Abstract
Denitrification and its products composition were evaluated in four typical Chinese paddy soils with pH (H2O) ranging from 4.80 to 8.29 after application of 50 or 100 mg kg−1 soil K15NO3 and subsequent anaerobic incubation. Denitrification rates, which were indicated by nitric oxide (NO), nitrous oxide (N2O), and dinitrogen gas (N2) production, significantly varied among different paddy soils. The denitrification rates of the neutral and alkaline paddy soils were 2.6 to 16.6 times higher, respectively, than those of acidic paddies. Furthermore, denitrification in paddy soils could produce end products other than N2, and the product composition depended on the paddy soil type. The percentage of total N gases (NO + N2O + N2) present as N2O was negatively and linearly correlated with denitrification rate (P < 0.05). Soil pH and C/N showed positive effects on denitrification rate (r = 0.800 and r = 0.781, respectively, P < 0.05 for both), but negative effects on the percentage of total N gases present as N2O (r = −0.976, P < 0.01 and r = −0.781, P < 0.05, respectively). Denitrification rate and the percentage of total gases present as N2O increased as the nitrate (NO3 −) concentration increased. However, there was no effect of NO3 − concentration on the percentage of total N gases present as NO. Our results indicate that the potential N loss through denitrification may be higher in alkaline paddies than that in neutral and acidic paddies. Moreover, the variation of the N2O percentage in denitrification products of different paddy soils should be considered when estimating the denitrification-derived N2O emission and when calculating the N budget in paddy soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactive nitrogen (Nr) levels have increased dramatically worldwide due to anthropogenic activities, particularly N-fertilizer production and use as well as fossil fuel combustion (Galloway et al. 2004). As a consequence, environmental Nr is accumulating at local, regional, and global levels and the excess of Nr can have negative impacts on the environment, such as the greenhouse effect, destruction of the ozone layer, acid rain, nitrate pollution in groundwater, eutrophication of lakes, and offshore water (Vitousek et al. 1997).
Denitrification is the reduction of NO3 − through the intermediates nitrite (NO2 −), nitric oxide (NO), and nitrous oxide (N2O) to form dinitrogen gas (N2). It is the only pathway by which Nr in terrestrial and aquatic ecosystems are transformed back into inert N2 gas (Galloway et al. 2004), but denitrification in soil can increase N2O and NO concentrations in the atmosphere.
The denitrification activity depends on the oxygen partial pressure, pH, NO3 − concentration, temperature, availability of electron donors, and the quality and quantity of organic materials (Šimek et al. 2000; D'Haene et al. 2003; Amha and Bohne 2011; Rahman et al. 2014). Losses resulting from the complete reduction of NO3 − to N2 are rarely measured directly in the field; the large atmospheric N2 background makes detecting slight increases in N2 caused by denitrification analytically difficult (Davidson and Seitzinger 2006). Although N2O is generally regarded as an intermediate of the denitrification pathway to N2, it can also be a denitrification end product by denitrifying bacteria that lack nitrous oxide reductase (Papen et al. 1989), further complicating the accurate estimation of the denitrification rate. Therefore, knowledge of the soil denitrification and its product composition are essential in determining the soil N budget.
China is one of the major rice growers in the world with approximately 20 % of the world’s total area dedicated to rice production (Frolking et al. 2002). The current average N-use efficiency of rice cultivation systems in China (% recovery of applied N in plants) ranges from 30 % to 40 % (Zhu and Chen 2002). A major reason for low N-use efficiency in rice paddy fields is the loss of gaseous N through denitrification (Xing and Zhu 2000; Zhu and Chen 2002; Li and Lang 2014).
Numerous studies have investigated denitrification rate in paddy soils, but mostly by the measurement of the amount of NO3 − lost (Aulakh et al. 2001; Xing et al. 2002; Zhu et al. 2003; Wang et al. 2011; Ma et al. 2013), detailed knowledge of the total gaseous (NO + N2O + N2) losses of N via denitrification in rice paddy soils remains inadequate, and the mechanisms underlying N2O and NO emissions in paddy soil through denitrification remain unclear. As reported by Zhang et al. (2009), the amount of total N gases produced is a better measure of denitrification rate than the amount of NO3 − lost because NO3 − may be consumed through immobilization or the dissimilatory reduction of NO3 − to NH4 + (DNRA). Furthermore, the denitrification rate in flooded soils is not controlled by the activity of denitrification enzymes but rather by the rate of NO3 − production through nitrification process (Zhou et al. 2012), which occurs in aerobic micro zone such as the rhizosphere and the interface between standing water and the soil (Nicolaisen et al. 2004). In other words, denitrification rate is substrate depended. Nevertheless, it is not clear whether NO3 − concentration would affect denitrification rate and its product composition.
