Abstract
Aims
For the sustainable development of agroecosystems in citrus orchards, we studied the short-term effects of bio-organic fertilizer substitution for chemical fertilizers on the dynamic changes in the soil environment.
Methods
We carried out a field experiment in citrus orchards. Five treatments were set up with a duration of six months and the substitution ratio was based on equivalent nitrogen substitution. Soil properties, soil labile organic carbon, and soil bacteria were measured and analyzed.
Results
Bio-organic fertilizers substituting chemical fertilizers could significantly increase soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), dissolved organic carbon (DOC), and easily oxidized organic carbon (EOC) content (P < 0.05). The carbon pool management index (CPMI) was the largest in 75% bio-organic fertilizer treatment. In addition, the substitution of chemical fertilizers with bio-organic fertilizers significantly increased the alpha diversity of bacterial communities, with a maximum Shannon index of 9.78 for SF75. The relative abundance of Actinobacteria, Actinobacteria and Bacteroidetes was higher than that of CK, while the relative abundance of Acidobacteria and Choloflexi was lower than that of the control group. Redundancy analysis showed that DOC, CPMI, available potassium (AK), and nitrate-nitrogen (NO3−-N) were the main driving factors affecting the bacterial community structure. The highest expression abundance of metabolic pathways in soil bacteria was predicted by KEGG to exist.
Conclusion
We conclude that the appropriate application of bio-organic fertilizer improved soil properties and reshaped bacterial ecology and 75% bio-organic fertilizer is a promising fertilization practice for citrus orchard soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Citrus is one of the main economic crops in China, and at present, citrus production in most areas of China is still dominated by the use of chemical fertilizers, and organic fertilizer resources are not fully utilized, and excessive chemical fertilizer application makes citrus orchards with serious soil consolidation and nutritional barriers to fruit tree growth, resulting in reduced yields and fruit quality, and substantially reducing the economic benefits of orchards (Lei et al. 2019). Chemical fertilizer misuse is currently causing serious adverse impacts globally, such as eutrophication, air pollution, and soil acidification (Savci 2012; Blanco-Canqui and Schlegel 2013; Sun et al. 2015). These environmental issues, therefore, worsen the condition and productivity of agricultural soils, endangering entire agroecosystems and reducing the sustainability of crop production. According to some studies, using organic fertilizers can enhance fruit quality, lessen soil environmental issues brought on by the misuse of chemical fertilizers, and promote the development of sustainable agriculture (Kim et al. 2022). Bio-organic fertilizers have the characteristics of both microbial and organic fertilizers and are a class of fertilizers that rely on the production and metabolic activities of microorganisms to provide a comprehensive nutritional balance and lasting fertility for plants and soils, with organic matter as the mainstay (Wu et al. 2009). Since the end of the last century, bio-organic fertilizer dispensing has been widely used in experimental studies, and the use of bio-organic fertilizers can compensate for the shortcomings of chemical fertilizers to a certain extent (Wang et al. 2017), not only to rapidly increase the content of nutrients such as soil nitrogen, potassium, phosphorus and organic matter (Li et al. 2021), but also to boost soil fertility, stimulate the improvement of microbial community structure, and increase the utilization and abundance of microbial carbon sources (Ansari and Mahmood 2017). The partial replacement of chemical fertilizers with organic fertilizers has been shown to improve soil fertility, crop productivity, and soil microbial abundance and diversity in long term (Qiao et al. 2019; Xiang et al. 2020). Therefore, our study chose bio-organic fertilizers to substitute chemical fertilizers, to explore the effect of bio-organic fertilizer substitution on soil fertility and soil microorganisms in citrus orchards in the short term.
Farmland is an important component of the soil organic carbon pool and is susceptible to human activities (Zhang et al. 2021). Frequent tillage and improper management tend to lead to a decline in soil organic carbon stocks (Varvel and Wilhelm 2011), thus causing soil fertility degradation and land productivity to decline (Yu et al. 2006). Soil as a carbon sink or source plays a crucial role in the global carbon cycle, global climate change, and the equilibrium of sustainable agricultural development (Liang et al. 2010). Root exudates, decomposing plant and animal remains, and dead microbial biomass are typical sources of soil labile organic carbon. (Bolan et al. 2014). Although the LOC is a small portion of the soil organic carbon, it can respond sensitively to the effects of different agricultural management measures on the rate and quality of SOC conversion and is an indispensable and sensitive indicator to reveal the dynamic changes of the soil carbon pool (Yang et al. 2020). Therefore, LOC is suitable for characterizing the turnover and availability of nutrients on farmlands where organic fertilizers are applied in the short term. Additionally, to evaluate the state and rate of change of soil carbon in agro-ecosystems, the carbon pool management index (CPMI) value is frequently utilized. CPMI is a comprehensive index of soil carbon pool and can reflect the impact coming from external factors on the SOC comprehensive and dynamic manner (Sodhi et al. 2009). Organic fertilizers contain C sources and additional macronutrients and micronutrients, so the application of organic manure can further improve soil SOC content and LOC concentration and promote soil carbon pool balance (Ishaq et al. 2002; Wang et al. 2003; Qiu et al. 2016). Thus, the LOC content and CPMI indices were used to indicate the changes in soil nutrients brought about by the application of different alternative proportions of bio-organic fertilizers in this study.
