Abstract
A field experiment was carried out to study the responses of soil respiration, soil C and N, composition of main microbial groups, enzyme activity, and microbial C source utilization to two organic amendments (i.e., straw and manure) added alone or combined with biochar for 9 months in an infertile soil. In comparison with the non-amended control, soil CO2 emission rates were significantly increased following straw and manure addition, but they were decreased by 19% and 36% on average when co-applied with biochar. Organic amendments significantly increased the size of labile C pool by 21%, total phospholipid-fatty acid (PLFA) concentrations by 32%, activities of α-glucosidase by 51%, β-glucosidase by 115%, β-D-cellobiosidase by 105%, β-xylosidase by 43%, phenol oxidase by 59%, and peroxidase by 91% on average relative to the non-amended control, whereas biochar co-application with organic amendments had no effect on soil PLFA concentrations but significantly decreased the enzyme activities by 38% and the sizes of labile C and N pools by 16% on average. The results of the substrate-induced respiration confirmed a lower capacity of biochar-treated soils to use the C sources of carbohydrates and carboxylic acids. Structural equation modeling indicated that the response of soil CO2 emission rates to organic amendment and biochar was directly linked to their effects on microbial activities. Therefore, our study demonstrated that biochar co-applied with straw or manure mitigated soil C loss by decreasing soil microbial activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Improving soil fertility while at the same time enhancing soil carbon (C) storage has been recognized as a global-scale soil climate mitigation strategy, because it is essential for sustainable agricultural production and food security as well as the mitigation of greenhouse gas emissions (Lal 2004; Pan et al. 2009; Schmidt et al. 2011; Amelung et al. 2020). Such improvements are particularly important for degraded lands that occupy nearly 33% of the global soils and have been subjected to long-term soil organic C losses (FAO 2019; Amelung et al. 2020). Application of organic amendments, such as straw and organic manure, has been widely recommended as an environmentally friendly practice to stop soil degradation and to enhance soil quality and crop production, as they increase organic C input and mineral nutrient availability while reducing the input of synthetic fertilizers (Bhattacharyya et al. 2012; Ventorino et al. 2013; Lin et al. 2019). However, both straw and manure applications have been shown to increase emissions of soil CO2 and CH4 with potential effects on global warming (Liu et al. 2014; Zhang et al. 2020). Converting crop residues into biochar via pyrolysis and its soil amendment can be an alternative way to increase soil C storage with other co-benefits for ecosystem functioning (Lehmann 2007; Sohi 2012), such as increase in nutrient retention and soil C stability, and amelioration of soil acidity (Singh and Cowie 2014; Dai et al. 2017). For this reason, biochar co-application with organic amendments has been suggested as a promising strategy to improve soil quality in recent years (Agegnehu et al. 2017; Singh et al. 2019). Although the sole effects of biochar and organic amendments on soil greenhouse gas emissions have been frequently reported (Wu et al. 2013; Singh et al. 2019), their combined effects are poorly known.
Soil respiration rate depends on soil organic matter quality, microbial community composition, and metabolic activities (Conant et al. 2011; Brookes et al. 2017). Change in the composition and partitioning of different soil organic matter pools with contrasting decomposability is one of the key factors affecting soil organic matter turnover (Rovira and Vallejo 2002; Belay-Tedla et al. 2009). The incorporation of organic amendments into soil increased soil labile C and N pools, thus enhancing soil respiration (Blagodatskaya and Kuzyakov 2008; Liu et al. 2014; Nguyen and Marschner 2016), whereas biochar application increased the recalcitrant C pools (Qayyum et al. 2014; Li et al. 2018; Chen et al. 2019), with contrasting effects on soil respiration likely due to diverse types of biochar and soil with different chemical and biological stabilities (Liu et al. 2016). For example, a few studies suggested that biochar increased soil respiration by increasing soil labile C fractions (Smith et al. 2010; Jones et al. 2011; Mitchell et al. 2015), whereas others found that biochar suppressed soil respiration by decreasing C availability because biochar particles adsorbed the dissolved organic C (Lu et al. 2014), or increased the chemical stabilization of organic C (Keith et al. 2011; Singh and Cowie 2014; Weng et al. 2017).
Soil microorganisms play an important role in nutrient cycling and organic matter decomposition also due to their enzyme production (Campbell et al. 2003; Nannipieri et al. 2003, 2018; Sinsabaugh 2010). Extractable C sources, such as non-cellulosic polysaccharides, were broken down mostly by hydrolytic enzymes, while acid-resistant C sources such as lignin and tannin were often broken down by oxidative enzymes produced by fungi and gram positive bacteria (Sinsabaugh 2010; German et al. 2011). Soil enzyme activities are often regulated by microbial populations as well as the availability and quality of nutrients, such as C, N, and P, both in soil and litter (Hill et al. 2014; Fanin and Bertrand 2016; Dong et al. 2021). Straw and manure amendments can increase microbial biomass and enzyme activities related to organic matter decomposition as a result of increased N and P nutrients and labile substrates (Maisto et al. 2010; Wu et al. 2013; Mackie et al. 2015; Elzobair et al. 2016). For example, straw and manure amendments have been reported to increase soil hydrolase activities, such as urease, β-glucosidase, cellobiohydrolase, and β-1,4-N-acetylglucosaminidase (Ai et al. 2012; García-Orenes et al. 2016). However, the effects of biochar on soil enzyme activities are contrasting with both positive, negative, or negligible effects (Lammirato et al. 2011; Elzobair et al. 2016; Chen et al. 2017), though biochar also contains some labile C compounds that can rapidly stimulate microbial growth and activity (Smith et al. 2010; Luo et al. 2011; Farrell et al. 2013). Indeed, acting as a non-significant source of nutrients for plant and microorganisms, how biochar in combination with other organic amendments affects soil microbial communities and enzymatic activities and their linkage with soil respiration are still unknown.
The objectives of this study were to (1) investigate the effects of biochar co-application with other organic amendments (i.e., straw and manure) on soil respiration and soil labile and recalcitrant C and N pools; (2) to determine whether biochar interferes with the effects of organic amendments on the composition of main soil microbial groups and enzyme activities involved in the C dynamics; and (3) to elucidate the linkages among soil respiration, soil organic C and N pools, composition of main microbial groups, and activity. We hypothesized that the stimulations of straw and manure amendments on soil respiration may be mitigated with the addition of biochar, because biochar can increase soil C and N recalcitrancy and decrease microbial activities involved in organic matter decomposition. To test this, a field experiment was performed to investigate the responses of soil C and N pools, composition of main microbial groups, enzyme activity, and microbial C source utilization to straw and manure added alone or combined with biochar after 9 months in an infertile soil, whereas soil respiration rates were monitored at different times during the experiment.