The measurement of denitrification rates in situ is problematic due to the presence of heterogeneous communities of denitrifers and the physical and chemical complexity of soils (Betlach and Tiedje 1981), thus, elucidating denitrification and its product compositions in paddy soils through laboratory culture is vital to deducing the actual soil N loss through denitrification under field conditions. Therefore, in this study, we first reported the use of total N gases (NO + N2O + N2) production as an indicator of denitrificaton rate of paddy soils, and the effect of NO3 − concentration on denitrification rate and its product composition was also investigated by a 15N labeling anaerobic incubation experiment under laboratory conditions. Four typical Chinese paddy soils that differed in pH, clay content, and C/N ratio were chosen, and two NO3 − concentrations (50 and 100 mg N kg−1 soil), which were within the range of observed NO3 − concentrations in paddy soils after fertilization (Cai and Mosier 2000; Cai 2002), were adopted.
Materials and methods
Site description and soil sampling
Two paddy soils were collected from Yixing (31°17′N, 119°54′E) and Huai’an (33°43′N, 118°86′E), situated in the North and South Jiangsu provinces, respectively, and two from the Ecological Experiment Station of Red Soil, the Chinese Academy of Sciences, Yingtan (28°15′N, 116°55′E), Jiangxi Province. The four paddy soils were developed from different parent materials. The Yixing soil was developed from alluvial deposits and classified as Hydragric Anthrosol (Hy). The Huai’an soil was developed from lacustrine sediment and classified as Anthraquic Cambisol (An). One of the soil samples from Yingtan was derived from red sandstone and classified as Haplic Acrisol (Ha), whereas the other was derived from quaternary red clay and classified as Ferralsol (Fe) according to the World Reference Base for Soil Resources system (IUSS Working Group WRB 2007). Fifteen soil cores from each site were pooled, sieved (<2 mm), and immediately stored at 4 °C until analysis. The key properties of the soils are shown in Table 1.
Anaerobic incubation and sampling
Denitrification rate was determined using the anaerobic incubation method of Xu and Cai (2007) and slightly modified by Zhang et al. (2009). In brief, for each paddy soil sample, a set of 250 mL each Erlenmeyer flask contained 40 g (oven-dried equivalent) of fresh soil and 40 mL of deionized water. The flasks for each soil were then divided into two groups for two levels of NO3 − concentration treatments; 2 mL of a K15NO3 (20 % 15 N atom % excess) solution containing 2.0 mg of NO3 − (equivalent to 50 mg of NO3 − kg−1 soil (N50)) or 4.0 mg of NO3 − (equivalent to 100 mg of NO3 − kg−1 soil (N100)) were uniformly added to each flask for groups 1 and 2, respectively, using a 2.5-mL syringe (no carbon addition). The flasks were immediately capped with airtight silicone rubber stopper fitted with butyl rubber septa. A silicone sealant was placed around the stoppers to ensure strictly airtight conditions. The flasks were connected to a multiport vacuum manifold to be vacuumed simultaneously and flushed with highly purified N2 gas. Such procedure was repeated three times (each for 12 min) to create anaerobic conditions (Zhang et al. 2009). After equilibration at atmospheric pressure, the flasks were incubated in the dark at 25 ± 1 °C. At 3, 6, 12, 24, 48, 96, 168, and 264 h after the addition of 15NO3 − solution, three flasks from each soil and nitrate treatment were randomly selected, and the headspace gases were sampled to determine the N2O and NO concentrations and N2O and N2 isotopic compositions using a 25-mL syringe. Immediately after gas sampling, soil in the flask was extracted with 160 mL 2.5 M KCl solution. The mixture (solution plus soil) was shaken at 25 °C for 1 h and filtered through a qualitative filter paper. The filtrates were then stored at 4 °C prior being analyzed for mineral N (NO3 − and NH4 +) concentrations and the relative 15N abundance. In order to determine the concentration and isotopic composition of insoluble organic N, the KCl-extracted soil was washed with distilled water to remove residual mineral N and subsequently oven-dried at 55 °C.
