Abstract
Partial substitution of chemical fertilizers by organic amendments is essential for improving the soil quality without yield loss. Fungi play an important role in soil quality because they decompose organic matter and cycle nutrients in the soil. However, there is limited information regarding the effect of different organic substitution rates (OSRs) on the soil quality and fungal community. This study investigated the relationship between the soil quality index and fungal community in a tea plantation under different OSRs of N, from a single application of synthetic fertilizer (NPK) to 100% N substitution with organic fertilizer (OM100). The OSRs were positively correlated with the soil physicochemical and biological soil quality index (SQI), but only the physicochemical SQI exhibited a significant relationship with tea production. The OSR also shifted the soil fungal community composition. Soil pH, soil organic C (SOC), microbial biomass C (MBC), and available potassium (AK) were the key characteristics that were significantly correlated with the variation of soil fungal community. Network analysis indicated that additional organic substitution can enhance the soil fungal network complexity, which also showed a positive correlation with the SQI. These results confirmed the feasibility of organic substitution for soil quality improvement, and implied that the soil fungal network complexity could be a new indicator for soil quality assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil quality is crucial for plant productivity and environmental quality sustainability (Karlen et al. 1997), and it is characterized by inherent and dynamic aspects, but only the latter can be affected by management practices (Seybold et al. 1999). Soil quality assessment is usually performed by selection of some sensitive soil properties to build a linkage with crop yield or other key functional traits. Soil microbes are the drivers of soil functional processes, such as nutrient cycling, C sequestration, and N fixation (Aryal et al. 2003; Allison et al. 2007; Finzi et al. 2015). Thus, in addition to the physicochemical properties, e.g., bulk density, pH, and soil organic C (SOC), Schloter et al. (2018) reviewed several molecular indicators for determining soil quality, such as microbial biomass (Nannipieri et al. 2003), potential enzymatic activities (Gil-Sotres et al. 2005), metabolic quotient (Nannipieri et al. 2003, 2018), and qPCR for soil microbial function genes.
It is well known that the addition of organic fertilizer has beneficial effects on soil quality, because it improves the soil porosity, it increases the SOC content (Edmeades 2003; Diacono and Montemurro 2011), and it contains all essential nutrients which are slowly released (Chen 2006). Partial substitution of chemical nutrients by organic fertilizer, also named organic substitution, is recommended, although ammonia loss by volatilization may increase after manure application (Ramanantenasoa et al. 2019). Proper organic substitution not only can sustain the crop yield but also can mitigate the impacts by the overuse of synthetic N fertilizer, such as waterbody eutrophication, greenhouse gas emissions, and biodiversity losses (Evenson and Gollin 2003; Canfield et al. 2010; Pingali 2012; Smith et al. 2013; Steffen et al. 2015).
Soil fungi play important roles in organic matter turnover, e.g., litter degradation, and SOC decomposition (Jones et al. 2011; Sun et al. 2016). In agricultural soils, farming practices can influence the soil nutrient status, with effects on the soil fungal community composition and function (Potthoff et al. 2006; Lauber et al. 2008). For example, the fungal community shifted under different fertilization regimes (Cwalina-Ambroziak and Bowszys 2009; Song et al. 2015; Zhou et al. 2016). N amendments increased the relative abundance of Ascomycota (Sun et al. 2016), while large application rates of chemical N fertilizer decreased the fungal community diversity (Zhou et al. 2016). Conversely, applied organic matter may increase diversity of soil fungi (Sun et al. 2016). Zhang et al. (2012) found that fungal richness significantly increased after adding pig manure and straw to soils when compared with adding chemical fertilizer. Nevertheless, most of the abovementioned studies focused on a single organic substitution rate (OSR), while the influence of different substitution rates is poorly known. Guenet et al. (2010) reported a nonlinear relationship between the amount of organic matter input and the mineralization rate of soil organic matter (SOM).
Although there have been some reports describing the response of the yield to the OSR in annual crops, e.g., rice (Bi et al. 2009) and corn (Huang et al. 2010), there are rare studies on the effect of the OSR on perennial crops. Tea (Camellia sinensis) is a perennial and an important cash crop in subtropical and tropical areas. Heavy applications of N fertilizer are always required in tea plantations to obtain good yield of harvested young shoots (Kamau et al. 2008; Ruan et al. 2010). To reduce the application and losses to leaching and volatilization of excess synthetic N fertilizers, organic substitution is considered as an important approach to sustain the yield production. Therefore, understanding the effect of different OSRs on tea yield and soil quality can be useful for fertilization strategy optimization.
To investigate the effect of organic fertilizer substitution on soil quality and fungal community of soil, a long-term field trial with different OSRs applied to the tea plantation was set up. The objectives of this research were to investigate (1) the response of soil quality to different OSRs, (2) whether and how OSRs shift the composition of soil fungal community, and (3) the optimal OSR for the tea plantation. Soil quality was evaluated as soil quality indices (SQI) calculated by physicochemical, microbiological, and biochemical properties.
Materials and methods
Field site and experiment design
The long-term field experiment was established in 2007 at Longhu Mountain, in Fu’an, a county in southeastern China (119°34′ E, 27°14′ N). This area has a warm temperature and subtropical monsoon climate with annual mean temperature of 19.3 °C and 1646 mm of annual precipitation. The studied soil was acidic red soil, developed from weathered granite material. Before the experiment, the soil properties were pH 4.15, SOC 14.10 g kg−1, TN1.00 g kg−1, available P (AP) 18.43 mg kg−1, and available K (AK) 132.10 mg kg−1. The experimental soil was formed by granite weathering, infertile, and acidic.