Materials and methods
Site description, biochar, and organic amendments
A field trial with biochar and organic amendments was established in April 2017 in a dry cropland located in Jincheng Township (31°15′N and 119°43′E), Lin’an, Hangzhou City, Zhejiang Province, China. The climate is characterized as a subtropical monsoon with a mean annual precipitation of 1420 mm and a mean annual temperature of 15.9 °C. The soil is classified as a Ferrasol by the Food and Agriculture Organization of the United Nations (FAO) soil classification system (WRB 2006); it had a pH of 4.74, organic C of 4.55 g kg−1, total N of 0.45 g kg−1, available N of 63.10 mg kg−1, available P of 1.58 mg kg−1, and available K of 89.00 mg kg−1and contained 10.4% sand, 43.5% silt, and 46.1% clay. Corn straw was collected from a local farmer, dried, and cut into small pieces (< 2 cm). Biochar was pyrolyzed from corn straw at 450 ~ 500 °C for 2 h under a slow-pyrolysis process in an oxygen-limited rotary furnace by Nanjing Qinfeng Science and Technology Corporation. The biochar material was ground to pass through a 2-mm sieve and homogenized prior to application. Sheep manure was obtained from unconfined piles from a local sheep farm and had a water content of 0.63 g g−1. The properties of the tested corn straw, manure, and biochar are shown in Table 1.
Field experiment
The experiment was set up with a two-way factorial design, namely biochar and organic amendment (i.e., straw and manure), with six treatments including a control (non-amendment), corn straw and manure and across biochar treatments (without and with biochar amendment, indicated by -Biochar and + Biochar, respectively). The six treatments were arranged in a randomized block design with three replicates (total 18 plots). Each plot has an area of 6.6 m2 and was separated from each other by a 0.5-m-wide border. Each plot was enclosed with a 60-cm-wide PVC board inserted into the soil to a depth of 40 cm. The corn straw, manure, and biochar were applied at 10.20 t ha−1, 33.47 t ha−1, and 8.49 t ha−1, respectively, equivalent to 3.60 t C ha−1. Thus, the amounts of added C were 0, 3.6, and 3.6 t C ha−1 for the control, straw, and manure treatments (without biochar), and 3.6, 7.2, and 7.2 t C ha−1 for those with biochar. In early April 2017, the biochar, straw pieces, and manure were evenly spread over the plots manually and incorporated into the plow layer (0 ~ 15 cm) with hoeing before seeding. All plots were planted with maize with a local cultivar (Zea mays Zhedan 11). Fertilizer was not applied during our experiment in order to not confound the effects of straw, manure, and biochar addition.
Soil respiration measurement
Soil respiration was monitored with CO2 fluxes in the field by the static closed chamber method and gas chromatography technique as already described (Chen et al. 2016). Each static chamber consisted of two parts: a base box with the size of 0.3 × 0.3 × 0.15 m and a chamber box with the size of 0.3 × 0.3 × 0.3 m. The chamber base was inserted approximately 0.13 m into the soil after organic amendments without covering the maize plants. Soil CO2 flux sampling was conducted between 9 and 11 a.m. on June 12, 2017 (jointing stage); July 13, 2017 (tassel stage); August 12, 2017 (milk stage); September 14, 2017 (harvest stage); October 12, 2017 (after harvest); and January 2, 2018 (the day of soil sampling). A 30 mL gas sample was collected with a syringe at 0, 10, 20, and 30 min after chamber closure. At each gas sampling, soil temperature at 5 cm depth was determined with a thermometer. The CO2 concentration in the gas samples was determined in the laboratory within 24 h following the sampling with a gas chromatography (Shimadzu, Japan) equipped with a flame ionization detector. Gas sample sets were eliminated if the linear regression value of r2 < 0.90. The soil CO2 emission rates were calculated as:
where F (mg CO2-C m−2 h−1) is the efflux rate of soil CO2; ρ is the density of the CO2 at 0 °C; V is the chamber volume; A is the base area of the chamber; \(\frac{dc}{dt}\) is the change of the CO2 concentration per unit in the closed chamber, and T is the absolute temperature of the sampling time, and T0 is the absolute temperature (273.15 K) under the standard condition.
Soil sampling and analysis of chemical properties
Soil samples were collected from each plot on January 2, 2018, which was 9 months after the biochar and organic material amendments. Five soil cores with a diameter of 3 cm were collected randomly from the 0–15 cm depth of each plot following a ‘X’ sampling pattern and were mixed thoroughly to form a composite sample. Each sample was placed in a sterilized plastic bag, and sealed and stored in an ice box before transport to the laboratory. The soil samples were sieved (< 2 mm) to remove large rocks, visible roots, and debris. A portion of the soil was air-dried at room temperature before chemical analysis. Another portion of each sample was freeze-dried and stored at − 70 °C until used for phospholipid fatty acid (PLFA) analysis, and the remaining soil was kept at 4 °C for microbial biomass and microbial activity analyses.
Soil organic C (SOC), total N (TN), and available N were determined by wet digestion using K2CrO7 oxidation, the Kjeldahl procedure, and the alkaline hydrolysis method, respectively, according to Lu (1999). Soil available phosphorus (P) (Bray 1-P, 0.03 M NH4F + 0.025 M HCl extractable) and available potassium (K) (1 M NH4OAc extractable) were determined according to the procedure described by Soil Science Society of China (1999). Soil moisture content was measured gravimetrically and soil pH was measured in a 1:2.5 ratio of soil to deionized water according to Lu (1999).
A two-step acid hydrolysis procedure was employed to determine the extractable and acid-resistant concentrations of soil labile and recalcitrant C and N pools (Rovira and Vallejo 2002). In brief, 500 mg dry soil was firstly hydrolyzed with 20 mL of 2.5 M H2SO4 in a Pyrex tube for 30 min at 105 °C (step 1). The hydrolysate was recovered by centrifugation (250 × g). The residue was flushed with 20 mL of de-ionized water, and the extract was added to the previous hydrolyzate. This initially extracted hydrolysate included labile C pool I (LPI-C) and labile N pool I (LPI-N). The remaining residue was dried at 60 °C and subsequently hydrolyzed with 2 mL of 13 M H2SO4 at room temperature for 12 h under continuous shaking (step 2). Then, 24 mL de-ionized water was added and the sample was kept at 105 °C for 3 h with occasional shaking. The hydrolyzate was recovered by centrifugation at 2250 × g and decantation. The residue was washed with 20 mL of de-ionized water and then added to the hydrolyzate. C and N in this hydrolyzate were defined as labile C pool II (LPII-C) and labile N pool II (LPII-N). The C and N concentrations in hydrolyzate were determined on the TOC-TN analyzer (Shimadzu, Japan). The remaining residue was washed with water and transferred into a pre-weight foil plate and dried at 60 °C. The recalcitrant C pool (RP-C) was calculated as the total concentration of the organic C minus those of labile pools.