Analyses
The N2O concentrations of samples were determined using an Agilent 7890 gas chromatograph (Agilent, USA). The NO concentrations in the samples were measured using an NO x analyzer (Model 42i, Thermo Environmental Instruments Inc, Franklin, MA, USA). A segmented flow analyzer (Skalar SAN++, Netherlands) was used to determine the concentrations of NH4 + and NO3 − in soil extracts. The N2O and N2 isotopic compositions were determined using a Finnigan MAT 253 isotopic ratio mass spectrometer. A method based on N2O production from hydroxylamine intermediates, after reduction with Cd/Cu, was used to determine 15N enrichment of NO3 − in KCl extracts (Stevens and Laughlin 1994), as described in detail by Lan et al. (2013). The 15N enrichment of NH4 + in the KCl extracts was determined by distillation with MgO, and by isotopic mass spectrometry (Finnigan MAT 251) after converting NH4 + in soil to molecular N2 using NaBrO. The concentration and 15N composition of insoluble organic N remaining in the soil after KCl extraction were determined by isotopic mass spectrometry (Finnigan MAT 251) after converting NH4 + in soil to molecular N2 by Kjeldahl digestion with NaBrO (Zhang et al. 2009).
Calculations and statistical analyses
N2 production
The total amount of N2 evolved in the Erlenmeyer flask was calculated as described by Zhang et al. (2009), by considering the flask headspace volume, N2 density, and dissolved N2 in the soil solution, as follows:
where C is the total amount of N2 (mg kg−1 soil), 1.15 is the density of N2 at standard pressure and 25 °C (kg m−3), V g is the headspace volume (m3), V l is the water volume (m3), α is the Bunsen correction coefficient (0.0143 at 25 °C), and W is the soil weight (kg). The change in air pressure in the flasks was insignificant during incubation and was ignored in the calculation of N2 production.
The amount of 15N2 and 14N2 produced from denitrification during the anaerobic incubation period was calculated based on the total amount of N2 in the flask calculated by Eq. (1), the measured atom % excess of 15N in N2, and by assuming that the isotopic composition of the produced N2 was the same as that of the reduced soil NO3 − (Zhang et al. 2009). To verify whether the atom % excess 15N in NO3 − could calculate N2 gas production through denitrification, the temporal variation in the average atom % excess 15N in NO3 − was evaluated. The preliminary results showed that the atom % excess 15N in NO3 − slightly decreased in all soils and NO3 − treatments during incubation (Fig. 1). The lowest atom % excess 15N in NO3 − was observed in An soil, whereas the highest was obtained in Fe soil. The temporal variations in the atom % excess 15N in NO3 − ranged from 1.52 % to 3.98 %, with the highest in the An-N50 treatment and the lowest in the Hy-N100 treatment. These results indicate the suitability of this approach.
Temporal variations in the 15N atom % excess of NO3 − in paddy soils. (a, 50 mg N kg−1 labeled NO3 − treatment; b, 100 mg N kg−1 labeled NO3 − treatment; Hy, Hydragric Anthrosol, An, Anthraquic Cambisol; Ha, Haplic Acrisol; and Fe, Ferralsol. Data are the mean of three replicates. Bars represent standard deviations)
Denitrification rate, percentage of total evolved N gases (NO + N2O + N2) present as N2O or NO, and recovery of added NO3 −-15N
The denitrification rate was expressed as the total N gases (NO + N2O + N2) production per hour during incubation. We also calculate the percentage of total N gases present as N2O or NO. The recovery of added 15NO3 − after the anaerobic incubation was calculated using Eq. (2). 15N enrichment in NO and dissolved organic N of KCl extracts were not considered because not determined.
Statistical analyses
The differences in the soil properties, denitrification rates, and percentage of total evolved N gases present as NO or N2O of the different treatments were evaluated by ANOVA and compared by the Tukey’s test at P < 0.05, using SPSS software package 18.0 for Windows. Spearman’s rank correlation coefficient analysis was used to determine the edaphic variables correlated with denitrification rate, and the percentage of total N gases present as NO or N2O using the SPSS software package 18.0 for Windows.
Results
NO3 − dynamics during incubation
Under anaerobic conditions, the added NO3 − was continuously consumed with increasing incubation time in all tested soils, except the Ha soil in which maintained a high NO3 − concentration during the incubation (Fig. 2a, b). The NO3 − level was negligible in the Fe soil when it was treated with 50 mg N kg−1 NO3 − after 168 h, and in the Hy and An soils after 264 h (Fig. 2a). By contrast, large amounts of NO3 − (>20 mg N kg−1) remained in all soils at the end of the incubation when treated with 100 mg of NO3 − (Fig. 2b). However, when treated with both 50 and 100 mg of NO3 −, the amount of consumed NO3 − varied significantly among the different soils after 264 h, soils could be ranked An > Hy > Fe > Ha (Fig. 2b).