The N, P, and K application rates in this study were 300 kg N ha−1, 32.75 kg P ha−1, and 62.23 kg K ha−1, respectively. Organic substitution was calculated according to N rate. The insufficient P and K in the organic substitution treatments were supplemented by synthetic P and K fertilizers if the total P and K was less than the demanded rate. Urea, calcium superphosphate, and potassium sulfate were applied as synthetic N, P, and K fertilizers, respectively. The applied organic fertilizer was pig manure, and its nutrient information have been detailed in Supplementary Table 1. There were two 100% synthetic treatments, NPK and PK without N, and then 25%, 50%, 75%, and 100% of the target N replaced with pig manure. The quantity of the applied fertilizers for each plot is reported in Table 1.
Organic fertilizer, calcium superphosphate, and 40% urea and potassium sulfate were applied in late November as base fertilizers. The remaining 60% urea and potassium sulfate were divided into two even applications in early March and mid-August as top dressings. All fertilizers were applied in field furrows so that they were approximately 10 cm in depth, following the recommendation of good practice.
Each plot was 30 m2 in area, and had been planted with tea trees (variety “purple peony”), with row spacing of 1.5 m and plant spacing of 0.3 m. The young tea shoots (approximately one bud with four leaves) were harvested using machinery in early May and autumn early September.
Soil sampling and analysis
Soil samples were collected before fertilization in February 2017. For each plot, 10 soil cores (2 cm in diameter) were collected to a depth of 10 cm by an auger sampler, and then mixed as one sample. The collected fresh soil was sieved (< 2 mm) and separated into two parts; one was stored at − 80 °C for DNA extraction, and the other was stored at − 4 °C for soil chemical properties analysis.
Soil fungal community composition measurement
Total soil DNA was extracted from 0.25 g fresh soil by the PowerSoil DNA Isolation Kit (MOBIO Laboratories, Carlsbad, CA, USA) according to the product’s protocol. The quality of the extracted DNA was then tested using a spectrophotometer (ND2000, Thermo Scientific, USA).
ITS3-F (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4-R (5′-TCCTCCGCTTATTGATATGC-3′) were used as the primers to amplify the ITS2 region (McKay et al. 1999). The PCR reaction mixture contained 2.5 μL of 10× PCR buffer (TaKaRa, Dalian, China), 1.5 mM Mg2+, 0.4 μM of each deoxynucleoside triphosphate, 1.0 μM of each primer, 0.5 U of Ex Taq (TaKaRa, Dalian, China), and 10 ng DNA template in a final volume of 25 μL. The following thermal cycling conditions were used: an initial denaturation at 94 °C for 3 min; 30 cycles at 94 °C for 40 s, 50 °C for 60 s, and 72 °C for 60 s; and a final extension at 72 °C for 10 min.
The PCR products were then purified with a QIAquick Gel Extraction Kit (Qiagen, CA, USA). The purified PCR products were sequenced using the Illumina MiSeq PE250 platform (Illumina, San Diego, CA, USA). Sequencing data were processed using the QIIME software package (version 1.9.0) (Caporaso et al. 2010). Briefly, forward and reverse reads were joined using fastq-join with a minimum of 10 bp overlap. Low quality sequences (Phred quality score Q < 20 or sequences shorter than 200 bp) were discarded, and chimeras were filtered by the UCHIME algorithm (Edgar et al. 2011) in the USEARCH tool (Edgar 2010). High-quality sequences were assigned to OTUs using UCLUST with a similarity threshold of 97% (Edgar 2010). The taxonomic classification of representative sequences from individual OTUs was performed with the UNITE database (Abarenkov et al. 2010). Singletons and non-fungal OTUs were removed and all samples were rarefied to 42,351 sequences per sample for further analysis. Sequence data was deposited in the National Center for Biotechnology Information (NCBI) database with accession number SAMN08891279.
Soil quality index
Soil quality integrates soil physical, chemical, and microbiological properties and is often defined as a comprehensive indicator of soil fertility (Doran and Parkin 1994). The soil chemical properties included the soil pH, TC, TN, AP, AK, mineral N (exchangeable NH4+ and NO3−); exchangeable Ca, Mg, Fe, Mn, Cu, and Zn; and heavy metals As, Cr, Pb, and Cu. The soil biochemical and microbiological properties included the activity of urease, acid phosphomonoesterase, catalase, nitratase, arylsuphatase, leucine aminopeptidase, polyphenol oxidase, β-glucosidase, N-acetyl-β-D- glucosaminidase and invertase, and MBC and MBN. These enzymes are involved in C, N, P, and S cycling in the soil, which indicates the ability of soil nutrition supply. All the methods and the references for measuring soil properties mentioned above are presented in Supplementary materials and methods, as well as the SQI calculation. In this study, the minimum data set (MDS) method was selected to calculate the SQI. Two different SQI values were calculated, with one representing the soil physicochemical properties and the other representing the soil biochemical and microbiological properties. The MDS for the soil physicochemical and biochemical and microbiological properties are displayed in Supplementary Files 1 and 2.
