitrification (Forte and Fierro 2019; Meng et al. 2020; Morse et al. 2012; Šimek et al. 2004). In this study, the inhibition of procyanidin on the denitrification activity was more effective in the JH and JT soils, which is consistent with the N2O emissions (Fig. 1) and the denitrification enzyme activity data (Fig. 3), indicating that there is great potential for procyanidin to regulate denitrification-derived N2O emissions in these two paddy soils. Such differences of the performance of procyanidin in different soils can be attributed to differences in the SOM content among the soils, which was significantly higher in the JH and JT soils than in the QHD soil (Table 1); SOM is a major control of denitrification activity (Malique et al. 2019; Wu et al. 2017). The SOM contains readily decomposable organic C and may trigger denitrification by enhancing respiration (through the creation of anoxic microsites) and by providing energy for denitrifiers (Butterbach-bahl et al. 2013; Köster et al. 2015). Our results suggest that denitrification activity is more prevalent in SOM-rich soils, in agreement with previous findings (Chen et al. 2018; Malique et al. 2019; Yin et al. 2015). Therefore, our results imply that the use of procyanidin reduced N2O emissions more effectively under conditions favoring denitrification, i.e., high soil moisture content (i.e., 80% WFPS) and high SOM content in the soil. The QHD soil typically had low denitrification activity (Fig. 3c), indicating that the contribution of denitrification to N2O emissions was weak in this soil.
In addition, the adsorption of procyanidin to surface-reactive soil particles has been regarded as a crucial factor governing its effectiveness as a BDI (Galland et al. 2019), as procyanidin strongly binds to soil components (e.g., SOM, and metals) and becomes less bioavailable (Kraal et al. 2009). In this study, a lower efficacy of procyanidin in the JH soil (47.3%) compared to the JT soil (57.1%) (Fig. 1a and b) can partly be attributed to a greater adsorption of procyanidin in the JH soil, which has a higher SOM content (Table 1). It has also been shown that procyanidin can chelate metals with their o-diphenol groups (Dixon et al. 2005). The available K concentration in our study was significantly higher in the QHD soil than in the other two soils (Table 1). Accordingly, the bioavailability of procyanidin might be impacted by the binding of K (Longo et al. 2018), which led to a low inhibition effect on N2O emissions in QHD soil.
The sensitivity of dominant denitrifying microorganisms in soils to procyanidin may also result in differences in the efficacy of the BDI. The sensitivity of denitrifiers can be modulated by changes in the composition of the denitrifying community (Bardon et al. 2016a). The different compositions of the nirS- and nirK-harboring denitrifiers in soils in this study (Figs. 5 and 6) provides an explanation for the differential effects of procyanidin in the three paddy soils. Additionally, procyanidin can be used by soil microbes as a C and nutrient source and become degraded, thereby influencing its bioavailability (Bardon et al. 2016a; Kraus et al. 2004). The degree of degradation depends on the composition of the soil microbial community (Bhat et al. 1998), which is different in different habitats (Martiny et al. 2006; Nelson et al. 2016). The different degradation potential of procyanidin affects its bioavailability among the soils tested. However, the fate of procyanidin in different soils needs to be further investigated.
Possible microbial mechanisms for procyanidin to reduce N2O emissions from paddy soils
Procyanidin drastically decreased N2O emissions in the JH and JT soils but to a smaller degree in the QHD soil by inhibiting denitrifying enzyme activities, which is consistent with procyanidin significantly inhibiting NaR activity in the JH and JT soils and the slight inhibition in the QHD soil (Fig. 4), as well as with the literature (Bardon et al. 2016b, 2017; Galland et al. 2019). This suggests that the inhibition of NaR activity by procyanidin was responsible for decreasing soil N2O emissions. However, no obvious inhibition of NiR activity was observed in any of the paddy soils (Fig. S1), likely due to NiR being distributed in the periplasm, making it less sensitive to the inhibitory effect of procyanidin than the membrane-bound NaR (Bardon et al. 2016b). These imply that procyanidin addition reduced N2O emissions by directly affecting NaR rather than NiR activity in our study.