Changes in the NH4 +-N and NO3 −-N concentrations in paddy soils during anaerobic incubation. (a and c, 50 mg N kg−1 labeled NO3 − treatment; b and d, 100 mg N kg−1 labeled NO3 − treatment; Hy, Hydragric Anthrosol; An, Anthraquic Cambisol; Ha, Haplic Acrisol; and Fe, Ferralsol. Data are the mean of three replicates. Bars represent standard deviations)
Nitrogen gas production patterns and denitrification rate
The dynamics of N gases production in different soils significantly varied. Both NO and N2O concentrations were detected in Hy and An soils 3 h after the start of incubation regardless of the added NO3 − concentration (Fig. 3a–d); NO and N2O production in Ha and Fe soils were detected only after 12 and 24 h, respectively (Fig. 3a–d). The N2O concentrations remained at high levels in the two acidic paddy soils (Ha and Fe), whereas the NO concentrations were at considerably lower levels (Fig. 3a–d). The NO production generally peaked earlier than that of N2O regardless of the NO3 − treatment (Fig. 3a–d), and NO was no longer detected in Hy and An soils after 96 h (Fig. 3a, b). Nitrous oxide remained detectable in all treatments at the end of incubation except in the An-N50 treatment, where it was undetectable after 168 h (Fig.3c, d). 15N-labeled N2 was not detected in Hy and An soils treated with both NO3 − concentrations until after 6 h, and it was until 168 h in the two acidic paddy soils (Ha and Fe; Fig. 3e, f). Dinitrogen gas production in all soils and NO3 − treatments continuously increased as the incubation proceeded (Fig. 3e, f). However, at the end of the incubation, the total N gases (NO + N2O + N2) that accumulated in the headspace significantly varied among soils and nitrate treatments (P < 0.05; Fig. 3g, h). The accumulated total N gases in the N100 treatment were approximately 1.2 to 1.4 times higher than those of the corresponding N50 treatment. The calculated denitrification rates significantly varied among the treatments after 264 h of incubation, ranging from 0.014 mg N kg−1 h−1 in the Ha-N50 treatment to 0.273 mg N kg−1 h−1 in the An-N100 treatment (Table 2). The denitrification rates of the neutral and alkaline paddy soils were 2.6 to 16.6 times higher, respectively, than those of acidic paddies (Table 2).
Production of NO-N, N2O-N, and N2-N in paddy soils during anaerobic incubation. (Hy, Hydragric Anthrosol; An, Anthraquic Cambisol; Ha, Haplic Acrisol; and Fe, Ferralsol. N50, 50 mg N kg−1 labeled NO3 − treatment; N100, 100 mg N kg−1 labeled NO3 − treatment. Data are the mean of three replicates. Bars represent standard deviations
Recovery of added 15NO3 −
Labeled 15N was not fully recovered as NO3 −, NH4 +, N2O, N2, and insoluble organic N. The 15NO3 − recovery rate in all soils was approximately 90 % 3 h after NO3 − addition to soil. This value gradually decreased to 56 % to 69 % in the N50 treatment and to 61 % to 78 % in the N100 treatment at the end of the incubation. Moreover, 15N was detected in NH4 + (particularly in Fe soil; Fig. 2c, d) and soil organic matter (data not shown) after incubation.
Percentage of the total evolved N gases (NO + N2O + N2) presented as NO and N2O
The percentage of total N gases present as NO was negligible (<1 %) in the two acidic paddy soils during the entire 264 h of incubation and it was significantly higher in Hy and An soils (Fig. 4a, b), where it decreased as the incubation proceeded and became undetectable after 96 h. The percentage of total N gases present as NO was higher in Hy soil than that in An soil (Fig. 4a, b). However, there was no difference in the percentage of total N gases present as NO when the N50 and N100 treatments were compared (Table 2).
Changes in the percentage of total evolved N gases present as NO (a, b) and as N2O (c, d) in paddy soils during anaerobic incubation (a, c 50 mg N kg−1 labeled NO3 − treatment; b, d 100 mg N kg−1 labeled NO3 − treatment; Hy hydragric anthrosol, An anthraquic cambisol, Ha haplic acrisol, Fe ferralsol. Data are the mean of three replicates. Bars represent standard deviations)
The percentage of total N gases present as N2O was generally higher than the percentage of total N gases present as NO during the all incubation in all treatments, particularly in the two acidic paddy soils (Fig. 4). The percentage of total N gases present as N2O increased between 3 and 12 h in Hy and An soils and between 3 and 96 h in Ha and Fe soils but subsequently decreased in all soils and NO3 − treatments (Figs. 4c, d). The acidic paddy soils (Ha and Fe) maintained higher percentage of total N gases present as N2O than the neutral (Hy) and alkaline (An) paddy soils. The average percentage of total N gases present as N2O in Hy and An soils were significantly lower than those in the acidic paddy soils, in which the percentages exceeded 60 % (Table 2). Furthermore, the addition of NO3 − to soil increased the percentage of total N gases present as N2O in all tested paddy soils (Table 2).