Network analysis
The co-occurrence network was inferred based on the Spearman correlation matrix to explore the co-occurrence patterns between microbial taxa in complex communities. Before we constructed the network, we preprocessed the data set. CK and NPK, OM25 and OM50, and OM75 and OM100 were summarized as no organic fertilizer (NOF) input, low organic fertilizer (LOF) input, and high organic fertilizer (HOF) input treatments, because the CK and NPK treatments had no organic fertilizer, the OM25 and OM50 had a lower rate of organic substitution, and the OM75 and OM100 had a higher rate of organic substitution. OTUs with relative abundance less than 0.01% and frequency of occurrence lower than 4 within 6 data columns were removed to reduce rare OTUs in the data set. Then, the psych and igraph packages in the R software (version 3.5.1) were used to analyze the preprocessed data and calculate the Spearman correlation and the network properties. Only the results with a cut-off at an absolute r value greater than 0.6 and a p value below 0.05 after adjusting by Benjamini–Hochberg’s false discovery rate were retained for further network visualization using the “gephi” software (version 0.9.2; https://gephi.org/) (Benjamini and Hochberg 1995). Further analysis for co-occurrence network was described in the Supplementary materials and methods.
Statistical analysis
The SQI under different OSRs treatments was evaluated by one-way ANOVA at p < 0.05, as well as the difference in the yield of tea leaves. The SQI of physicochemical and biochemical and microbiological properties under the NOF (CK and NPK), LOF (OM25 and OM50), and HOF (OM75 and OM100) groups was evaluated by Kruskal–Wallis test at p < 0.05. The soil fungal α-diversity indices and the relative abundance at different taxonomy levels were compared by one-way ANOVA and Fisher’s LSD multiple comparison test with a significance level at p < 0.05.
Linear regression was also carried to evaluate the relationship between OSRs and yield of tea leaves, and other parameters, i.e., SQI, and α-diversity indices. The relationship between the SQI and yield of fresh tea leaves was also explored by linear regression.
The Pearson correlation analysis was performed for evaluating the relationship between the SQI and soil fungal index (i.e., fungal α-diversity indices, fungal β-diversity, and network modularity), and the Spearman correlation analysis was performed for SQI and eigengenes in each module of networks. Nonmetric multidimensional scaling (NMDS) plots were used to estimate the overall differences in the fungal community composition, based on the Bray–Curtis distances.
Analysis of similarities (ANOSIM) was used to test the significance of the treatment effect on the soil fungal community composition. Distance-based redundancy analysis (dbRDA) was carried out to test the effect of environmental factors on fungal community composition. The ANOVA for dbRDA was performed by “term” in the R software (version 3.5.1).
Simultaneously, variance inflation (VIFs) were computed to confirm the presence of collinearities among environmental properties, and the environmental properties were removed until all the VIFs were less than 10 (Supplementary Table 2). In addition, we also performed variance decomposition to test the effect of environmental factors on fungal community composition. All analyses mentioned in this paragraph were performed in R software using the “vegan package”.
Results
Soil quality index and tea yield change under different OSRs
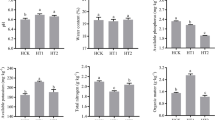
In the present study, the SQI values of physicochemical and biochemical and microbiological properties ranged from 0.31 to 0.75 and 0.31 to 0.77, respectively. The OM100 showed the highest value in the SQI of physicochemical and biochemical and microbiological properties. The CK treatment showed the lowest physicochemical SQI, and the NPK treatment showed the lowest biochemical and microbiological SQI (Fig. 1). Additionally, significant positive correlations were found between the OSRs and physicochemical (R2 = 0.44, p < 0.01) and biochemical and microbiological (R2 = 0.77, p < 0.001) SQI (Supplementary Fig. 1). The Fisher’s LSD multiple comparison testing showed no difference in physicochemical and biochemical and microbiological SQI values between CK and NPK treatments, and values of physicochemical and biochemical and microbiological SQI in high OSRs OM75 and OM100 were significantly higher than CK and NPK treatment (data not shown).
Compared with CK, tea yields were all higher under fertilization during both spring and autumn seasons. The spring yield was generally higher than that in autumn. With the OSR increase, the spring tea yield did not show significant change, but the autumn tea yield decreased. The annual total yield slightly decreased with the OSRs, but OM100 showed a significant decrease if compared with low substitution treatments, e.g., NPK and OM25 (Fig. 2a).
The linear regression analysis between (a) the tea yield and the OSRs, (b) the tea yield and the soil physicochemical SQI, and (c) the tea yield and the biochemical and microbiological SQI. The different letters in a plot (a) represent the yield difference under different OSRs. The red triangle, green inverted triangle, and blank dot represent the spring tea yield, autumn tea yield, and total annual yield, respectively. The curves represent the relationships between two variables, and a p value less than 0.05 in the equation indicates significant correlation
The soil physicochemical SQI exhibited a significant quadratic relationship with the spring tea yield (R2 = 0.50, p < 0.01), autumn tea yield (R2 = 0.36, p < 0.05), and the total tea yield (R2 = 0.43, p < 0.05) (Fig. 2b). However, we did not observe any significant linear relationship between the yields and soil biological SQI (Fig. 2c).
Soil fungal diversity change under different OSRs
For the soil fungal α-diversity, the (richness) Chao1 (R2 = 0.42, p < 0.01) and the (diversity) Shannon (R2 = 0.48, p < 0.01) indices displayed a positive correlation with the OSRs (Supplementary Fig. 2). Compared with CK and NPK, only high OSR treatments (OM75 and OM100) showed a significantly higher Chao1 index than the other treatments (p < 0.05), while low OSR treatments (OM25 and OM50) did not show any significant change (Supplementary Fig. 2a). The Shannon index exhibited a slight increase with the OSR, but it was insignificant among OSR treatments. The NPK treatment showed the lowest Shannon index (p < 0.05) (Supplementary Fig. 2b).