Different clades of nirS-/nirK-denitrifiers played different roles in producing N2O in the paddy soils, with the abundance of nirS clade I denitrifiers significantly related to N2O emissions in all three soils (Fig. 7). This might be because nirS clade I shared high homology with the sequences of denitrifying isolates, which have a high capacity to produce N2O as the end product of denitrification (Wei et al. 2015). Furthermore, N2O emissions significantly increased with the abundance of clade I and III nirK-denitrifiers in the JH soil, but increased with the abundance of clade II and III nirK-denitrifiers in the JT and QHD soils, suggesting that N2O emissions were associated with different denitrifier clades in different soils (Chen et al. 2019; Saito et al. 2008).
We found that the response patterns of denitrifier clades to procyanidin addition varied among soils (Figs. 5 and 6). Our data therefore suggest that the reduction in N2O emissions in the procyanidin-treated soils likely resulted from procyanidin-induced inhibition of the different clades of nirS-/nirK-denitrifiers. The divergent response patterns of these denitrifier clades to procyanidin may be linked to the differences in SOM content in different soils, as SOM strongly influences the size, composition, and activity of nirS- and nirK-type denitrifiers (Tables 1 and 2; Cui et al. 2016; Kandeler et al. 2006). Our data suggest that procyanidin was more effective when the denitrification-derived N2O emissions were high. It is worth noting that procyanidin decreased nirS- and nirK-type denitrifiers abundance, but not NiR activity in the paddy soils, suggesting an indirect effect of less NO2¯-N due to reduction of NaR activity. Additionally, the abundances of nirS- and nirK-harboring denitrifiers were higher than those quantified by traditional primers, which only targeted nirS clade I/nirK clade I (Figs. 5a–c and 6a–c; Table 2). We believe conventional primers targeting nirS and nirK poorly cover some potential abundant denitrifiers, such as Burkholderiales and Rhizobiales bacteria (Wei et al. 2015; Yoshida et al. 2009). These denitrifying bacteria are known to be potential N2O emitters in rice paddy soil (Saito et al. 2008).
Conclusions
Our study demonstrates for the first time that procyanidin, a biological denitrification inhibitor, can mitigate N2O emissions from paddy soils, most likely by inhibiting the activity of nitrate reductase and the growth of the different clades of nirS-/nirK-denitrifiers. In addition, the efficacy of procyanidin in reducing N2O varied dramatically among soils. The use of procyanidin has great potential for mitigating N2O fluxes, especially in soils with a high soil moisture or organic matter content that favors denitrification. Identifying the key factors that control the efficacy of procyanidin is a crucial step in understanding the effect of BDI on denitrification in soils, thus encouraging wider adoption of this BDI to achieve environmental benefits. Moreover, it is important to examine whether applying procyanidin is feasible to limit gaseous N losses and increase rice yield under field conditions. Future studies need to evaluate the different types of environmental benefits of applying procyanidin in paddy fields.
References
Abdelmagid HM, Tabatabai MA (1987) Nitrate reductase activity of soils. Soil Biol Biochem 19:421–427
Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Glob Chang Biol 16:1837–1846
Bardon C, Piola F, Bellvert F, Haichar FZ, Comte G, Meiffren G, Pommier T, Puijalon S, Tsafack N, Poly F (2014) Evidence for biological denitrification inhibition (BDI) by plant secondary metabolites. New Phytol 204:620–630
Bardon C, Piola F, Haichar FZ, Meiffren G, Comte G, Missery B, Balby M, Poly F (2016a) Identification of B-type procyanidins in Fallopia spp. involved in biological denitrification inhibition. Environ Microbiol 18:644–655
Bardon C, Poly F, Piola F, Pancton M, Comte G, Meiffren G, Haichar FZ (2016b) Mechanism of biological denitrification inhibition: procyanidins induce an allosteric transition of the membrane-bound nitrate reductase through membrane alteration. FEMS Microbiol Ecol 92:fiw034