Discussion
The four paddy soils developed from different parent materials significantly differed in NO3 − consumption, which ranged from negligible to complete disappearance of NO3 − at the end of incubation at 25 °C when NO3 − was added at a rate of 50 mg N kg−1 soil (Fig. 2). The obtained denitrification rates of Hy and An soils collected from Jiangsu Province were higher than those of Ha and Fe soils collected from Jiangxi Province when the N50 treatments were compared (Table 2). This confirms the reported positive correlation between denitrification rate and latitude in the forest soils of eastern China by Zhang et al. (2009). However, the denitrification rates of the four paddy soils (from 0.014 mg N kg−1 h−1 to 0.273 mg N kg−1 h−1) were generally higher than those of forest soils (from 0.011 mg N kg−1 h−1 to 0.127 mg N kg−1 h−1) collected from different climatic zones of China and measured using the same method (Zhang et al. 2009). Similar results were reported by Xu and Cai (2007), who found that rice cultivation significantly increases denitrification rate (based on NO3 − concentration measurements) compared with four other land uses (tea garden land, forestland, brush land, and upland). According to Xu and Cai (2007), this phenomenon is most probably due to increases in the organic C and total N contents in the soil, which promote the population growth and activities of microbial under anaerobic conditions of flooded rice fields.
Parent materials generally affect soil formation processes and lead to differences in soil physicochemical properties, which subsequently affect N transformations (Miller and Donahue 1990). Denitrification rate was reported to be correlated with the amount of easily-decomposable soil organic C, as well as soil pH and C/N (D'Haene et al. 2003; Amha and Bohne 2011). In this study, the four paddy soils developed from different parent materials exhibited different properties such as pH, clay content, and C/N ratio. Spearman’s correlation coefficients indicate that the denitrification rates of four Chinese paddy soils were significantly correlated with soil pH (r = 0.800, P < 0.05) and C/N ratio (r = 0.781, P < 0.05). Thus, soil pH and C/N may be important regulators of denitrification in paddy soils. The slightly alkaline paddy soil (An) had higher denitrification rate than the neutral (Hy) and acidic (Ha and Fe) paddy soils, which confirming that the optimum pH for denitrifiers is between 7.0 and 8.0 as reported by Wijler and Delwiche (1954) and Davidson et al. (1985). However, Xu and Cai (2007) and Zhang et al. (2009) were unable to establish any relationship between pH and denitrification rate. Denitrification is an electron-consuming and heterotrophic process (Ahn 2006), in which the available C provides electrons for NO3 − reduction, thereby promoting denitrification (Heinen 2006). This phenomenon may explain the positive relationship between denitrification rate and the C/N ratio. Therefore, low denitrification rates in Ha and Fe soils may due to the small amount of easily-decomposable soil organic C, which worth further investigation.
The denitrification rates of the four tested paddy soils increased as the NO3 − concentration increased. The added NO3 − possibly affected denitrification through two different ways: (a) by directly regulating the availability of the denitrification substrate and (b) by indirectly affecting denitrifying microbes (Drury et al. 1991; Amha and Bohne 2011). The NO3 − supply in flooded paddy soils is primarily derived from the nitrification of added ammonia or urea fertilizers. Evidence showed that the nitrification rate increases with increasing pH (from 6.0 to 8.5), with the optimum pH at approximately 8.5 (Sahrawat 1982, 2008). Thus, the denitrification-induced loss of the NO3 − produced through the higher nitrification rates of alkaline paddy soils would probably be higher than those in neutral and acidic paddy soils. However, our results also show that when the added NO3 − concentration was increased from 50 mg N kg−1 to 100 mg N kg−1, the denitrification rates in the paddy soils exhibited only an approximately 1.2- to 1.4-fold increase, which indicates that denitrification rate gradually increased when the NO3 − concentration exceeded 50 mg N kg−1. This is in agreement with many models that consider denitrification to be a function of NO3 − concentration, as described by Michaelis–Menten relationship (Heinen 2006).
The added of NO3 −-N was not recovered by summing up the N gas products, NH4 +-N, and organic N at the end of incubation. The amount of N gases produced is a more precise measure of the denitrification rate than the amount of NO3 − consumed, as also reported by Zhang et al. (2009) and Yu et al. (2014). The greater increase in the NH4 + concentration was observed in Fe soil (Fig. 2c, d). Ammonium production through the dissimilatory reduction of NO3 − to NH4 + (DNRA) has been observed in rice paddy soils (reaching 21 % of NO3 − consumption; Chen et al. 1995a, 1995b; Yin et al. 2002). There is evidence that many soil bacteria and fungi have the ability to perform DNRA. Redox status and ratio of C/NO3 − have been identified as the most important factors regulating DNRA in soil (Rutting et al. 2011). However, factors affecting DNRA in paddy soils are still poorly known.