Soil microorganisms play a critical role in regulating various ecosystem functions and processes, such as climate moderation, environmental purification, nutrient cycling, and soil organic decomposition (Ji et al. 2018; Manjunath et al. 2018). Numerous experiments have confirmed that soil microorganisms can be used to indicate changes in soil quality and the effects of fertilization management because they can modify the community structure in time to respond to environmental changes (García-Delgado et al. 2019; Mganga et al. 2016). Increases in soil fertility, fungal diversity, and fungal abundance have been seen as a result of some chemical fertilizers being partially replaced by organic fertilizers. (Xiang et al. 2020). The application of organic fertilizer contributes to increasing the diversity of soil microbial communities in fields with long-term fertilization (Ding et al. 2016). According to Wang et al. (2017), bio-fertilizer had a significant impact on the soil bacterial community composition in apple orchards. Liu et al. (2021) observed that helpful microbial activity in the rhizosphere of kiwi is promoted by long-term bio-fertilizer application, leading to a microbial network conducive to plant growth. Moreover, the application of bio-fertilizer can not only provide C and N sources and energy for soil microbial activities but also stimulates the secretion of carbohydrates, amino acids, organic acids, proteins, and enzymes by rhizosphere microorganisms, thus suppressing the formation of related soil-borne diseases (Badri and Vivanco 2009; Gu et al. 2020; Kautz et al. 2006; Lehmann and Kleber 2015). The majority of recent studies have shown that a promising tactic to improve soil fertility and microbial diversity is the partial replacement of chemical fertilizers with organic fertilizers (Huang et al. 2020; Qiao et al. 2019; Xiang et al. 2020). Hence, it is crucial to investigate the short-term effects of partially substituting chemical fertilizers with bio-organic fertilizers on soil fertility and bacterial characteristics of the soil in citrus orchards.
In this study, we hypothesized that the short-term effects of partial substitution of chemical fertilizers with bio-organic fertilizers would improve soil properties, soil labile organic carbon, and soil bacterial communities in citrus orchards. Additionally, we anticipate obtaining the ideal substitution ratio to enhance soil fertility and the microbiological ecosystem of the soil in order to support the sustainable growth of agriculture.
Materials and methods
Experimental design and details
Field experiments were carried out from May 1, 2021, to November 30, 2021, in a citrus orchard in Dayang Town, Jiande City, Zhejiang Province, China (29°23′36″N, 119°31′32″E). It has a north subtropical monsoon climate. The average annual temperature is 16.9 °C, and there is 1500 mm of precipitation on average per year. The soil type is Fluventic Eutrochrept (USDA soil taxonomic classification) and the citrus species is Ponkan (Citrus reticulata ‘Ponkan’). Following were the physicochemical characteristics of the soil before the experiment: pH 4.15, TN,1.44 g·kg− 1, TP,2.53 g·kg− 1, total potassium (TK,10.54 g·kg− 1), SOC, 14.58 g·kg− 1, available phosphorus (AP, 201.67 mg·kg− 1), and AK, 364.5 mg·kg− 1.
Chemical fertilizer was a potassium sulfate compound fertilizer (N-P2O5-K2O:18-8-14), which was provided by Taizhou Agricultural Materials Co., Ltd. The bio-organic fertilizer (TN, 22.50 g·kg− 1, TK, 37.97 g·kg− 1, TP, 5.20 g·kg− 1) was developed by Yuanqi Technology Co., Ltd. and was made of natural silkworm guano, vegetable meal, and walnut shell as the main raw materials, fermented with compound ecological flora (specific Bacillus and Lactobacillus, etc.), with an effective live bacterial count of about 50 million/g, organic matter ≥ 50%.