Under the long-term different fertilization regimes, the soil fungal community composition was well separated by Bray–Curtis distance-based NMDS (stress = 0.09). The ANOSIM analysis confirmed that fungal communities of each treatment was significantly different (R = 0.79, p = 0.001) (Supplementary Fig. 3).
Distance-based RDA found that the first two components accounted for 46.31% of the variation in the fungal community (Fig. 3a). The soil pH (F = 6.5156, p = 0.001), SOC (F = 2.4006, p = 0.034), MBC (F = 2.2803, p = 0.046), and AK (F = 2.3778, p = 0.049) had a significant impact on the composition of the fungal community in the dbRDA model (Table 2). Variance decomposition showed that 62.64% of the variance could be explained by soil pH, SOC, MBC, and available nutrients (Fig. 3b).
Distance-based (distance = bray) redundancy analysis (dbRDA) (a) depicting the correlation between fungal communities and soil properties, and (b) variance decomposition, with the percentages of variance explained by soil properties. Available nutrients include nitrate, exchangeable NH4+-N, available P, and available K. SOC, soil organic C; MBC, microbial biomass C
Soil fungal community and its co-occurrence pattern
The soil fungal communities were dominated by Ascomycota, Zygomycota, and Basidiomycota at the phylum level, which accounted for approximately 90% of the relative abundance in all samples (Supplementary Table 3). NPK showed the highest (76.53%), and CK (43.56%) the lowest relative abundance of the phylum Ascomycota, which gradually decreased by increasing OSR (Supplementary Table 3). Conversely, with respect to the relative abundance of the phylum Zygomycota, CK showed the highest value (40.94%), while NPK showed the lowest value (10.94%), and the relative abundance increased with the OSRs. The relative abundance of Basidiomycota ranged from 6.59 (OM100) to 15.35% (OM75), and did not exhibit any linear relationship with the OSR (Supplementary Table 3).
The co-occurrence network of NOF, LOF, and HOF was distinctly different (Fig. 4). Compared with NOF, LOF and HOF increased the number of nodes (from 28 to 56/59) and edges (from 69 to 140/143), respectively (Table 3). Furthermore, the number of negative correlation edges increased with the OSRs (Table 3). The ratio of negative edges accounted for approximately 2.90% in NOF, approximately 4.29% in LOF, and approximately 30.77% in HOF.
The fungal co-occurrence networks under the NOF (CK and NPK), LOF (OM25 and OM50), and HOF (OM75 and OM100) treatments based on Spearman correlations. The (a, d) NOF, (b, e) LOF, and (c, f) HOF networks are colored by the fungal phyla and modules. The size of each node is proportional to the number of connections (degree), and the thickness of each edge is proportional to the value of Spearman’s correlation coefficients. The blue edges indicate negative interactions between two bacterial nodes, while red edges indicate positive interactions
Seven, 13, and 9 keystone species were identified in the fungal co-occurrence network of NOF, LOF, and HOF, respectively (Fig. 4 and Supplementary File 3). Most keystone species in the three networks were from the phylum Ascomycota. NOF and LOF shared four keystone species, i.e., OTU16, OTU48, OTU74, and OTU90, while HOF did not share any keystone species with NOF or LOF (Fig. 5). All 16 keystone species in the NOF and LOF network belonged to three classes, i.e., Sordariomycetes, Agaricomycetes, and Eurotiomycetes (Fig. 5a, b). In contrast, HOF keystone species belonged to class Leotiomycetes, Sordariomycetes, Eurotiomycetes, Dothideomycetes, and Agaricomycetes (Fig. 5c). With respect to their functions, four and six keystone species in the NOF and LOF networks, respectively, were identified as potential pathogens. However, keystone species of the HOF network exhibited more diverse functions, e.g., producing enzymes and decomposing wood material, but no function related to pathogenicity (Supplementary File 3).
Phylogenetic tree for active nonorganic fertilizer (CK and NPK) OTUs, low-organic fertilizer (OM25 and OM50) OTUs, and high-organic fertilizer (OM75 and OM100) OTUs in networks. Shared OTUs in the network were bolded. The scale bars represent sequence divergence, and bootstrap values (> 50%) are shown at branch points
The relationship between the SQI and soil fungal indices
The physicochemical SQI was more related with soil fungal species richness, while the biochemical and microbiological SQI was more related with soil fungal species diversity. The physicochemical SQI showed a stronger positive correlation with the Chao1 index (R = 0.72, p < 0.01) than the Shannon index (R = 0.53, p < 0.05), while the biological SQI exhibited a stronger positive correlation with the Shannon index (R = 0.84, p < 0.001) than the Chao1 index (R = 0.68, p < 0.01) (Table 4). In addition, soil fungal β-diversity also exhibited a significantly positive correlation with both the SQI (R = 0.78, p < 0.01, R = 0.87, p < 0.001) (Table 4).
In the network analysis, the modularity of the soil fungal network showed a strong positive correlation with the physicochemical SQI (R = 0.79, p < 0.01) and biochemical and microbiological SQI (R = 0.91, p < 0.001) (Table 4). The modularity of each network also increased with the addition of more organic fertilizer (from 0.420 to 0.579) and showed a significant positive correlation with physicochemical (R = 0.79, p < 0.01) and biochemical and microbiological (R = 0.91, p < 0.001) SQI (Table 4). However, the module eigengene of each network did not show any significant correlation with both physicochemical and biochemical and microbiological SQI (Table 5).