Soil phospholipid fatty acid (PLFA) analysis
The composition of main microbial groups was characterized by PLFA analysis according to Bossio and Scow (1998). In brief, 4 g of freeze dried soil was placed in a PTFE centrifuge tube along with a mixture of chloroform, methanol, and citrate buffer (with a volume ratio of 1:2:0.8). The mixture was shaken for 4 h in the dark, then separated from glycolipids and neutral lipids using silica acid columns (Supelco, Inc., Bellefonte, PA, USA). The methanol fraction containing PLFAs was evaporated to dryness using N2, and the PLFAs were converted to methyl esters with a mild alkaline methanolysis reaction. After this reaction, the resulting fatty acid methyl esters were extracted with 5 mL of 4:1 hexane:chloroform, evaporated to dryness and re-dissolved in 50 μL of hexane. Finally, soil PLFA extracts were identified using an Agilent 6850 Gas Chromatograph (Agilent, USA). PLFA concentrations were expressed as nmol PLFA g−1 dry soil. Fatty acids are denoted according to the PLFA nomenclature by Zak et al. (2000). The dominant PLFAs were assigned to major microbial groups according to previous studies (Frostegård and Bååth 1996; Zelles 1997; Bossio and Scow 1998): cy17:0, cy19:0, 16:1 ω7c, 17:1 ω8c, 18:1 ω7c for gram-negative bacteria (G −); i14:0, i15:0, a15:0, i16:0, a16:0, i17:0, a17:0 for gram-positive bacteria (G +); 18:1 ω9c and 18:2 ω6c for fungi; 16:1 ω5c for arbuscular mycorrhizal fungi (AMF); and 10Me16:0, 10Me17:0, and 10Me18:0 for actinomycetes. Total microbial PLFA concentrations were calculated as the sum of all determined PLFAs. Bacterial biomass was calculated by summing G + and G − bacterial biomass plus 14:0, 16:0, 17:0, 18:0, and 20:0. The fungal to bacterial biomass ratio (F:B ratio) was calculated by dividing the sum of all fungal PLFAs by the sum of all bacterial PLFAs.
Analysis of soil enzyme activities
Activities of seven hydrolytic enzymes including α-glucosidase (AG), β-glucosidase (BG), cellobiohydrolase (CB), N-acetylglucosaminidase (NAG), β-xylosidase (XYL), leucine aminopeptidase (LAP), and acid phosphomonoesterase (PHOS) were determined according to Saiya-Cork et al. (2002) (Table S1). This protocol measures yields of the fluorescent cleavage products MUB (4-methylumbelliferyl). In brief, 2 g of fresh soil was homogenized with 100 mL of 50 mM acetate buffer (pH 5.0). Then, a 200 μL aliquot was dispensed into a 96-well microplate from the obtained suspension, which was continuously stirred using a magnetic stir plate. The microplates containing enzyme and substrate mixtures were incubated in the dark at 25 °C for 3 h and the fluorescence quantified using a microplate fluorometer (Synergy™ H1, Biotek) at 365 nm excitation and 450 nm emission wavelengths. L-3, 4-dihydroxyphenylalanine (L-DOPA) was used as substrate for spectrophotometrical analysis for the activities of phenol oxidase (PHOX) and peroxidase (PERO) according to DeForest (2009). All enzyme activities were expressed as nmol product g−1 dry soil h−1. A geometric mean enzyme activity (GMEA) (a general index to integrate information from variables that possess different units and range of variation) for each sample was calculated as follows: GMEA = (AG × BG × CB × XYL × NAG × LAP × PHOS × PHOX × PERO)1/9. The GMEA was used to estimate the total enzyme activity by considering that it is sensitive to changes in soil management (García-Ruiz et al. 2008).
Measurements of soil microbial activities
Microbial C source utilization profiles were employed to assess soil microbial activities using the MicroResp™ CO2 detection system following the addition of a range of substrates according to Campbell et al. (2003). In brief, sieved (< 2 mm) soil was pre-incubated at 40% of WHC for 7 days to allow the respiratory activity of the microbial community to settle. Colorimetric CO2-traps were prepared in microplate wells by dispensing 150 mL of a warm (55 °C) Noble agar-(1%) based gel containing cresol red (12.5 ppm, w/w), potassium chloride (150 mM), and sodium bicarbonate (2.5 mM). A total of 15 C substrates, including carbohydrates (D-glucose, D-galactose, D-fructose, and N-acetylglucosamine), amino acids (aspartic acid, glycine, γ-amino-butyric acid, and arginine), carboxylic acids (citric acid, oxalic acid, malic acid, and succinic acid), and phenolic acids (vanillic acid, syringic acid, and protocatechuic acid) were prepared. A sterile water was used for soil basal respiration measurement. Soil was delivered to a deep-well microplate (0.30 g per well) containing a solution of pre-dispensed C sources in relevant wells. The final soil moisture content was adjusted to 60% WHC before the system was sealed and incubated at 25 °C for 6 h. The initial and final CO2-trap absorbance values at 570 nm of the detection plates were determined. Absorbance values were then converted to CO2 concentration, following construction of a standard curve as described by Campbell et al. (2003); then, respiration rates (μg CO2-C g−1 h−1) from individual soil were calculated.
Statistical analyses
A three-way analysis of variance (ANOVA) was used to determine the effects of biochar, organic amendment and time and their interactions on soil CO2 emission rates, soil temperature, and moisture in the field condition. A two-way ANOVA was used to determine the effects of biochar and organic amendment and their interactions on soil properties, microbial PLFAs, enzyme activities, and substrate-induced respiration. Data sets were checked for normality and homogeneity of variance and transformed when necessary. Relationships between soil respiration rates, soil characteristics, microbial PLFAs, and enzyme activities were tested using Pearson’s correlation. To assess how biochar and organic amendments influence the composition of main microbial groups, a two-way permutational multivariate analysis of variance (PERMANOVA) was carried out using the adonis function in the package “vegan” in R. Redundancy analysis (RDA) was performed to determine how the soil chemical properties, and soil C and N pools were related to composition of main microbial groups by using CANOCO for Windows 4.5. The significance of the relations between the ordination and explanatory variables was tested by using a permutation test with 999 permutations.