The denitrification of these paddy soils produce end products other than N2, and product composition depended on the paddy soil type (Table 2). The percentage of total N gases present as NO were smaller than those percentage of total N gases present as N2O regardless of the soil and NO3 − treatment (Fig. 4). The acidic paddy soils showed a significantly smaller NO than N2O percentage than the alkaline and neutral paddy soils (Table 2). This result probably lead to the absence of a significant correlation between the NO and N2O percentages (r = 0.161, P > 0.05). The percentage of total N gases present as NO was correlated with total C content, but not with soil pH and NO3 − concentration.
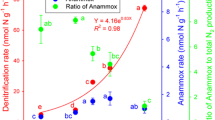
In the two acidic paddy soils (Ha and Fe), the percentage of total N gases present as N2O was approximately 60 % (Table 2); whereas in Hy and An paddy soils, it was below 25 % and N2 was the main product. The decreased N2O production at the end of incubation was probably due to the strong reducing conditions, which promoted the complete reduction of NO3 − to N2. The percentage of total N gases present as N2O was negatively and linearly correlated with denitrification rate when the same amount of NO3 − was added to soil (Fig. 5), which implies that the soil with lower denitrification rate tends to have a higher N2O proportion in the denitrification products.
The variations in composition of denitrification products of the different paddy soils could be ascribed to their different soil properties. The N gases produced during soil denitrification are reportedly dependent on various edaphic and environmental factors (Zhang et al. 2009; Senbayram et al. 2012). In the present study, a negative Spearman’s correlation was established between the percentage of total N gases produced as N2O and soil pH (r = −0.976, P < 0.01, Table 3), which confirms previous laboratory and field studies, showing that the N2O/N2 ratio decreases when the soil pH increases (Goodroad and Keeney 1984; Šimek and Cooper 2002; Liu et al. 2010). Moreover, the percentage of total N gases present as N2O was also negatively correlated with soil C/N ratio (r = −0.781, P < 0.05, Table 3), probably because higher available organic C substrates in soil promote the complete reduction of low to moderate levels of NO3 to N2 gas, thus reducing the evolved N2O (Ullah et al. 2005). The addition of NO3 − to soil increased the percentage of total N gases present as N2O in all tested paddy soils (Table 2), probably because NO3 reduction is more energy-efficient than that of N2O (Ullah et al. 2005). Our results suggest that soils that had lower denitrification rates had higher percentage of total N gases present as N2O in their denitrification products at the same NO3 − concentration, whereas increased NO3 − concentration promoted denitrification and increased the percentage of N2O in the produced N gases.
Conclusions
Denitrification rate and its product compositions significantly varied among the studied paddy soils. The potential N loss through denitrification was higher in the alkaline paddy soils than that in the neutral and acidic paddy soils, and the N loss was also higher in soils with high C/N ratios. Denitrification in paddy soils could produce end products other than N2, therefore, the variation of the N2O proportion in denitrification products of different paddy soils should be considered when estimating the denitrification-derived N2O emission and when calculating the N budget in paddy soils. However, further studies must be conducted to confirm our results regarding the denitrification and associated N gas production mechanisms in paddy soils. Future work should include an increased number of soil types to establish more definitively the factors affecting denitrification in the paddy soils of China.