Each citrus tree represents a plot in the field experiment, which was set up as a three-replicate, entirely randomized design. Five treatments were: application of 100% chemical fertilizer (CK), 75% chemical fertilizer + 25% bio-organic fertilizer (SF25), 50% chemical fertilizer + 50% bio-organic fertilizer (SF50), 25% chemical fertilizer + 75% bio-organic fertilizer (SF75) and 100% bio-organic fertilizer (SF100). The substitution ratio followed equal nitrogen substitution, with the total amount of chemical fertilizer applied decreasing in a 25% gradient using the control group as a base, while the total amount of bio-organic fertilizer also increased in a 25% gradient. The basal fertilizer was applied in early June 2021 by spreading method with bio-organic fertilizer as basal fertilizer applied to five treatments at one time and chemical fertilizer as basal fertilizer added to both CK and SF25 treatments. Chemical fertilizer was applied in mid-July 2021 as an additional fertilizer, using the spreading method. Details are shown in Table 1. The irrigation, insecticide, and weeding measures in the citrus orchard were the same as conventional management.
Soil sampling and analysis
Soil samples were gathered from citrus orchards in October 2021. Five soil cores were sampled from 0 to 20 cm depth in each plot and mixed into one composite soil sample. The soil was separated into three sections, one section air-dried for determining physicochemical properties, one section fresh sample for the determination of ammonium nitrogen (NH4+-N) and NO3−-N, and the other section stored in a -80 °C refrigerator for bacterial analysis.
The pH of soil was determined with a pH meter (FE20-FiveEasy™ pH, Mettler Toledo, German).TP was measured using the molybdenum blue method after being digested with HClO4-H2SO4 (Gong et al. 2019).TN and SOC were determined by Element Analyzer (Elementar Vario EL Cube, German) (Liu et al. 2021). AK was extracted with 1 M neutral ammonium acetate and then estimated with Atomic absorption spectrometry (AAS, Analytik Jena novAA 300, German) (Li et al. 2021). AP was assessed using the molybdenum blue method after being extracted with HCl-NH4F (Li et al. 2021). NH4+-N and NO3−-N were extracted with 1 M potassium chloride and measured using an AA3 continuous-flow autoanalyzer (SAN++, SKALAR, Netherlands) (Gong et al. 2019). Using Blair’s suggested methods, the easily oxidized organic carbon (EOC) portion was examined (Blair et al. 1995). Three different concentrations of KMnO4(33 mmol·L− 1, 167 mmol·L− 1, and 333 mmol·L− 1) were used to extract highly labile organic carbon (HLOC), moderately labile organic carbon (MLOC), and low labile organic carbon (LLOC), respectively.DOC was determined in water extracts and processed through 0.45-µm filters, then estimated via a TOC analyzer (Multi N/C 3100 HT1300, Analytik Jena AG, Germany) (Yu et al. 2019).
The extraction of total DNA and sequencing of 16SrDNA (V4-V5 region) of soil microorganisms were entrusted to Guangdong Magigene Technology Co.
Soil DNA extraction, amplification, and sequencing
Standard protocols were followed to extract soil DNA using the ALFA-SEQ Advanced Soil DNA Kit (Megigene, Guangzhou, China), and the quantity and quality of DNA were assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The V4-V5 region of the bacterial 16S rDNA gene was targeted by 515F(5’-GTGCCAGCMGCCGCGGTAA-3’) and 907R(5’-CCGTCAATTCMTTTRAGTTT-3’). The Illumina Miseq platform (Illumina, San Diego, CA, USA) was used to sequence the amplified libraries. After obtaining the raw sequences for quality control through Trimmomatic and FLASH. UCLUST was used to group high-quality sequences into operational taxonomic units (OTUs) at a 97% sequence similarity level. Taxonomic annotation was performed against the SILVA Database to obtain the corresponding bacterial taxonomic information. Soil total DNA extraction, amplification, library construction, sequencing, and data analysis were performed by Guangdong Magigene Biotechnology Co., Ltd. (Guangzhou, China).
Data analyses
IBM SPSS Statistics v26.0(IBM Corp) was used to perform the statistical analysis. Tukey’s-HSD one-way analysis of variance (ANOVA) was used to analyze the significant differences(P < 0.05) in soil physicochemical indicators among different treatments. Alpha diversity indices (chao1, good_coverage, Shannon) were calculated by the “vegan” R package and were plotted using the “ggplot2” R package. A redundancy analysis (RDA) was conducted using the “vegan” R package to explore the correlations between bacterial community structure and selected soil properties at the phylum level. on the Magigene Cloud platform (http://cloud.magigene.com/), phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) was performed to predict bacterial function.