Discussion
The effect of OSRs on fungal communities
Fertilization with organic amendments usually alters the fungal communities, such as richness, diversity, and community composition (Lucheta et al. 2016; Yao et al. 2017). This can be confirmed by the current study, which demonstrated that both soil fungal α-diversity and community composition were significantly changed with the OSRs (Supplementary Fig. 2 and Fig. 3). Most previous studies found that soil pH, SOM, and MBC are the key factors in explaining fungal community differences (Sun et al. 2016; Tao et al. 2016) and diversity changes (Sterner and Elser 2002; Chen and Xu 2005). In the current study, soil pH, MBC, and MBN were significantly correlated with the fungal α-diversity (Supplementary Table 4), while pH, SOC, MBC, and available nutrients explained > 60% of the community composition variance in the dbRDA (Fig. 3).
During the last two decades, a pH-driven microbial diversity pattern has been widely detected (Lentendu et al. 2011; Bardgett and van der Putten 2014; Wang et al. 2015). Tao et al. (2016) proposed that soil pH is a key factor in shaping the soil fungal community composition. It was found that in the same field, with an increase in the OSR and the addition of organic matter, the soil pH was significantly increased (Ji et al. 2018). Thus, this implied that organic substitution mitigated soil acidification and led to an increase in the soil fungal richness, because less fungal taxa could survive at lower pH values (Rousk et al. 2010).
Organic amendments will usually improve the soil nutrient and organic C content (Maeder et al. 2002; Chang et al. 2007; Liang et al. 2012), which consequently results in a change in fungal communities (Lucheta et al. 2016; Yao et al. 2017). Moreover, soil fungal community alteration may also depend on different types of C sources. Hoppe et al. (2016) found significantly different fungal community composition in the deadwood of different tree species. Deacon et al. (2006) found that there were distinct differences in the resource use of fungal isolates from grassland. However, in the current study, only a sole C source condition was created, because only sole organic fertilizer was applied in the field, albeit with different amounts.
In the studied soil, the fungal community mainly consisted of Ascomycota, Basidiomycota, and Zygomycota (Supplementary Table 3), which confirms what was found by Sun et al. (2016). However, the relative abundance of Ascomycota and Zygomycota exhibited different growth strategies with organic fertilizer application (Supplementary Table 3). Similar result was also found in the study by Dai et al. (2018). This may be due to the Zygomycota saprotroph exhibiting increased sensitivity to C sources than the Ascomycota saprotroph. In addition, five fungal functional groups (i.e., arbuscular mycorrhizal fungi, ectomycorrhizal fungi, ericoid mycorrhizal fungi, plant pathogens, and saprotrophs) were identified according to FUNGuild (http://www.stbates.org/guilds/app.php) (Nguyen et al. 2016; Guo et al. 2019). Our results showed that saprotroph fungi and plant pathogen fungi were the dominant function fungi and account for ~ 90% of the whole community, and plant pathogen fungi increased with the OSRs (Supplementary Fig. 4), which indicated a risk of plant disease. Nevertheless, results on fungal diversity depend on the used primers and database. Indeed, Xue et al. (2019) reported that the choice of genetic marker and classification database resulted in different relative abundances among the 30 most abundant fungal groups, which had a great impact on the next analysis, such as the level of particular taxa or for defining potential indicator species. Besides, due to the missing sequencing data at the beginning of this study, the difference of fungal community composition also might be attributed to the spatial heterogeneity of soils. In addition, soil samples collected from 0 to 10 cm depth may not take into account the root effect as affected by fertilization. Indeed, tea plant can influence soil microbial communities because of the root architectures and metabolisms (Chen et al. 2006).
The effect of OSRs on fungal co-occurrence networks
Previous studies showed that when organic fertilizer was added to soils, a more complex co-occurrence network developed than that due to the addition of inorganic fertilizer (Ling et al. 2016; Wang et al. 2017). A similar result was found in our study, where the fungal network modularity and negative edges increased with the OSRs (Fig. 4 and Table 3).
In the present study, a greater addition of organic matter (higher OSR) increased C, and this caused an increase in the soil pH; this change increased the niche width (Dundore-Arias et al. 2019) and niche differentiation (Dumbrell et al. 2010), respectively, and the results might have been diversity with beneficial coexistence of species in the soil habitat. This may also explain why there were more nodes and edges in the co-occurrence networks of LOF and HOF than those in the NOF (Fig. 4 and Table 3). This also partly supports the previous findings that applying organic fertilizer increases the degree of cooperation and exchange events within the soil ecosystem (Chaffron et al. 2010; Faust and Raes 2012). Furthermore, increasing the C input into soil may also enhance the microbial competition for the C source due to the niche overlap (Dundore-Arias et al. 2019). Chen et al. (2019) reported that biochar amendments induced competitive interactions in co-occurrence networks. Thus, the increase in negative edges in HOF was mainly due to the greater C input (Fig. 4 and Table 3). Overall, we presumed that the amount of C input might be a key factor in determining the complexity of the fungal co-occurrence network.
The effect of OSR on soil quality and yield
Under organic fertilizer addition, both the physicochemical and biochemistry and microbiological SQI were significantly increased with the OSR (Fig. 1), which indicated the beneficial effect of OSR on soil quality. This confirmed results by Liu et al. (2017), who found that application of NPK plus pig manure enhanced the soil physicochemical properties and microbiological properties when compared with NPK or no fertilization.