Bardon C, Poly F, Haichar FZ, Le Roux X, Simon L, Meiffren G, Comte G, Rouifed S, Piola F (2017) Biological denitrification inhibition (BDI) with procyanidins induces modification of root traits, growth and N status in Fallopia x bohemica. Soil Biol Biochem 107:41–49
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41:379–388
Bhat TK, Singh B, Sharma OP (1998) Microbial degradation of tannins – a current perspective. Biodegradation 9:343–357
Bhatia A, Sasmal S, Jain N, Pathak H, Kumar R, Singh A (2010) Mitigating nitrous oxide emission from soil under conventional and no-tillage in wheat using nitrification inhibitors. Agr Ecosyst Environ 136:247–253
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. Chemical and microbiological properties. American Society of Agronomy, Madison, pp 595–624
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 368:20130122
Chen H, Yin C, Fan X, Ye M, Peng H, Li T, Zhao Y, Wakelin SA, Chu G, Liang Y (2019) Reduction of N2O emission by biochar and/or 3,4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci Total Environ 694:133658
Chen S, Wang F, Zhang Y, Qin S, Wei S, Wang S, Hu C, Liu B (2018) Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ Microbiol 20:980–992
Cheng Y, Wang J, Wang J, Chang SX, Wang S (2017) The quality and quantity of exogenous organic carbon input control microbial NO3¯ immobilization: a meta-analysis. Soil Biol Biochem 115:357–363
Cui P, Fan F, Yin C, Song A, Huang P, Tang Y, Zhu P, Peng C, Li T, Wakelin SA, Liang Y (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol Biochem 93:131–141
Dassonville N, Guillaumaud N, Piola F, Meerts P, Poly F (2011) Niche construction by the invasive Asian knotweeds (species complex Fallopia): impact on activity, abundance and community structure of denitrifiers and nitrifiers. Biol Invasions 13:1115–1133
Denmead OT, Freney JR, Simpson JR (1976) Closed ammonia cycle within a plant canopy. Soil Biol Biochem 8:161–164
Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins-a final frontier in flavonoid research? New Phytol 165:9–28
Enwall K, Philippot L, Hallin S (2005) Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Appl Environ Microbiol 71:8335–8343
Fan X, Yin C, Chen H, Ye M, Zhao Y, Li T, Wakelin SA, Liang Y (2019) The efficacy of 3,4-dimethylpyrazole phosphate on N2O emissions is linked to niche differentiation of ammonia oxidizing archaea and bacteria across four arable soils. Soil Biol Biochem 130:82–93
Forte A, Fierro A (2019) Denitrification rate and its potential to predict biogenic N2O field emissions in a Mediterranean maize-cropped soil in southern Italy. Land 8:97
Galland W, Piola F, Burlet A, Mathieu C, Nardy M, Poussineau S, Blazère L, Gervaix J, Puijalon S, Simon L, Haichar FZ (2019) Biological denitrification inhibition (BDI) in the field: a strategy to improve plant nutrition and growth. Soil Biol Biochem 136:107513
Galland W, Haichar FZ, Czarnes S, Mathieu C, Demorge J-L, Simon L, Puijalon S, Piola F (2021) Biological inhibition of denitrification (BDI) in the field: effect on plant growth in two different soils. Appl Soil Ecol 159:103857
Hu HW, Chen D, He JZ (2015) Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiol Rev 39:729–749
Huang T, Gao B, Hu XK, Lu X, Well R, Christie P, Bakken LR, Ju XT (2014) Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous fluvo-aquic soil. Sci Rep 4:3950
IPCC (2014) Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK. and New York, NY, USA
Jones CM, Stres B, Rosenquist M, Hallin S (2008) Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol Biol Evol 25:1955–1966
Kalembasa SJ, Jenkinson DS (1973) A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J Sci Food Agric 24:1085–1090
Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L (2006) Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol 72:5957–5962
Köster JR, Cárdenas LM, Bol R, Lewicka-Szczebak D, Senbayram M, Well R, Giesemann A, Dittert K (2015) Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification – an N2O isotopomer case study. Soil Biol Biochem 84:65–74
Kraal P, Nierop KGJ, Kaal J, Tietema A (2009) Carbon respiration and nitrogen dynamics in Corsican pine litter amended with aluminium and tannins. Soil Biol Biochem 41:2318–2327
Kraus TEC, Zasoski RJ, Dahlgren RA, Horwath WR, Preston CM (2004) Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species. Soil Biol Biochem 36:309–321