References
Ahn YH (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721. doi:10.1016/j.procbio.2006.03.033
Amha Y, Bohne H (2011) Denitrification from the horticultural peats: effects of pH, nitrogen, carbon, and moisture contents. Biol Fertil Soils 47:293–302. doi:10.1007/s00374-010-0536-y
Aulakh MS, Khera TS, Doran JW, Bronson KF (2001) Denitrification, N2O and CO2 fluxes in rice-wheat cropping system as affected by crop residues, fertilizer N and legume green manure. Biol Fertil Soils 34:375–389. doi:10.1007/s003740100420
Betlach MR, Tiedje JM (1981) Kinetic explanation for accumulation of nitrite, nitric-oxide, and nitrous-oxide during bacterial denitrification. Appl Environ Microbiol 42:1074–1084
Cai Z (2002) Ammonium transformation in paddy soils affected by the presence of nitrate. Nutr Cycl Agroecosyst 63:267–274. doi:10.1023/A:1021154909229
Cai ZC, Mosier AR (2000) Effect of NH4Cl addition on methane oxidation by paddy soils. Soil Biol Biochem 32:1537–1545. doi:10.1016/S0038-0717(00)00065-1
Chen DL, Chalk PM, Freney JR (1995a) Distribution of reduced products of 15N-labelled nitrate in anaerobic soils. Soil Biol Biochem 27:1539–1545. doi:10.1016/0038-0717(95)00098-Y
Chen DL, Chalk PM, Freney JR, Smith CJ, Luo QX (1995b) Estimation of nitrification rates in flooded soils. Microb Ecol 30:269–284. doi:10.1007/BF00171934
Davidson EA, Seitzinger S (2006) The enigma of progress in denitrfication research. Ecol Appl 16:2057–2063. doi:10.1890/1051-0761(2006)016
Davidson EA, Strand MK, Galloway LF (1985) Evaluation of the most probable number method for enumerating denitrifying bacteria. Soil Sci Soc Am J 49:642–645. doi:10.2136/sssaj1985.03615995004900030023x
D'Haene K, Moreels E, Neve S, Chaves Daguilar B, Boeckx P, Hofman G, Cleemput O (2003) Soil properties influencing the denitrification potential of Flemish agricultural soils. Biol Fertil Soils 38:358–366. doi:10.1007/s00374-003-0662-x
Drury CF, McKenney DJ, Findlay WI (1991) Relationships between denitrification, microbial biomass and indigenous soil properties. Soil Biol Biochem 23:751–755. doi:10.1016/0038-0717(91)90145-A
Frolking S, Qiu J, Boles S, Xiao X, Liu J, Zhuang Y, Li C, Qin X (2002) Combining remote sensing and ground census data to develop new maps of the distribution of rice agriculture in China. Global Biogeochem Cycles 16:1091–1101. doi:10.1029/2001GB001425
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vöosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226. doi:10.1007/s10533-004-0370-0
Goodroad LL, Keeney DR (1984) Nitrous oxide production in aerobic soils under varying pH, temperature and water content. Soil Biol Biochem 16:39–43. doi:10.1016/0038-0717(84)90123-8
Heinen M (2006) Simplified denitrification models: overview and properties. Geoderma 133:444–463. doi:10.1016/j.geoderma.2005.06.010
IUSS Working Group WRB (2007) World Reference Base for Soil Resources 2006, first update 2007. World Soil Resources Reports No. 103, FAO, Rome
Lan T, Han Y, Roelcke M, Nieder R, Cai ZC (2013) Processes leading to N2O and NO emissions from two different Chinese soils under different soil moisture contents. Plant Soil 371:611–627. doi:10.1007/s11104-013-1721-1
Li P, Lang M (2014) Gross nitrogen transformations and related N2O emissions in uncultivated and cultivated black soil. Biol Fertil Soils 50:197–206. doi:10.1007/s00374-013-0848-9
Liu B, Morkved PT, Frostegard A, Bakken LR (2010) Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol Ecol 72:407–417. doi:10.1111/j.1574-6941.2010.00856.x
Ma YC, Sun LY, Zhang XY, Yang B, Wang JY, Yin B, Yan XY, Xiong ZQ (2013) Mitigation of nitrous oxide emissions from paddy soil under conventional and no-till practices using nitrification inhibitors during the winter wheat-growing season. Biol Fertil Soils 49:627–635. doi:10.1007/s00374-012-0753-7
Miller RW, Donahue RL (1990) Soils: an introduction to soils and plant growth. Prentice Hall, Englewood Cliffs
Nicolaisen MH, Petersen NR, Revsbech NP, Reichardt W, Ramsing NB (2004) Nitrification–denitrification dynamics and community structure of ammonia oxidizing bacteria in a high yield irrigated Philippine rice field. FEMS Microbiol Ecol 49:359–369. doi:10.1016/j.femsec.2004.04.015
Papen H, Vonberg R, Hinkel I, Thoene B, Rennenberg H (1989) Heterotrophic nitrification by Alcaligenes Faecalis: NO2 −, NO3 −, N2O, and NO production in exponentially growing cultures. Appl Environ Microb 55:2068–2072
Rahman MM, Basaglia M, Vendramin E, Boz B, Fontana F, Gumiero B, Casella S (2014) Bacterial diversity of a wooded riparian strip soil specifically designed for enhancing the denitrification process. Biol Fertil Soils 50:25–33. doi:10.1007/s00374-013-0828-0