It has been reported that about 30% organic substitution significantly increased soil fertility and quality (Zhao et al. 2016; Li et al. 2017). However, most of these studies described experiments characterized by only one or two substitution rates.
In the current study, although the soil physicochemical properties increased by increasing OSRs (Supplementary File 3), the application of OM25 resulted in the maximum yield (Fig. 2a). Therefore, the pattern of the improvement in the soil quality differed from that of the tea yield, because the tea yield had a significant quadratic relationship with the soil physicochemical quality (Fig. 2b). Whereby, the positive effect of the OM25 may be attributed to the improvement in the soil quality (Saleque et al. 2004; Jiang et al. 2006), while the negative effect by the OM 75 and OM100 could be due to the insufficient mineral N supply before the harvest, which can also cause yield loss (Ladha et al. 2003; Bi et al. 2009). Moreover, the fertilization strategy may also be an important reason for the negative effect of high OSR on yield. In order to meet the nutritional demand of the tea plants, it was necessary to split three times the application of synthetic N fertilizer. Therefore, the high OSR treatments did not supply sufficient mineral N for the young shoot growth before the harvest, thus reducing the autumn yield compared with the spring yield in treatments with high OSR (Fig. 2a).
Generally, the application of organic fertilizer has positive effects on plant disease suppression and nutrient cycling (Termorshuizen et al. 2006; Lang et al. 2011; Zhao et al. 2016). Li et al. (2017) reported that the addition of organic fertilizer had a greater suppressive effect on Fusarium and Verticillium dahlia. Tao et al. (2015) also found that partial substitution of mineral NPK with organic matter suppressed soil-borne pathogens by increasing the Bacillus abundance in cotton fields. Our results confirmed these findings because the treatment with high OSR (HOF) resulted in less pathogenic species than with NOF and LOF treatments, as shown by the Supplementary File 3. In addition, more species related to degradation were found in HOF co-occurrence networks (Supplementary File 3), likely due to the increased availability of SOC and nutrients (Scotti et al. 2016).
Relationship between soil quality index and fungal network
Both physicochemical and biochemical and microbiological SQI showed positive relationships with soil fungal α-diversity and β-diversity, which indicated that SQI improvement changed the composition of soil fungal community. This may be related to the enhancement of the competition between microbes (Fontaine et al. 2003) and the maintenance of microbial α-diversity (Siciliano et al. 2014).
The soil fungal network modularity was significantly correlated with both physicochemical and biochemical and microbiological SQI (Table 4). This indicated the possibility of using network modularity in the fungal co-occurrence network analysis as an indicator for SQI assessment. This may save both time and labor costs if automated high throughput sequencing (HTS) is used, compared with traditional physicochemical and biochemical and microbiological SQI assessment, which requires the measurements of several properties, such as pH, SOC, TN, AP, AK, MBC, MBN, and enzymatic activities (Doran and Parkin 1994; Liu et al. 2013; Schloter et al. 2018).
Conclusions
Our study investigated the soil quality and the changes in the composition of fungal community alteration under different OSRs over a 10-year period in a mono-cultured tea plantation. Both the soil physicochemical and biochemical and microbiological SQI had a strongly positive relationship with the OSRs. However, after taking into consideration the yield and the benefit of soil quality, 25% OSR should be recommended for practice for tea plantations’ fertilizer treatment. An increase in the OSR resulted in a more complex fungal co-occurrence network. The fungal network complexity was positively correlated with SQI, which indicated the possibility of using fungal network complexity in the SQI assessment. However, additional investigations with other soils are required to verify the accuracy of using fungal networks for SQI assessment.
References
Abarenkov K, Henrik Nilsson R, Larsson KH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T (2010) The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol 186:281–285
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39:1770–1781
Aryal UK, Xu HL, Fujita M (2003) Rhizobia and AM fungal inoculation improve growth and nutrient uptake of bean plants under organic fertilization. J Sustain Agric 21:27–39
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515:505–511
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Bi L, Zhang B, Liu G, Li Z, Liu Y, Ye C, Yu X, Lai T, Zhang J, Yin J, Liang Y (2009) Long-term effects of organic amendments on the rice yields for double rice cropping systems in subtropical China. Agric Ecosyst Environ 129:534–541
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of Earth’s nitrogen cycle. Science 330:192–196
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chaffron S, Rehrauer H, Pernthaler J, von Mering C (2010) A global network of coexisting microbes from environmental and whole-genome sequence data. Genome Res 20:947–959
Chang EH, Chung RS, Tsai YH (2007) Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci Plant Nutr 53:132–140