A structural equation modeling (SEM) was constructed to identify how organic amendment and biochar influenced soil CO2 efflux based on the relationships among soil respiration, soil C quality, microbial biomass, and enzyme and microbial activities. Firstly, we assumed that organic amendment and biochar induced contrasting effects on soil labile C concentrations and microbial biomass, which further affected soil enzyme and microbial activities; and such changes ultimately modulated microbial response of organic C decomposition to biochar and organic amendment addition. In our model, biochar and organic amendment were regarded as exogenous variables; labile C pool, microbial PLFAs, total enzyme activities, and microbial C utilization were treated as endogenous variables, and soil CO2 efflux was considered a response variable. Adequate model fits were indicated by the χ2 test (df > 5; P > 0.05), goodness-of-fit index (GFI), and a low root-mean-square error of approximation (RMSEA) (< 0.05) (Hooper et al. 2008). Furthermore, we calculated the standardized total effects of each variable on soil CO2 efflux by summing all direct and indirect effects. All the SEM analyses were conducted using AMOS 18.0 software (IBM, Chicago, IL, USA).
Results
Soil respiration
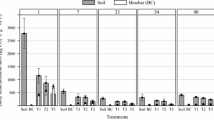
Soil CO2 emission rates significantly (P < 0.05) decreased from June 2017 to January 2018 following the decrease of soil temperature (Fig. 1 and Fig. S1). Straw and manure amendments alone significantly increased soil CO2 emission rates by 89% and 127% on average, respectively. Biochar addition had a significant effect on soil CO2 emission rates. Biochar co-applied with straw or manure significantly decreased soil CO2 emission rates by 19% and 36% on average compared with the sole straw and manure addition. There were significant (P < 0.001) interactive effects of biochar and organic amendment on soil CO2 emission rates, whereas the interactions of biochar × time (P = 0.46), biochar × organic amendment × time (P = 0.31) were not significant in any case.
Main soil properties, labile and recalcitrant C and N pools
The two-way ANOVA shows that organic amendment and biochar significantly (P < 0.05) affected soil pH, and the contents of SOC and TN, but they had no significant interactions on them (Table 2). Straw and manure addition alone slightly increased pH, SOC, and TN, whereas biochar co-applied with straw and manure remarkably increased the contents of SOC and TN relative to the biochar-untreated soils. In biochar-treated soils, the C/N ratios were significantly decreased (by 12% and 22%) by straw and manure addition. Straw and manure addition alone significantly increased soil available N and P contents, whereas biochar addition had little effect on them with the exception of an increase in soil available K content. Biochar and organic amendment had no significant effects on soil temperature and moisture (Fig. S1).
Straw and manure addition alone significantly increased the size of soil labile C pool I (LPI-C) by 38% and 15% on average, respectively, in comparison with the control (Fig. 2). In contrast, biochar in combination with straw or manure decreased the LPI-C contents by 24% and 11% compared to the straw or manure addition alone, respectively. The sizes of recalcitrant C pool (RP-C) were remarkably higher in biochar-treated soils relative to the biochar-untreated soils. There was a significant interactive effect of biochar and organic amendment on the size of LPI-C. Straw and manure addition alone decreased the size of LPI-N, while manure slightly increased the size of RP-N. Biochar-treated soils had significantly lower size of LPI-N but higher size of RP-N than the biochar-untreated soils.
Effects of straw, manure, and biochar amendments on labile C and N pools I (LPI-C and LPI-N), labile C and N pools II (LPII-C and LPII-N), and recalcitrant C and N pools (RP-C and RP-N). Treatments amended and non-amended with biochar are shown with (+ Biochar) and (− Biochar). Vertical bars are standard deviations of the mean (n = 3)
Phospholipids fatty acid (PLFA)
The concentrations of total PLFAs and all the individual groups were significantly (P < 0.05) increased by organic amendments in both biochar-treated and non-biochar-treated soils with the exception of actinobacteria (Fig. 3). Biochar addition hardly affected the concentrations of total PLFAs and individual groups. Straw and manure addition induced a higher F/B ratio regardless of biochar application. The PERMANOVA showed no effects of organic amendment (P > 0.05) on the composition of main microbial groups in the topsoil. There were no significant differences in their composition between biochar-treated and untreated soils as indicated by the pattern of RDA (Fig. 4A). TN, AP, LPII-C, pH, LPII-N, and RP-N were positively correlated with the main microbial groups (Fig. 4A and Table S2).
Effects of straw, manure, and biochar amendments on the concentrations of phospholipids acid (PLFA) biomarkers. Treatments amended and non-amended with biochar are shown with (+ Biochar) and (− Biochar), respectively. G + , G − , and F/B represent gram-positive bacteria, gram-negative bacteria, and fungi/bacteria ratio, respectively. Vertical bars are standard deviations of the mean (n = 3)
Redundancy analysis (RDA) of soil microbial community composition (A) and enzyme activities (B) under straw, manure, and biochar amendments. Treatments amended (+ Biochar) and non-amended with biochar (− Biochar) are shown in red and blue, respectively. TN, total N; LPII-C, soil labile C pool II; LPII-N, soil labile N pool II; RP-N, soil recalcitrant N pool. AK, soil available K content; AN, soil available N content; AP, soil available P content; PLFA, phospholipid fatty acid; AG, α-glucosidase; BG, β-glucosidase; CB, cellobiohydrolase; NAG, N-acetylglucosaminidase; XYL, β-xylosidase; LAP, leucine aminopeptidase; PHOS, acid phosphatase; PHOX, phenol oxidase; PERO, peroxidase; GMEA, geometric mean of the assayed enzyme activities. The red arrows with asterisks indicate significant (*, P < 0.05; **, P < 0.01) factors affecting the microbial communities and enzyme activities
Soil enzyme activities and microbial metabolic profiles
Straw and manure amendments had positive effects on the enzyme activities, whereas the presence of biochar had negative effects on them (Fig. 5). Compared to manure alone, the combined application of biochar and manure significantly (P < 0.05) decreased the activities of β-glucosidase (BG) by 49%, β-D-cellobiosidase (CB) by 60%, β-xylosidase (XYL) by 25%, phenol oxidase (PHOX) by 40%, peroxidase (PERO) by 31%, and GMEA by 36%, respectively. Significant interactive effects of biochar and organic amendment were detected in the activities of CB, XYL, PHOX, and enzyme C/N ratio. There were significant (P < 0.05) differences in the enzyme activities between biochar-treated and untreated soils (Fig. 4B).