The CPMI was calculated according to the following equations:
-
$$\mathrm{Carbon}\;\mathrm{Pool}\;\mathrm{Index}\;(\mathrm{CPI})=\mathrm{SOC}\;\mathrm{in}\;\mathrm{the}\;\mathrm{treatment}\;\mathrm{group}/\mathrm{SOC}\;\mathrm{in}\;\mathrm{the}\;\mathrm{control}\;\mathrm{group}$$
-
$$\mathrm{Lability}\;\mathrm{of}\;\mathrm{Carbon}\;(\mathrm L)=\mathrm{Labile}\;\mathrm{Organic}\;\mathrm{Carbon}\;(\mathrm{LOC})/\mathrm{Nonlabile}\;\mathrm{Organic}\;\mathrm{Carbon}\;(\mathrm{NLOC})$$
-
$$\mathrm{Lability}\;\mathrm{Index}\;(\mathrm{LI})=\mathrm L\;\mathrm{in}\;\mathrm{the}\;\mathrm{treatment}\;\mathrm{group}/\mathrm L\;\mathrm{in}\;\mathrm{the}\;\mathrm{control}\;\mathrm{group}\;({\mathrm L}_0)$$
-
$$\mathrm{Carbon}\;\mathrm{Pool}\;\mathrm{Management}\;\mathrm{Index}\;(\mathrm{CPMI})=\mathrm{CPI}\times\mathrm{LI}\times100$$
Result
Soil chemical properties change under different substitution ratios
Short-term bio-organic fertilizers substituting chemical fertilizers has changed soil chemical properties (Table 2). Compared to CK, the pH of SF25, SF50, SF75, and SF100 increased significantly with the substitution rate. The TN content also showed a significant increase with increasing substitution ratio, with increases of 15.04%, 31.58%, 65.41% and 105.26% for SF25, SF50, SF75 and SF100 treatments, respectively, compared to CK (P < 0.05). The SOC content increased significantly with increasing substitution ratio, with SF25, SF50, SF75, and SF100 being 1.2, 1.3, 1.7, and 2.2 times higher than the CK treatment, respectively. SF25, SF50, SF75, and SF100 treatments all showed significant increases in TP content compared to CK, with SF100 showing the largest increase of 27.98%. SF50, SF75, and SF100 treatments all significantly increased the content of AP and AK, and showed a trend of increasing and then decreasing with increasing substitution ratio. The application of bio-organic fertilizer significantly increased the NO3−-N content, with the SF50 treatment increasing the most, 2.1 times more than CK. While there was no discernible difference between the SF100 treatment and CK, the NH4+-N content was noticeably higher in the SF25 and SF75 treatments compared to the CK treatment.
Variations in Soil labile carbon fractions and CPMI
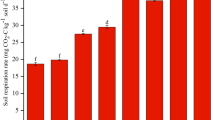
As depicted in Fig. 1, the DOC content of SF50, SF75 and SF100 treatments was notably higher than that of CK treatment by 1.1, 2.7 and 3.4 times, respectively. Bio-organic fertilizer application increased the EOC content and increased with the percentage of substitution, SF50, SF75, and SF100 treatments were significantly higher than CK treatment and SF25 was not significantly different from CK. The HLOC content was the highest, ranging from 4.81 to 12.95 g·kg− 1, accounting for 40.2–61.18%. MLOC was followed, ranging from 3.59 to 5.17 g·kg− 1, accounting for 28.12–39.68%. LLOC had the lowest content, ranging from 2.21 to 3.01 g·kg− 1, accounting for 15.16–22.07%.
The CPMI of soil at different substitution ratios is shown in Table 3. Compared to CK, SF25, SF50, SF75, and SF100 all increased the soil CPMI to different degrees. SF7 improved the most significantly, with an increase of 69.52 compared to CK. Both CPI and LI of the bio-organic fertilizer application treatment were greater than the control group, where CPI increased with the increase of substitution ratio, while LI showed a decreasing trend.