Chen J (2006) The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. International Workshop on Sustained Management of the Soil-Rhizosphere System for Efficient Crop Production and Fertilizer Use, Bangkok, pp 1–11
Chen CR, Xu ZH (2005) Soil carbon and nitrogen pools and microbial properties in a 6-year-old slash pine plantation of subtropical Australia: impacts of harvest residue management. For Ecol Manag 206:237–247
Chen YM, Wang MK, Zhuang SY, Chiang PN (2006) Chemical and physical properties of rhizosphere and bulk soils of three tea plants cultivated in Ultisols. Geoderma 136:378–387
Chen L, Jiang Y, Liang C, Luo Y, Xu Q, Han C, Zhao Q, Sun B (2019) Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7:77
Cwalina-Ambroziak B, Bowszys T (2009) Changes in fungal communities in organically fertilized soil. Plant Soil Environ 55:25–32
Dai Z, Enders A, Rodrigues JLM, Hanley KL, Brookes PC, Xu J, Lehmann J (2018) Soil fungal taxonomic and functional community composition as affected by biochar properties. Soil Biol Biochem 126:159–167
Deacon LJ, Janie Pryce-Miller E, Frankland JC, Bainbridge BW, Moore PD, Robinson CH (2006) Diversity and function of decomposer fungi from a grassland soil. Soil Biol Biochem 38:7–20
Diacono M, Montemurro F (2011) Long-term effects of organic amendments on soil fertility. In: Lichtfouse E, Hamelin M, Navarrete M, Debaeke P (eds) Sustainable agriculture volume 2. Springer Netherlands, Dordrecht, pp 761–786
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. SSSA, Madison, pp 3–21
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345
Dundore-Arias JP, Felice L, Dill-Macky R, Kinkel LL (2019) Carbon amendments induce shifts in nutrient use, inhibitory, and resistance phenotypes among soilborne Streptomyces. Front Microbiol 10:498
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Edmeades DC (2003) The long-term effects of manures and fertilisers on soil productivity and quality: a review. Nutr Cycl Agroecosyst 66:165–180
Evenson RE, Gollin D (2003) Assessing the impact of the green revolution, 1960 to 2000. Science 300:758–762
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Change Biol 21:2082–2094
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843
Gil-Sotres F, Trasar-Cepeda C, Leirós MC, Seoane S (2005) Different approaches to evaluating soil quality using biochemical properties. Soil Biol Biochem 37:877–887
Guenet B, Neill C, Bardoux G, Abbadie L (2010) Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl Soil Ecol 46:436–442
Guo J, Ling N, Chen Z, Xue C, Li L, Liu L, Gao L, Wang M, Ruan J, Guo S, Vandenkoornhuyse P, Shen Q (2019) Soil fungal assemblage complexity is dependent on soil fertility and dominated by deterministic processes. New Phytol, Accepted Author Manuscript. https://doi.org/10.1111/nph.16345
Hoppe B, Purahong W, Wubet T, Kahl T, Bauhus J, Arnstadt T, Hofrichter M, Buscot F, Krüger D (2016) Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Divers 77:367–379
Huang S, Zhang W, Yu X, Huang Q (2010) Effects of long-term fertilization on corn productivity and its sustainability in an Ultisol of southern China. Agric Ecosyst Environ 138:44–50
Ji L, Wu Z, You Z, Yi X, Ni K, Guo S, Ruan J (2018) Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: a 10-year field trial in a tea plantation. Agric Ecosyst Environ 268:124–132
Jiang D, Hengsdijk H, Dai TB, de Boer W, Jing Q, Cao WX (2006) Long-term effects of manure and inorganic fertilizers on yield and soil fertility for a winter wheat-maize system in Jiangsu, China. Pedosphere 16:25–32
Jones MDM, Forn I, Gadelha C, Egan MJ, Bass D, Massana R, Richards TA (2011) Discovery of novel intermediate forms redefines the fungal tree of life. Nature 474:200–203
Kamau DM, Spiertz JHJ, Oenema O (2008) Carbon and nutrient stocks of tea plantations differing in age, genotype and plant population density. Plant Soil 307:29
Karlen DL, Mausbach MJ, Doran JW, Cline RG, Harris RF, Schuman GE (1997) Soil quality: a concept, definition, and framework for evaluation. Soil Sci Soc Am J 61:4–10
Ladha JK, Dawe D, Pathak H, Padre AT, Yadav RL, Singh B, Singh Y, Singh Y, Singh P, Kundu AL, Sakal R, Ram N, Regmi AP, Gami SK, Bhandari AL, Amin R, Yadav CR, Bhattarai EM, Das S, Aggarwal HP, Gupta RK, Hobbs PR (2003) How extensive are yield declines in long-term rice–wheat experiments in Asia? Field Crop Res 81:159–180
Lang J, Hu J, Ran W, Xu Y, Shen Q (2011) Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol Fertil Soils 48:191–203
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415
Lentendu G, Zinger L, Manel S, Coissac E, Choler P, Geremia RA, Melodelima C (2011) Assessment of soil fungal diversity in different alpine tundra habitats by means of pyrosequencing. Fungal Divers 49:113–123
Li R, Tao R, Ling N, Chu G (2017) Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: implications for soil biological quality. Soil Till Res 167:30–38
Liang Q, Chen H, Gong Y, Fan M, Yang H, Lal R, Kuzyakov Y (2012) Effects of 15 years of manure and inorganic fertilizers on soil organic carbon fractions in a wheat-maize system in the North China Plain. Nutr Cycl Agroecosyst 92:21–33
Ling N, Zhu C, Xue C, Chen H, Duan Y, Peng C, Guo S, Shen Q (2016) Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol Biochem 99:137–149
Liu Z, Zhou W, Shen J, Li S, Ai C (2013) Soil quality assessment of yellow clayey paddy soils with different productivity. Biol Fertil Soils 50:537–548
Liu Z, Rong Q, Zhou W, Liang G (2017) Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS One 12:e0172767
Lucheta AR, de Souza CF, Roesch LFW, Tsai SM, Kuramae EE (2016) Fungal community assembly in the Amazonian dark earth. Microb Ecol 71:962–973