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276
Lan T, Han Y, Cai Z (2015) Denitrification and its product composition in typical Chinese paddy soils. Biol Fertil Soils 51:89–98
Li J, Kwak JH, Chen J, An Z, Gong X, Chang SX (2021a) Canola straw biochars produced under different pyrolysis temperatures and nitrapyrin independently affected cropland soil nitrous oxide emissions. Biol Fertil Soils 57:319–328
Li Z, Zeng Z, Song Z, Wang F, Tian D, Mi W, Huang X, Wang J, Song L, Yang Z, Wang J, Feng H, Jiang L, Chen Y, Luo Y, Niu S (2021b) Vital roles of soil microbes in driving terrestrial nitrogen immobilization. Glob Chang Biol 27:1848–1858
Linquist BA, Anders MM, Adviento-Borbe MA, Chaney RL, Nalley LL, da Rosa EF, van Kessel C (2015) Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems. Glob Chang Biol 21:407–417
Longo E, Rossetti F, Merkyte V, Obiedzińska A, Boselli E (2018) Selective binding of potassium and calcium ions to novel cyclic proanthocyanidins in wine by high-performance liquid chromatography/high-resolution mass spectrometry. Rapid Commun Mass Spectrom 32:1637–1642
Malique F, Ke P, Boettcher J, Dannenmann M, Butterbach-Bahl K (2019) Plant and soil effects on denitrification potential in agricultural soils. Plant Soil 439:459–474
Martiny JB, Bohannan BJ, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Øvreås L, Reysenbach AL, Smith VH, Staley JT (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112
Mathieu O, Henault C, Leveque J, Baujard E, Milloux MJ, Andreux F (2006) Quantifying the contribution of nitrification and denitrification to the nitrous oxide flux using 15N tracers. Environ Pollut 144:933–940
Meng X, Li Y, Yao H, Wang J, Dai F, Wu Y, Chapman S (2020) Nitrification and urease inhibitors improve rice nitrogen uptake and prevent denitrification in alkaline paddy soil. Appl Soil Ecol 154:103665
Montzka SA, Dlugokencky EJ, Butler JH (2011) Non-CO2 greenhouse gases and climate change. Nature 476:43–50
Morse JL, Ardón M, Bernhardt ES (2012) Using environmental variables and soil processes to forecast denitrification potential and nitrous oxide fluxes in coastal plain wetlands across different land uses. J Geophys Res-Biogeo 117:G02023
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nelson MB, Martiny AC, Martiny JB (2016) Global biogeography of microbial nitrogen-cycling traits in soil. Proc Natl Acad Sci U S A 113:8033–8040
Norman RJ, Edberg JC, Stucki JW (1985) Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci Soc Am J 49:1182–1185
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939:1–19
Pandey A, Suter H, He JZ, Hu HW, Chen D (2021) Dissimilatory nitrate ammonification and N2 fixation helps maintain nitrogen nutrition in resource-limited rice paddies. Biol Fertil Soils 57:107–115
Pu Y, Zhu B, Dong Z, Liu Y, Wang C, Ye C (2019) Soil N2O and NOx emissions are directly linked with N-cycling enzymatic activities. Appl Soil Ecol 139:15–24
Putz M, Schleusner P, Rütting T, Hallin S (2018) Relative abundance of denitrifying and DNRA bacteria and their activity determine nitrogen retention or loss in agricultural soil. Soil Biol Biochem 123:97–104
Qiao C, Liu L, Hu S, Compton JE, Greaver TL, Li Q (2015) How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Glob Chang Biol 21:1249–1257
Qiu Y, Jiang Y, Guo L, Burkey KO, Zobel RW, Shew HD, Hu S (2018) Contrasting warming and ozone effects on denitrifiers dominate soil N2O emissions. Environ Sci Technol 52:10956–10966
R Core Team (2021) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available online at https://www.R-project.org/
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Rodrigues JM, Lasa B, Aparicio-Tejo PM, Gonzalez-Murua C, Marino D (2018) 3,4-Dimethylpyrazole phosphate and 2-(N-3,4-dimethyl-1H-pyrazol-1-yl) succinic acid isomeric mixture nitrification inhibitors: quantification in plant tissues and toxicity assays. Sci Total Environ 624:1180–1186
Saito T, Ishii S, Otsuka S, Nishiyama M, Senoo K (2008) Identification of novel betaproteobacteria in a succinate-assimilating population in denitrifying rice paddy soil by using stable isotope probing. Microbes Environ 23:192–200
Shaukat M, Samoy-Pascual K, Maas E, Ahmad A (2019) Simultaneous effects of biochar and nitrogen fertilization on nitrous oxide and methane emissions from paddy rice. J Environ Manage 248:109242