Effects of straw, manure, and biochar amendments on soil enzyme activities. AG, α-glucosidase; BG, β-glucosidase; CB, cellobiohydrolase; NAG, N-acetylglucosaminidase; XYL, β-xylosidase; LAP, leucine aminopeptidase; PHOS, acid phosphatase; PHOX, phenol oxidase; PERO, peroxidase; GMEA, geometric mean of the assayed enzyme activities. Treatments amended and non-amended with biochar are shown with (+ Biochar) and (− Biochar), respectively. Vertical bars are standard deviations of the mean (n = 3)
Soil basal respiration and microbial activities of most of the tested substrates were increased by straw or manure addition, but significantly decreased in the biochar-treated soils (Fig. 6 and Fig. S2). Significant interactive effects of biochar and organic amendment were detected in the carbohydrates and carboxylic acids oxidation.
Linkages among soil C and N pools, enzyme activities, microbial biomass, metabolic function, and soil respiration
The enzyme activities showed weak correlations with the soil chemical properties, but the total enzyme activities (indicated by GMEA) and those involved in C dynamics significantly (P < 0.05) correlated with the microbial PLFAs (Fig. S3 and Table S3). Soil basal respiration and those induced by the tested substrates (except for phenolic acids) had weak correlations with soil C and N pools, but significantly and positively correlated with the PLFAs and the activities of AG, BG, PHOX, PERO, and NAG (Fig. S4). The soil respiration rate almost had no significant correlations with the soil chemical properties and organic C and N composition, but significantly and positively correlated with PLFAs, enzyme activities, and substrate-induced respirations (Fig. 7).
Soil CO2 efflux rate (on the day of soil sampling) in relation to soil chemical properties, soil labile and recalcitrant C and N pools, microbial abundance, enzyme activities and microbial C source utilization rates. SOC, soil organic C; TN, total N; AK, soil available K content; AN, soil available N content; AP, soil available P content; LPI-C, soil labile C pool I; LPI-N, soil labile N pool I; LPII-C, soil labile C pool II; LPII-N, soil labile N pool II; RP-C, soil recalcitrant C pool; RP-N, soil recalcitrant N pool. PLFA, phospholipid fatty acid; G + , gram-positive bacteria; G − , gram-negative bacteria; Actino., actinomycetes; AG, α-glucosidase; BG, β-glucosidase; CB, cellobiohydrolase; XYL, β-xylosidase; PHOX, phenol oxidase; PERO, peroxidase; BR, soil basal respiration; Chy, carbohydrates; CxA, carboxylic acids; AmA, amino acids; PhA, phenolic acids; TCU, total microbial C utilization rate. The asterisks indicate significant correlations (*, P < 0.05; **, P < 0.01; ***, P < 0.001)
The SEM provided good fits to the data (χ2 = 8.144, df = 8, P = 0.420, RMSEA = 0.033), and explained 62% of the variance in soil basal respiration (Fig. 8A and Table S4). Overall, organic amendment affected soil CO2 efflux through its significant impact on total PLFA contents and GMEA, while biochar addition affected soil CO2 efflux through affecting LPI-C and GMEA. Standardized total effects revealed that organic amendment had a positive effect whereas biochar had a negative effect on the soil CO2 efflux, which was mainly driven by the GMEA, followed by the microbial C utilization capacity (Fig. 8B).
Structural equation models showing the effects of biochar and organic amendments on soil CO2 efflux rate (A) through soil labile C pool, microbial biomass (total PLFA), GMEA (geometric mean of the assayed enzyme activities) and total C utilization rate of microbial communities. The standardized total effects (direct plus indirect effects) (B) derived from the structural equation model depicted above. Numbers adjacent to arrows are path coefficients, and arrow widths are proportional to the magnitude of the path coefficients. Blue and red arrows indicate positive and negative correlations, respectively. Continuous and dashed arrows indicate significant and insignificant relationships, respectively. Significant level: *, P < 0.05; **, P < 0.01; ***, P < 0.001
Discussion
Effect of biochar co-applied with organic amendments on soil respiration
In this study, we observed a contrasting effect of biochar and organic amendment on soil respiration (Fig. 1). The significant increase in CO2 emission rate under straw and manure addition during the whole experiment suggested the stimulation of straw and manure amendments on soil respiration, and was consistent with a large number of studies conducted both in laboratory and in field (Troy et al. 2013; Wu et al. 2013; Liu et al. 2014; Hu et al. 2014; Duan et al. 2020). It depended on the fact that straw and manure contain degradable substances, such as sugar, fat, hemicellulose, and cellulose, which can be decomposed and utilized by microorganisms, resulting in a significant increase in the release of CO2. In contrast, biochar contained less labile compounds than straw and manure. However, a few studies indicated that biochar application can enhance soil respiration as it provides some labile C for soil microorganisms within a short period after application (Smith et al. 2010; Jones et al. 2011; Wang et al. 2016). Similarly, Yang et al. (2017) reported that soil CO2 emissions were increased by 129.7% and 24.4% on average under maize stover and its biochar treatment, respectively, in a 3-year experiment.
Although biochar alone did not affect soil respiration in this study, it is interesting to find that biochar co-applied with straw and manure significantly decreased CO2 emission rate (by 19% and 36% on average) compared with the sole straw and manure addition (Fig. 1). The three-way ANOVA result also demonstrated that biochar and organic amendment had an interactive effect on soil respiration. Our findings were inconsistent with the observations by Hu et al. (2014) and Duan et al. (2020) reporting that biochar co-application with straw did not change soil CO2 flux in comparison with the straw addition. The reported discrepancies may be related to the differences in soil type and fertility, biochar type, and addition rate. Decreased CO2 emissions under biochar addition indicated that biochar application had an inhibitory effect on organic matter decomposition and thus supported our first hypothesis. Moreover, addition of biochar together with manure showed a lager reduction in soil CO2 emission than biochar co-applied with corn straw. In this study, the decrease in soil CO2 emissions under biochar addition can be explained by the changes in soil C quality, activities of enzymes involved in organic C decomposition, and microbial metabolic activities (Lehmann et al. 2011; Luo et al. 2017; Chen et al. 2019) as discussed in the following sections.