Rutting T, Boeckx P, Muller C, Klemedtsson L (2011) Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 8:1779–1791. doi:10.5194/bg-8-1779-2011
Sahrawat KL (1982) Nitrification in some tropical soils. Plant Soil 65:281–286. doi:10.1007/BF02374659
Sahrawat KL (2008) Factors affecting nitrification in soils. Commun Soil Sci Plant 39:1436–1446. doi:10.1080/00103620802004235
Senbayram M, Chen R, Budai A, Bakken L, Dittert K (2012) N2O emission and the N2O/(N2O + N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agric Ecosyst Environ 147:4–12. doi:10.1016/j.agee.2011.06.022
Šimek M, Cooper JE (2002) The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci 53:345–354. doi:10.1046/j.1365-2389.2002.00461.x
Šimek M, Cooper JE, Picek T, Šantrůčková H (2000) Denitrification in arable soils in relation to their physico-chemical properties and fertilization practice. Soil Biol Biochem 32:101–110. doi:10.1016/S0038-0717(99)00137-6
Stevens RJ, Laughlin RJ (1994) Determining nitrogen-15 in nitrite or nitrate by producing nitrous oxide. Soil Sci Soc Am J 58:1108–1116. doi:10.2136/sssaj1994.03615995005800040015x
Ullah S, Breitenbeck GA, Faulkner SP (2005) Denitrification and N2O emission from forested and cultivated alluvial clay soil. Biogeochemistry 73:499–513. doi:10.1007/s10533-006-9040-8
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750. doi:10.1890/1051-0761
Wang JY, Zhang M, Xiong ZQ, Liu PL, Pan GX (2011) Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol Fertil Soils 47:887–896. doi:10.1007/s00374-011-0595-8
Wijler J, Delwiche CC (1954) Investigations on the denitrifying process in soil. Plant Soil:155–169. doi:10.1007/BF01343848
Xing GX, Zhu ZL (2000) An assessment of N loss from agricultural fields to the environment in China. Nutr Cycl Agroecosyst 57:67–73. doi:10.1023/A:1009717603427
Xing GX, Cao YC, Shi SL, Sun GQ, Du LJ, Zhu JG (2002) Denitrification in underground saturated soil in a rice paddy region. Soil Biol Biochem 34:1593–1598. doi:10.1016/S0038-0717(02)00143-8
Xu YB, Cai ZC (2007) Denitrification characteristics of subtropical soils in China affected by soil parent material and land use. Eur J Soil Sci 58:1293–1303. doi:10.1111/j.1365-2389.2007.00923.x
Yin SX, Chen D, Chen LM, Edis R (2002) Dissimilatory nitrate reduction to ammonium and responsible microorganisms in two Chinese and Australian paddy soils. Soil Biol Biochem 34:1131–1137. doi:10.1016/S0038-0717(02)00049-4
Yu YJ, Zhang JB, Chen WW, Zhong WH, Zhu TB, Cai ZC (2014) Effect of land use on the denitrification, abundance of denitrifiers, and total nitrogen gas production in the subtropical region of China. Biol Fertil Soils 50:105–113. doi:10.1007/s00374-013-0839-x
Zhang J, Cai Z, Cheng Y, Zhu T (2009) Denitrification and total nitrogen gas production from forest soils of Eastern China. Soil Biol Biochem 41:2551–2557. doi:10.1016/j.soilbio.2009.09.016
Zhou S, Sakiyama Y, Riya S, Song XF, Terada A, Hosomi M (2012) Assessing nitrification and denitrification in a paddy soil with different water dynamics and applied liquid cattle waste using the 15Nisotopic technique. Sci Total Environ 430:93–100. doi:10.1016/j.scitotenv.2012.04.056
Zhu ZL, Chen DL (2002) Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosyst 63:117–127. doi:10.1023/A:1021107026067
Zhu JG, Liu G, Han Y, Zhang YL, Xing GX (2003) Nitrate distribution and denitrification in the saturated zone of paddy field under rice/wheat rotation. Chemosphere 50:725–732. doi:10.1016/S0045-6535(02)00212-6
Acknowledgments
This project was financially supported by the following Sino-German collaborative projects: “Innovative nitrogen management technologies to improve agricultural production and environmental protection in intensive Chinese agriculture” funded by the German Ministry of Education and Research (BMBF FKZ: 0330800C), “Cultivated land conservation and nitrogen fertilizer management technology” funded by the Ministry of Science and Technology of China (MOST grant no. 2007DFA30850), and the DAAD-PPP project (project ID 50751522) with the China Scholarship Council (personal approval no. 2011016097).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Submitted to: Biology and Fertility of Soils
Rights and permissions
About this article
Cite this article
Lan, T., Han, Y. & Cai, Z. Denitrification and its product composition in typical Chinese paddy soils. Biol Fertil Soils 51, 89–98 (2015). https://doi.org/10.1007/s00374-014-0953-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0953-4