Silver WL, Herman DJ, Firestone MK (2001) Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology 82:2410–2416
Šimek M, Elhottová D, Klimeš F, Hopkins DW (2004) Emissions of N2O and CO2, denitrification measurements and soil properties in red clover and ryegrass stands. Soil Biol Biochem 36:9–21
Song A, Fan F, Yin C, Wen S, Zhang Y, Fan X, Liang Y (2017) The effects of silicon fertilizer on denitrification potential and associated genes abundance in paddy soil. Biol Fertil Soils 53:627–638
Standford S, English L (1949) Use of flame photometer in rapid soil test for K and Ca. Agron J 41:446–447
Tao R, Wakelin SA, Liang Y, Hu B, Chu G (2018) Nitrous oxide emission and denitrifier communities in drip-irrigated calcareous soil as affected by chemical and organic fertilizers. Sci Total Environ 612:739–749
Thomas RL, Sheard RW, Moyer JR (1967) Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron J 59:240–243
Volpi I, Laville P, Bonari E, di Nasso NNo, Bosco S, (2017) Improving the management of mineral fertilizers for nitrous oxide mitigation: the effect of nitrogen fertilizer type, urease and nitrification inhibitors in two different textured soils. Geoderma 307:181–188
Wang S, Shan J, Xia Y, Tang Q, Xia L, Lin J, Yan X (2017) Different effects of biochar and a nitrification inhibitor application on paddy soil denitrification: a field experiment over two consecutive rice-growing seasons. Sci Total Environ 593–594:347–356
Wei W, Isobe K, Nishizawa T, Zhu L, Shiratori Y, Ohte N, Koba K, Otsuka S, Senoo K (2015) Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J 9:1954–1965
Wu D, Senbayram M, Well R, Brüggemann N, Pfeiffer B, Loick N, Stempfhuber B, Dittert K, Bol R (2017) Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol Biochem 104:197–207
Xia L, Li X, Ma Q, Lam SK, Wolf B, Kiese R, Butterbach-Bahl K, Chen D, Li Z, Yan X (2020) Simultaneous quantification of N2, NH3 and N2O emissions from a flooded paddy field under different N fertilization regimes. Glob Chang Biol 26:2292–2303
Yin C, Fan F, Song A, Cui P, Li T, Liang Y (2015) Denitrification potential under different fertilization regimes is closely coupled with changes in the denitrifying community in a black soil. Appl Microbiol Biot 99:5719–5729
Yin C, Fan F, Song A, Fan X, Ding H, Ran W, Qiu H, Liang Y (2017) The response patterns of community traits of N2O emission-related functional guilds to temperature across different arable soils under inorganic fertilization. Soil Biol Biochem 108:65–77
Yoon S, Cruz-García C, Sanford R, Ritalahti KM, Löffler FE (2015) Denitrification versus respiratory ammonification: environmental controls of two competing dissimilatory NO3¯/NO2¯ reduction pathways in Shewanella loihica strain PV-4. ISME J 9:1093–1104
Yoshida M, Ishii S, Otsuka S, Senoo K (2009) Temporal shifts in diversity and quantity of nirS and nirK in a rice paddy field soil. Soil Biol Biochem 41:2044–2051
Zhang J, Lan T, Müller C, Cai Z (2015a) Dissimilatory nitrate reduction to ammonium (DNRA) plays an important role in soil nitrogen conservation in neutral and alkaline but not acidic rice soil. J Soil Sediment 15:523–531
Zhang J, Müller C, Cai Z (2015b) Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol Biochem 84:199–209
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol R 61:533–616
Acknowledgements
We thank the Soil and Fertilizer Technical Guidance Station of Jintan City for managing the field experiment and for the assistance in the field. We are also grateful to two anonymous reviewers and the Editor-in-Chief for their instructive comments and suggestions that improved an earlier version of this manuscript.
Funding
This work was jointly supported by grants from Zhejiang Provincial Science and Technology Programs (2018C02036), National Key Research and Development Programs of China (2017YFD0200707), and the Fundamental Research Funds for the Central Universities (2019FZJD007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, M., Yin, C., Fan, X. et al. Procyanidin inhibited N2O emissions from paddy soils by affecting nitrate reductase activity and nirS- and nirK-denitrifier populations. Biol Fertil Soils 57, 935–947 (2021). https://doi.org/10.1007/s00374-021-01576-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01576-y