Effect of biochar and organic amendments on soil C quality and microbial abundance and its linkage with soil respiration
Because labile organic matter acts as a key substrate for microbial decomposition (Wang et al. 2003; Brookes et al. 2017), the significantly increased size of soil labile C pool I (LPI-C) under straw and manure addition (Fig. 2) partly explained the remarkable increases in soil CO2 emission rate. As shown by Rovira and Vallejo (2002), soil LPI-C predominantly contains polysaccharides derived from plants (such as hemi-cellulose and starch) and from microorganisms, the increase in the size of soil LPI-C can be attributed to the decomposition of straw and manure. In contrast, biochar alone did not change but its co-application with straw and manure decreased the size of LPI-C relative to straw and manure, which was in line with our previous finding showing that biochar decreased soil labile C pool 20 months after a single amendment in a rice paddy (Chen et al. 2019). The significant interactive effect of biochar and organic amendment on the size of LPI-C suggested that the decreased soil respiration could be related to the decreased soil labile C pool as a result of the decelerated decomposition of organic materials under biochar co-application. As no additional chemical fertilizers were used in the study, it is possible that the abundant C compounds and high C/N ratio of biochar may have induced a stronger microbial N limitation, leading to decreased organic matter decomposition (Guo et al. 2020). It is also possible that biochar may increase soil organic C stability by adsorbing the dissolved organic C in biochar particles (Lu et al. 2014; Weng et al. 2017), leading to reduction in soil respiration. In accordance with a few studies suggesting that organic amendments, such as straw and manure, slightly increase soil recalcitrant C concentration in a short period (Liu et al. 2014; Peng et al. 2016), this study showed that biochar rather than straw and manure amendment significantly increased the size of soil recalcitrant C and N pools, likely due to the aromatic C and N contents of biochar (Smith et al. 2010; Cao et al. 2012; Chen et al. 2019). This confirms what found by Li et al. (2018) who observed a decrease in the soil heterotrophic respiration due to the increased soil aromatic C content of biochar, which decreased soil labile C pool and increased recalcitrant C pool when co-applied with straw and manure.
In this study, soil respiration rate had significant and positive correlations with the concentrations of total PLFAs and with those assigned to G + and G − bacteria and fungi (Fig. 7), suggesting that microbial abundance may be a key driver for soil respiration (Liu et al. 2018) and confirming the findings by Elzobair et al. (2016) and Zhu et al. (2018). In contrast, biochar alone had no significant effect on microbial abundance and the composition of main groups, suggesting that biochar had less amount of labile substrates for microbial growth than straw and manure (Lehmann et al. 2011; Wang et al. 2016). Our findings contradict those reporting that biochar amendment improves microbial biomass and clearly changed their community composition due to improvements in soil nutrient contents and microhabitat conditions (Mitchell et al. 2015; Chen et al. 2015, 2019; Zhou et al. 2017). Moreover, there were no interactive effects of biochar and straw and manure on microbial abundance and community structure (Fig. 3 and Fig. 4A), supporting our hypothesis that biochar did not interfere with the effects of organic amendments on soil microbial communities. Therefore, the decreased soil respiration rates under biochar in combination with either straw or manure could not be attributed to the unchanged microbial abundance and the composition of main groups.
Effect of biochar and organic amendments on soil enzyme activity and microbial C utilization and its relationship with soil respiration
The decreased soil respiration rate under co-application of biochar and organic amendments could be attributed to the inhibitory effect of biochar on the enzyme and microbial activities involved in the decomposition of organic matter (Figs. 5 and 6). Previous studies performed both in short-term and long-term showed that biochar addition decreased the activity of soil hydrolases, such as β-glucosidase and β-D-cellobiosidase involved in the degradation of carbohydrates in soil (Tian et al. 2016; Chen et al. 2017). Our study showed that both β-glucosidase and β-D-cellobiosidase as well as two oxidative enzymes (phenol oxidase and peroxidase) were enhanced under straw and manure treatment, but they were significantly decreased when co-applied with biochar. Phenol oxidase and peroxidase are among the most important C-degrading enzymes targeting the degradation of more recalcitrant materials, such as lignin, lignocellulose, and cellulose (Sinsabaugh 2010). The decreases in oxidative enzyme activities and their significant correlations with soil respiration rate support a decrease in degradation of organic matter (Fig. 7). Therefore, reductions in the enzyme activities involved in the organic matter decomposition can be one of the key mechanisms of declined soil respiration under the treatment of biochar in combination with straw and manure. Moreover, we found that the activities of N-acetylglucosaminidase and acid phosphatase were also decreased in biochar-treated soils, which were contrary to short-term studies (Paz-Ferreiro et al. 2015; Wang et al. 2015). We infer that the lower activities of N-acetylglucosaminidase and acid phosphatase in this study could be related to the increased N and P contents of biochar-treated soils. Guo et al. (2020) suggested that increased N and P availability under biochar could decrease microbial nutrient limitation, thereby decreasing the enzyme activities involved in N and P dynamics. Changes in enzyme activities under biochar application depend on enzyme type and soil property as well as feedstocks (Bailey et al. 2011; Elzobair et al. 2016; Liu et al. 2020). Furthermore, it is interesting to find that biochar addition induced a lower enzyme and microbial activities but unchanged microbial biomass, which may suggest a higher microbial metabolic efficiency. A few studies reported that biochar soil amendment decreased soil enzymes and induced a higher microbial C use efficiency (Jiang et al. 2016; Chen et al. 2017; Guo et al. 2020).
In the present study, decrease in microbial metabolic activity in terms of C source utilization could be another possible mechanisms of reduced soil respiration under biochar-treated soils. The rates of soil basal respiration and those induced by the addition of carbohydrates and carboxylic acids were higher under straw and manure addition, but they were significantly lower under biochar treatment (Fig. 6), suggesting a lower capacity of biochar-treated soil to catabolize these substrates and supported our second hypothesis. Our findings contradict those by Rutigliano et al. (2014) and Liu et al. (2020) who reported enhancements in the capacity of soil microorganisms to utilize C sources, in particular those of recalcitrant substrates. Because microbial metabolic activity is directly linked with living microbial cells, intra- and extracellular enzyme activities (Campbell et al. 2003), the lower C utilization rate under biochar-treated soils could be attributed to the lower enzyme activities as mentioned above. In support, the microbial utilization rates of carbohydrates, carboxylic acids, and amino acids had significant and positive correlations with the activities of enzymes involved in organic matter decomposition (Fig. 7). SEM analysis also confirmed that organic amendment had a positive effect while biochar addition had a negative effect on soil CO2 efflux, and biochar decreased soil CO2 efflux mainly through affecting soil microbial activities in terms of enzyme activities rather than microbial biomass (Fig. 8).