Abundance and alpha diversity of the bacterial community
The 16 S rDNA (V4-V5 region) of soil bacteria from the five treatments were sequenced by high-throughput sequencing technology for amplicons and analyzed for bacterial community diversity under different treatments. A total of 223,110 valid sequences were available. The number of OTUs common or unique to the five treatments of bacterial communities can be shown by the Venn diagram (Fig. 2A), with a total of 8965 OTUs of different species for the five treatments. The OTU types of CK5, SF25, SF50, SF75, and SF100 are 2693, 3354, 3426, 3613, and 3828, respectively. There are 733 OTU types common to the five treatments. OTUs unique to CK was 781, accounting for 8.71% of the total. The unique OTUs of CK5, SF25, SF50, SF75, and SF100 were 781, 1018, 1122, 1105, and 1376, respectively, accounting for 8.71%, 11.36%, 12.52%, 12.33%, and 15.35% of the total OTUs, respectively.
The effects of substitution ratios on the abundance and diversity of bacteria are shown in Fig. 2B. The Good’s coverage values ranged between 98.0% and 98.9%, indicating that its sequencing depth could demonstrate diversity reliably. The Chao1 index was higher than CK in all four treatments. SF75 had the highest chao1 index of 4006.3, which indicates that SF75 has the highest species abundance. A larger Shannon index denotes a higher diversity of the flora. The Shannon index is used to quantify the average or homogeneity of species abundance and diversity in microbial communities. The Shannon index of SF75 was noticeably higher than the control other treatments. In comparison to the other treatments, the SF75 treatment’s bacterial diversity was higher and more evenly distributed.
Composition and effect of the bacterial community in the soil
All samples’ bacterial OTUs were classified into 35 phyla, of which a total of 11 had relative abundances greater than 1%. The dominant phyla are Proteobacteria, Acidobacteria, Actinobacteria, Choloflexi, and Bacteroidetes, with a relative abundance of more than 80%. Compared with CK, the relative abundance of Proteobacteria increased in all bio-organic fertilizer application treatments and showed a tendency of growing, followed by a decrease as the substitution ratio increased. The relative abundances of Bacteroidetes, Planctomycetes, and Firmicutes were higher in SF25, SF50, SF75, and SF100 than in CK. However, Acidobacteria and Choloflexi had higher relative abundances in CK than in other treatments.
The heatmap of soil physicochemical factors and bacteria (phylum level) Pearson correlation analysis is shown in Fig. 3. Actinobacteria was significantly and positively associated with AP, MLOC, and CPMI (P < 0.05). Acidobacteria was significantly negatively related to AP, AK, MLOC, CPMI, DOC, SOC, EOC, HLOC, pH, TP, and TN (P < 0.01). Planctomycetes was found to be significant positive correlated with CPMI, DOC, SOC, EOC, HLOC, pH, TP, TN (P < 0.01), and AK, MLOC (P < 0.05). Bacteroidetes had notable and positive correlations with AP, AK, and pH (P < 0.01) and with MLOC, CPMI, DOC, SOC, EOC, HLOC, TP, and TN (P < 0.05). Verrucomicrobia was notably and negatively related to MLOC (P < 0.01) and NO3−-N (P < 0.05). Nitrospirae was positively correlated with NO3−-N, significantly negatively correlated with NH4+-N, CPMI, and TP (P < 0.01). Proteobacteria were significantly and positively correlated with NO3−-N (P < 0.01). Firmicutes were significantly and positively correlated with LLOC, CPMI, DOC, SOC, EOC, HLOC, pH, TP, and TN (P < 0.01). Chloroflexi showed highly significant negative correlations (P < 0.01) with NO3−-N, MLOC, CPMI, and significant negative correlations (P < 0.05) with AP, EOC, pH, SOC, and TN. Gemmatimonadetes was significantly and positively correlated with AK and AP (P < 0.01).
Bacterial community affected by soil environment
Redundancy analysis (RDA) was performed with dominant bacterial phylum as the response variable and soil physicochemical properties and soil organic carbon fraction as explanatory variables, and the results are shown in Fig. 4. The X and Y canonical axes of Fig. 5A explained 64.64% and 21.61% of the dominant bacterial phyla dynamics, respectively. The results showed that AP(P < 0.05), AK, NO3−-N (P < 0.01) significantly affected soil bacterial community composition. The RDA analysis plot of soil organic carbon fraction and bacterial community is shown in Fig. 5B. The explanation rate of the X and Y canonical axes was 61.62% and 21.47%, respectively. LLOC(P < 0.05), DOC, and CPMI(P < 0.01) significantly affected soil bacterial community composition. CK was less affected by soil organic carbon fraction than the treatment with bio-organic fertilizer. The phylum composition of SF75 and SF100 treatments was more correlated with the organic carbon fraction.