Maeder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
McKay GJ, Egan D, Morris E, Scott C, Brown AE (1999) Genetic and morphological characterization of Cladobotryum species causing cobweb disease of mushrooms. Appl Environ Microbiol 65:606–610
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Nguyen NH, Song ZW, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Pingali PL (2012) Green revolution: impacts, limits, and the path ahead. Proc Natl Acad Sci U S A 109:12302–12308
Potthoff M, Steenwerth KL, Jackson LE, Drenovsky RE, Scow KM, Joergensen RG (2006) Soil microbial community composition as affected by restoration practices in California grassland. Soil Biol Biochem 38:1851–1860
Ramanantenasoa MMJ, Genermont S, Gilliot JM, Bedos C, Makowski D (2019) Meta-modeling methods for estimating ammonia volatilization from nitrogen fertilizer and manure applications. J Environ Manag 236:195–205
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Ruan J, Haerdter R, Gerendás J (2010) Impact of nitrogen supply on carbon/nitrogen allocation: a case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants*. Plant Biol 12:724–734
Saleque MA, Abedin MJ, Bhuiyan NI, Zaman SK, Panaullah GM (2004) Long-term effects of inorganic and organic fertilizer sources on yield and nutrient accumulation of lowland rice. Field Crop Res 86:53–65
Schloter M, Nannipieri P, Sørensen SJ, van Elsas JD (2018) Microbial indicators for soil quality. Biol Fertil Soils 54:1–10
Scotti R, Pane C, Spaccini R, Palese AM, Piccolo A, Celano G, Zaccardelli M (2016) On-farm compost: a useful tool to improve soil quality under intensive farming systems. Appl Soil Ecol 107:13–23
Seybold CA, Herrick JE, Brejda JJ (1999) Soil resilience: a fundamental component of soil quality. Soil Sci 164:224–234
Siciliano SD, Palmer AS, Winsley T, Lamb E, Bissett A, Brown MV, van Dorst J, Ji M, Ferrari BC, Grogan P, Chu H, Snape I (2014) Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol Biochem 78:10–20
Smith P, Haberl H, Popp A, Erb K, Lauk C, Harper R, Tubiello FN, de Siqueira PA, Jafari M, Sohi S, Masera O, Böttcher H, Berndes G, Bustamante M, Ahammad H, Clark H, Dong H, Elsiddig EA, Mbow C, Ravindranath NH, Rice CW, Robledo Abad C, Romanovskaya A, Sperling F, Herrero M, House JI, Rose S (2013) How much land-based greenhouse gas mitigation can be achieved without compromising food security and environmental goals? Glob Change Biol 19:2285–2302
Song G, Chen R, Xiang W, Yang F, Zheng S, Zhang J, Zhang J, Lin X (2015) Contrasting effects of long-term fertilization on the community of saprotrophic fungi and arbuscular mycorrhizal fungi in a sandy loam soil. Plant Soil Environ 61:127–136
Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, de Vries W, de Wit CA, Folke C, Gerten D, Heinke J, Mace GM, Persson LM, Ramanathan V, Reyers B, Sörlin S (2015) Planetary boundaries: guiding human development on a changing planet. Science 347:1259855
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Sun R, Dsouza M, Gilbert JA, Guo X, Wang D, Guo Z, Ni Y, Chu H (2016) Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ Microbiol 18:5137–5150
Tao R, Liang Y, Wakelin SA, Chu G (2015) Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl Soil Ecol 96:42–51
Tao Z, Wang NF, Liu HY, Zhang YQ, Yu LY (2016) Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund region, Svalbard (High Arctic). Front Microbiol 7:277
Termorshuizen AJ, van Rijn E, van der Gaag DJ, Alabouvette C, Chen Y, Lagerlöf J, Malandrakis AA, Paplomatas EJ, Rämert B, Ryckeboer J, Steinberg C, Zmora-Nahum S (2006) Suppressiveness of 18 composts against 7 pathosystems: variability in pathogen response. Soil Biol Biochem 38:2461–2477
Wang JT, Zheng YM, Hu HW, Zhang LM, Li J, He JZ (2015) Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J Soils Sediments 15:1224–1232
Wang S, Chen X, Gong H, Cai Z (2017) Response of soil microbial abundance and diversity in Sacha Inchi (Plukenetia volubilis L.) farms with different land-use histories in a tropical area of southwestern China. Arch Agron Soil Sci 89:588–596
Xue C, Hao Y, Pu X, Ryan Penton C, Wang Q, Zhao M, Zhang B, Ran W, Huang Q, Shen Q, Tiedje JM (2019) Effect of LSU and ITS genetic markers and reference databases on analyses of fungal communities. Biol Fertil Soils 55:79–88
Yao Q, Liu J, Yu Z, Li Y, Jin J, Liu X, Wang G (2017) Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol Biochem 110:56–67
Zhang QC, Shamsi IH, Xu DT, Wang GH, Lin XY, Jilani G, Hussain N, Chaudhry AN (2012) Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl Soil Ecol 57:1–8
Zhao J, Ni T, Li J, Lu Q, Fang Z, Huang Q, Zhang R, Li R, Shen B, Shen Q (2016) Effects of organic–inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice–wheat cropping system. Appl Soil Ecol 99:1–12
Zhou J, Jiang X, Zhou B, Zhao B, Ma M, Guan D, Li J, Chen S, Cao F, Shen D, Qin J (2016) Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol Biochem 95:135–143
Acknowledgments
This work was financially supported by the National Key R&D Project of China (grant number 2016YFD0200900), the Natural Science Foundation of China (grant number 41601325), and the Chinese Academy of Agricultural Sciences (grant number CAAS-XTCX2016015, 1610212016012, 1610212018018).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, L., Ni, K., Wu, Z. et al. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization. Biol Fertil Soils 56, 633–646 (2020). https://doi.org/10.1007/s00374-020-01439-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-020-01439-y