Conclusions
In conclusion, the present study clearly demonstrated that biochar co-applied with organic amendments significantly reduced soil respiration in comparison with the sole straw and manure addition in a dry cropped soil. Reduction in soil respiration could, on one hand, be attributed to the decrease in the size of soil labile C pool and increase in the size of recalcitrant C pool, and, on the other hand, be strongly related to the decreases in soil enzyme and microbial activities involved in organic matter decomposition, rather than decrease in soil microbial biomass. These findings, therefore, provide direct evidence that biochar co-application could mitigate the stimulation of organic amendments on soil respiration by decreasing microbial activities. Our study highlights that biochar co-applied with organic amendment could be a more effective way than organic amendment alone to promote soil C storage and fertility in infertile soils. However, a long-term monitoring of C flux in the soil–crop system is required to determine whether such decrease in soil C turnover suggests a potential and sustainable increase in C stability in agricultural soil after biochar application. Meanwhile, shorter times than that used are needed to follow the temporal dynamic of the studied soil and microbial properties, especially their changes due to the addition of easily degradable C.
References
Agegnehu G, Srivastava AK, Bird MI (2017) The role of biochar and biochar-compost in improving soil quality and crop performance: a review. Appl Soil Ecol 119:156–170
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173–174:330–338
Amelung W, Bossio D, de Vries W, Kögel-Knabner I, Lehmann J, Amundson R, Bol R, Collins C, Lal R, Leifeld J, Minasny B, Pan G, Paustian K, Rumpel C, Sanderman J, van Groenigen JW, Mooney S, van Wesemael B, Wander M, Chabbi A (2020) Towards a global-scale soil climate mitigation strategy. Nat Commun 11:5427
Bailey VL, Fansler SJ, Smith JL, Bolton H Jr (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43:296–301
Belay-Tedla A, Zhou X, Su B, Wan S, Luo Y (2009) Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol Biochem 41:110–116
Bhattacharyya P, Roy KS, Neogi S, Adhya TK, Rao KS, Manna MC (2012) Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil till Res 124:119–130
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Bossio DA, Scow KM (1998) Impact of carbon and flooding on PLFA profiles and substrate utilization patterns of soil microbial communities. Microb Ecol 35:265–278
Brookes PC, Chen Y, Chen L, Qiu G, Luo Y, Xu J (2017) Is the rate of mineralization of soil organic carbon under microbiological control? Soil Biol Biochem 112:127–139
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microb 69:3593–3599
Cao XY, Pignatello JJ, Li Y, Lattao C, Chappell MA, Chen N, Miller LF, Mao JD (2012) Characterization of wood chars produced at different temperatures using advanced solid-state 13C NMR spectroscopic techniques. Energ Fuel 26:5983–5991
Chen J, Chen D, Xu Q, Fuhrmann JJ, Li L, Pan G, Li Y, Qin H, Liang C, Sun X (2019) Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol Fertil Soils 55:185–197
Chen J, Li S, Liang C, Xu Q, Li Y, Qin H, Fuhrmann JJ (2017) Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci Total Environ 574:24–33
Chen J, Liu X, Li L, Zheng J, Qu J, Zheng J, Zhang X, Pan G (2015) Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from South China. Appl Soil Ecol 91:68–79
Chen J, Sun X, Li L, Liu X, Zhang B, Zheng J, Pan G (2016) Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar. Eur J Soil Sci 67:857–867
Conant RT, Ryan MG, Ågren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvönen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Megan Steinweg J, Wallenstein MD, Martin Wetterstedt JÅ, Bradford MA (2011) Temperature and soil organic matter decomposition rates – synthesis of current knowledge and a way forward. Global Change Biol 17:3392–3404
Dai Z, Zhang X, Tang C, Muhammad N, Wu J, Brookes PC, Xu J (2017) Potential role of biochars in decreasing soil acidification – a critical review. Sci Total Environ 581:601–611
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol Biochem 41:1180–1186
Dong H, Ge J, Sun K, Wang B, Xue J, Wakelin SA, Wu J, Sheng W, Liang C, Xu Q, Jiang P, Chen J, Qin H (2021) Change in root-associated fungal communities affects soil enzymatic activities during Pinus massoniana forest development in subtropical China. Forest Ecol Manag 482:118817
Duan M, Wu F, Jia Z, Wang S, Cai Y, Chang SX (2020) Wheat straw and its biochar differently affect soil properties and field-based greenhouse gas emission in a Chernozemic soil. Biol Fertil Soils 56:1023–1036
Elzobair KA, Stromberger ME, Ippolito JA, Lentz RD (2016) Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 142:145–152
Fanin N, Bertrand I (2016) Aboveground litter quality is a better predictor than belowground microbial communities when estimating carbon mineralization along a land-use gradient. Soil Biol Biochem 94:48–60
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465:288–297
Food and Agriculture Organisation of the United Nations (FAO) (2019) Recarbonization of Global Soils - a dynamic response to offset global emissions, FAO, http://www.fao.org/3/i7235en/I7235EN.pdf
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
García-Orenes F, Roldán A, Morugán-Coronado A, Linares C, Cerdà A, Caravaca F (2016) Organic fertilization in traditional Mediterranean grapevine orchards mediates changes in soil microbial community structure and enhances soil fertility. Land Degrad Dev 27:1622–1628
García-Ruiz R, Ochoa V, HinojosaMB CJA (2008) Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol Biochem 40:2137–2145
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Guo K, Zhao Y, Liu Y, Chen J, Wu Q, Ruan Y, Li S, Shi J, Zhao L, Sun X, Liang C, Xu Q, Qin H (2020) Pyrolysis temperature of biochar affects ecoenzymatic stoichiometry and microbial nutrient-use efficiency in a bamboo forest soil. Geoderma 363:114162
Hill BH, Elonen CM, Jicha TM, Kolka RK, Lehto LLP, Sebestyen SD, Seifert-Monson LR (2014) Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 120:203–224
Hooper D, Coughlan J, Mullen M (2008) Structural equation modelling: guidelines for determining model fit. Articles 2
Hu YL, Wu FP, Zeng DH, Chang SX (2014) Wheat straw and its biochar had contrasting effects on soil C and N cycling two growing seasons after addition to a Black Chernozemic soil planted to barley. Biol Fertil Soils 50:1291–1299
Jiang X, Denef K, Stewart C, Cotrufo MF (2016) Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol Fertil Soils 52:1–14
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ Sci Technol 45:9611–9618
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627
Lammirato C, Miltner A, Kaestner M (2011) Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol Biochem 43:1936–1942