Soil bacterial biomarkers and functions prediction
Linear discriminant effect size (LEfSe) analysis was performed to identify and select unique bacterial taxa significantly associated with different substitution ratios of bio-organic fertilizer (P < 0.05, Fig. 6). The bacterial community LDA analysis detected 39 (CK = 10, SF25 = 2, SF50 = 8, SF75 = 9, SF100 = 10) biomarkers. The phyla Chloroflexi, Acidobacteria, and Elusimicrobia were higher in the CK than in the other treatments, while Bacteroidetes, Proteobacteria, Firmicutes, and Actinobacteria were higher in the bio-organic fertilizer treatments. The SF75 treatment had a higher score in Gemmatimonadetes.
Based on the OTU table, the relative abundance under different treatments of soil bacterial pathways prediction was obtained by comparing the KEGG database(Fig. 7). Bacteria could be classified into six functional categories, namely Metabolism, Genetic Information Processing, Human Diseases, Cellular Processes, Environmental Information Processing, and Organismal Systems (Han et al. 2021). The four main pathways are shown in Fig. 7, with the relative abundance of Metabolism, Genetic Information Processing, Cellular Processes, and Environmental Information Processing, in descending order. The relative abundance of Metabolism of cofactors and vitamins, Metabolism of terpenoids and polyketide, and Biosynthesis of other secondary metabolites in the subpathways of Metabolism were SF75 > SF100 > SF50 > SF25 > CK. The relative abundance of Folding, sorting, and degradation and Translation in the Genetic Information Processing subpathways were SF100 > SF75 > SF50 > SF25 > CK. The relative abundance of SF75 was highest in the Cell motility subpathway of Cellular Processes and the Membrane transport subpathway of Environmental Information Processing.
Discussion
Effects of bio-organic fertilizer on soil proprieties
Our results suggested that the applied bio-organic fertilizer significantly increased soil pH, TP, TN, and SOC contents compared to the control group, which is consistent with Liu et al. (2021). This is mainly due to a large amount of organic matter in bio-organic fertilizer and its ability to provide the full amount of nutrients to the soil, in addition, bio-organic fertilizer contains soil beneficial microorganisms, which can effectively modify the soil micro-ecological environment and promote the release of plant root secretions, thus alleviating soil acidification and increasing soil pH (Akhtar et al. 2018). The results of Jiang et al. (2018) indicated that organic matter can enhance the soil’s ability to buffer acids and lessen the amount of Ca, Mg, and K that leaches out of acidic soils. Qiu et al. (2019) observed an increasing trend of soil TN, TP, and TK content with the increasing percentage of bio-organic fertilizer substitution, which is in agreement with our findings.
Plant roots are able to use available nutrients from the soil directly, therefore soil available nutrient is a crucial part of soil propriety (Wang and Zabowski 1998). Concerning soil available nutrients of citrus orchard soils, both AP and AK contents markedly increased with bio-fertilizer application except SF25. AP, AK, and NO3−-N contents were highly associated with substitution ratios and as the proportion of substitution increases, they tend to increase and then decrease. SF75 has the highest AP, AK, and NO3−-N content. This could be mainly due to the decomposition of organic matter in bio-fertilizer thus promoting the release of trace elements in the soil (Baumann et al. 2013). In addition, the available nutrients contained in the bio-organic fertilizer itself also increase the soil available nutrients to a certain extent (Zhu et al. 2022). Previous studies suggested that bio-organic fertilizers had a major role in enhancing soil available nutrients, which is in line with our findings (Li et al. 2017b; Ye et al. 2020).
Impact on soil LOC and CPMI
Soil is a potential carbon sink and its ability to sequester organic carbon is determined by the dynamic balance between carbon input from primary biomass production and exogenous organic additions and carbon output from mineralization (Gong et al. 2009). LOC accounts for about 7–32% of SOC, which is unstable and vulnerable to human activities, easily oxidizable, decomposable, and mineralized (Biederbeck et al. 1994). Thus, LOC can be used as an indicator to monitor the effect of bio-organic fertilizer substitution rate on soil SOC and soil quality (Lou et al. 2011). Many studies have demonstrated that the usage of organic fertilizers can directly or indirectly increase the proportion of LOC (Ding et al. 2012; Liang et al. 2012). In the present study, we separated EOC which was extracted from KMnO4 into three fractions HLOC, MLOC, and LLOC. Bai et al. (2010) suggested that the application of organic fertilizer significantly increased the EOC content in the cultivated soil layer, and the treatment with bio-organic fertilizer in the present study also notably increased the EOC content compared to the control group. The aggregates formed by organic fertilizers can adsorb EOC and prevent its loss, thus improving the ability of soil water and nutrient supply (Yang et al. 2021). Bio-organic fertilizer input at different rates significantly increased HLOC and MLOC contents, which is in agreement with the study of Zhang et al. (2021), where the input of organic fertilizer brought numerous organic matter, thereby increasing the total organic carbon stock in the soil carbon pool and enhancing the contents of all other components (Gong et al. 2009). DOC is primarily generated from the microbial-mediated decomposition of plant residues and organic matter, which are water-soluble organic carbon and make up a tiny fraction of SOC (Li et al. 2017a). The significant increase in soil DOC content after organic fertilizer application may be because organic fertilizers improve the soil physical environment (e.g., porosity) and provide C and N sources for microorganisms to apply, promoting soil microbial activity (Yang et al. 2012), which is consistent with the changes in DOC content in this study, and the content of DOC was higher with the increasing proportion of bio-organic fertilizer substitution.
Wei et al. found a significant correlation between CPMI and carbon fractions, and CPMI is a useful indicator for assessing changes in soil quality and nutrient cycling due to soil management practices (Gong et al. 2009). In general, lower CPMI means that soil carbon stocks are being lost, while higher CPMI represents a greater capacity for soil carbon sequestration (Sainepo et al. 2018). The CPMI value of bio-organic fertilizer application in our study was higher than that of CK, and the highest CPMI value was found in SF75. Li et al. (2018) also indicated that CPMI was notably higher with organic fertilizer application than with chemical fertilizer application, and CPMI was closely related to the EOC and SOC contents. These results all showed that the input of organic fertilizers increased soil SOC and soil LOC content, and also increased soil carbon pool activity and carbon storage, thus improving soil carbon sequestration capacity and promoting soil carbon cycling (Jiang et al. 2022).
Effects on soil bacterial community
Soil microorganisms are an important part of terrestrial ecosystems, and the diversity and community structure of microbes are not only closely related to the effectiveness of biotransformation but also reflect soil fertility status and plant health (Trivedi et al. 2013; Manasa et al. 2020; Moreira et al. 2020). Bacteria is considered to be a more accurate assessment of soil fertility because of their much shorter turnover time than fungi for the utilization of C substrate (Lazcano et al. 2013; Siciliano et al. 2014; Ai et al. 2018) found that partial substitution of chemical fertilizers with organic fertilizers significantly affected soil bacterial alpha diversity. Our results showed a remarkable effect of bio-organic fertilizer addition on the diversity and species composition of soil bacterial communities in citrus orchards and was closely associated with the proportion of bio-organic fertilizer substitution. Compared with the CK, the Chao1 index of bacterial community abundance and the Shannon index of bacterial diversity were noticeably increased in the bio-organic fertilizer substitution treatment, indicating that bio-organic fertilizer substitution for chemical fertilizers could improve the abundance and diversity of soil bacterial communities. SF75 treatment showed the most significant performance, which is consistent with Ren et al. (2021).
Some studies have suggested that organic fertilizers can provide more adequate nutrients for heterotrophic bacterial activity than inorganic fertilizers, reducing competition between bacterial communities and that the introduction of naturally occurring microorganisms in organic fertilizers into the soil can increase microbial diversity (Dong et al. 2014; Han et al. 2021; Lin et al. 2019). In the present study, the dominant soil bacterial phyla in all treatments were Acidobacteria, Proteobacteria, Choloflexi, Actinobacteria, and Bacteroidetes, which were in agreement with previous studies (Hartmann et al. 2015; Ding et al. 2016). Proteobacteria and Bacteroidetes prefer eutrophic environments, and their abundance in this study was higher in the bio-organic fertilizer treatment than in the CK treatment. The bio-organic fertilizer application not only increased the soil nutrients but also promoted the symbiotic nitrogen fixation of Proteobacteria and the utilization of carbon sources by Bacteroidetes (Liu et al. 2016; Xu et al. 2016; Liang et al. 2018). Acidobacteria is acidophilic bacteria, and the application of bio-organic fertilizers increases the pH of the soil, thus harming the growth and multiplication of Acidobacteria (Männistö et al. 2013; Huang et al. 2015) found that Acidobacteria were involved in carbon cycling via the degradation of plant residues. Our study found that bio-organic fertilizer application enhanced the relative abundance of Chloroflexi, which was consistent with what was found in banana plantation soils by Li et al. (2021). Furthermore, in the present study, the abundance of Firmicutes was higher in the bio-organic fertilizer treatment than that of the control treatment and soil pH and LOC fractions were significantly increased with the addition of bio-organic fertilizers. The remarkable positive relationship between Firmicutes and soil pH and LOC fraction showed that the addition of bio-organic fertilizer could provide an appropriate environment for the growth and multiplication of Firmicutes and promote its participation in organic matter decomposition and carbohydrate metabolism (Zhao et al. 2017).