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836
Lehmann J (2007) A handful of carbon. Nature 447:143–144
Li Y, Li Y, Chang SX, Yang Y, Fu S, Jiang P, Luo Y, Yang M, Chen Z, Hu S, Zhao M, Liang X, Xu Q, Zhou G, Zhou J (2018) Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol Biochem 122:173–185
Lin Y, Ye G, Kuzyakov Y, Liu D, Fan J, Ding W (2019) Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol Biochem 134:187–196
Liu C, Lu M, Cui J, Li B, Fang C (2014) Effects of straw carbon input on carbon dynamics in agricultural soils: a meta-analysis. Glob Chang Biol 20:1366–1381
Liu S, Zhang Y, Zong Y, Hu Z, Wu S, Zhou J, Jin Y, Zou J (2016) Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: a meta-analysis. GCB Bioenergy 8:392–406
Liu Y, Guo K, Zhao Y, Li S, Wu Q, Liang C, Sun X, Xu Q, Chen J, Qin H (2020) Change in composition and function of microbial communities in an acid bamboo (Phyllostachys praecox) plantation soil with the addition of three different biochars. Forest Ecol Manag 473:118336
Liu YR, Delgado-Baquerizo M, Wang JT, Hu HW, Yang Z, He JZ (2018) New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol Biochem 118:35–41
Lu R (1999) Analytical methods for soils and agricultural chemistry. China Agricultural Science and Technology Press, Beijing
Lu W, Ding W, Zhang J, Li Y, Luo J, Bolan N, Xie Z (2014) Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76:12–21
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35
Mackie KA, Marhan S, Ditterich F, Schmidt HP, Kandeler E (2015) The effects of biochar and compost amendments on copper immobilization and soil microorganisms in a temperate vineyard. Agr Ecosyst Environ 201:58–69
Maisto G, De Marco A, De Nicola F, Arena C, Vitale L, Virzo De Santo A (2010) Suitability of two types of organic wastes for the growth of sclerophyllous shrubs on limestone debris: a mesocosm trial. Sci Total Environ 408:1508–1514
Mitchell PJ, Simpson AJ, Soong R, Simpson MJ (2015) Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol Biochem 81:244–254
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Nguyen TT, Marschner P (2016) Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol Fertil Soils 52:165–176
Pan G, Smith P, Pan W (2009) The role of soil organic matter in maintaining the productivity and yield stability of cereals in China. Agr Ecosyst Environ 129:344–348
Paz-Ferreiro J, Fu S, Méndez A, Gascó G (2015) Biochar modifies the thermodynamic parameters of soil enzyme activity in a tropical soil. J Soil Sediment 15:578–583
Peng X, Zhu QH, Xie ZB, Darboux F, Holden NM (2016) The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope Ultisol. CATENA 138:30–37
Qayyum MF, Steffens D, Reisenauer HP, Schubert S (2014) Biochars influence differential distribution and chemical composition of soil organic matter. Plant Soil Environ 60:337–343
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107:109–141
Rutigliano F, Romano M, Marzaioli R, Baglivo I, Baronti S, Miglietta F, Castaldi S (2014) Effect of biochar addition on soil microbial community in a wheat crop. Eur J Soil Biol 60:9–15
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Singh BP, Cowie AL (2014) Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci Rep 4:3687
Singh R, Singh P, Singh H, Raghubanshi AS (2019) Impact of sole and combined application of biochar, organic and chemical fertilizers on wheat crop yield and water productivity in a dry tropical agro-ecosystem. Biochar 1:229–235
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347
Sohi SP (2012) Carbon storage with benefits. Science 338:1034–1035
Soil Science Society of China (1999) Soil physical and chemical analysis. agricultural science and technology, Beijing, 146–226 (in Chinese)
Tian J, Wang J, Dippold M, Gao Y, Blagodatskaya E, Kuzyakov Y (2016) Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci Total Environ 556:89–97
Troy SM, Lawlor PG, O’Flynn CJ, Healy MG (2013) Impact of biochar addition to soil on greenhouse gas emissions following pig manure application. Soil Biol Biochem 60:173–181
Ventorino V, De Marco A, Pepe O, Virzo De Santo A, Moschetti G (2013) Impact of innovative agricultural practices of carbon sequestration on soil microbial community. In: Piccolo A (ed) Carbon sequestration in agricultural soils. Springer, Berlin, pp 145–177
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8:512–523
Wang WJ, Dalal RC, Moody PW, Smith CJ (2003) Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol Biochem 35:273–284
Wang X, Song D, Liang G, Zhang Q, Ai C, Zhou W (2015) Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl Soil Ecol 96:265–272
Weng Z, Van Zwieten L, Singh BP, Tavakkoli E, Joseph S, Macdonald LM, Rose TJ, Rose MT, Kimber SWL, Morris S, Cozzolino D, Araujo JR, Archanjo BS, Cowie A (2017) Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat Clim Change 7:371
World Reference Base for Soil Resources (WRB) (2006) A framework for international classification, correlation and communication. Food and Agriculture Organization of the United Nations, Rome
Wu F, Jia Z, Wang S, Chang S, Startsev A (2013) Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils 49:555–565
Yang X, Meng J, Lan Y, Chen W, Yang T, Yuan J, Liu S, Han J (2017) Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agr Ecosyst Environ 240:24–31
Zak DR, Pregitzer KS, Curtis PS, Holmes WE (2000) Atmospheric CO2 and the composition and function of soil microbial communities. Ecol Appl 10:47–59
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294
Zhang X, Fang Q, Zhang T, Ma W, Velthof GL, Hou Y, Oenema O, Zhang F (2020) Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: a meta-analysis. Global Change Biol 26:888–900
Zhou H, Zhang D, Wang P, Liu X, Cheng K, Li L, Zheng J, Zhang X, Zheng J, Crowley D, van Zwieten L, Pan G (2017) Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: a meta-analysis. Agr Ecosyst Environ 239:80–89
Zhu Z, Ge T, Luo Y, Liu S, Xu X, Tong C, Shibistova O, Guggenberger G, Wu J (2018) Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol Biochem 121:67–76
Acknowledgements
We thank Dr. Paolo Nannipieri, the Editor-in-Chief, and the anonymous reviewers for their very valuable comments in improving both the language and scientific quality of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China under grant numbers 41977083, the National College Students’ Innovation Training Program (No. 201910341020), the Fundamental Research Funds for the Provincial Universities of Zhejiang (2020YQ004), and the Zhejiang Provincial Natural Science Foundation of China under grant number LGF18C160001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Q., Lian, R., Bai, M. et al. Biochar co-application mitigated the stimulation of organic amendments on soil respiration by decreasing microbial activities in an infertile soil. Biol Fertil Soils 57, 793–807 (2021). https://doi.org/10.1007/s00374-021-01574-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01574-0