Relations between bacterial and soil properties, and bacterial functional prediction
Soil properties are closely related to soil microbial community structure and can affect soil microorganisms to varying degrees (Xun et al. 2015). In the present study, the main contributors of the bacterial structure were AK, DOC, CPMI, and NO3−-N based on RDA analysis which was conducted on soil properties and soil bacterial community (phylum level). Liu et al. (2021) and Li et al. (2021) found that SOC, AK, and pH were the main factors affecting the bacterial community structure of applied bio-organic fertilizers. These findings may be attributable to the specific effects of bio-organic fertilizers application on soil properties (e.g. SOC, pH) and the rhizosphere environment, which affect the soil microbial community structure in turn (Liu et al. 2021). Our study indicated that the presence of microorganisms and organic matter in the bio-organic fertilizer itself might have influenced the DOC content and bacterial community composition. Acidobacteria was found the ability to contribute to the productivity of carbon cycling in soils as many members of the Acidobacteria phylum can accelerate the C cycle (Wu et al. 2019). Moreover, bacteria play a crucial role in N cycling because they can absorb and utilize nitrogen from soil to further synthesize biomolecules with important physiological functions (e.g., proteins and nucleotides) (Sarathchandra et al. 2001; Shen et al. 2014) found that Gemmatimonadetes and Bacteroidetes were highly correlated with the available nutrients in the soil, for instance, AP and AK contents, which was consistent with our results.
To further understand the effects of bio-fertilizer and its substitution rate on the bacterial community, LEfSe analysis, and KEGG prediction were used to find biomarkers and the relative abundance of functional pathways, respectively. Biomarkers differed significantly among different bio-organic fertilizer substitution rates in our study. Acidobacteria and Proteobacteria were the Most of all significant biomarkers in bacteria, which was consistent with Liu et al. (2021). In this study, metabolism was found to be the main function of soil bacteria and its relative abundance was the highest. And the application of bio-organic fertilizers effectively promotes amino acid metabolism, metabolism of cofactors and vitamins, Metabolism of terpenoids and polyketides, and Biosynthesis of other secondary metabolites. Soil microorganisms are mainly involved in the material cycle and transformation of the soil through their metabolic activities, regulating the metabolic processes of the organism (Wu et al. 2016). The metabolism of amino acids, carboxylic acids, polymers, and amines is susceptible to organic fertilization (Yang et al. 2011), and bio-organic fertilization in this study enhanced the abundance of Amino acid metabolism, especially in SF75.
Conclusion
The partial substitution of chemical fertilizers with bio-organic fertilizers improved citrus orchard soil nutrient content, soil LOC content, and bacterial community diversity. Combining these three aspects, 75% bio-organic fertilizer substitution was the most effective in this study. Soil bacterial community composition was significantly correlated with CPMI and DOC content. The PICRUSt functional prediction suggested that the application of bio-organic fertilizer increased the relative abundance of bacterial metabolism. Suitable bio-organic fertilizer substitution helps to improve soil properties and provide sufficient substrate for bacterial communities, thus improving the soil micro-ecological environment and achieving sustainable agricultural development.
References
Ai C, Zhang S, Zhang X, Guo D, Zhou W, Huang S (2018) Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 319:156–166. https://doi.org/10.1016/j.geoderma.2018.01.010
Akhtar K, Wang W, Ren G, Khan A, Feng Y, Yang G (2018) Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res 182:94–102. https://doi.org/10.1016/j.still.2018.05.007
Ansari RA, Mahmood I (2017) Optimization of organic and bio-organic fertilizers on soil properties and growth of pigeon pea. Sci Hortic 226:1–9. https://doi.org/10.1016/j.scienta.2017.07.033
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Bai W, Zhang H, Liu B, Wu Y, Song J (2010) Effects of super-absorbent polymers on the physical and chemical properties of soil following different wetting and drying cycles: Effects of SAPs on soil properties. Soil Use Manag 26:253–260. https://doi.org/10.1111/j.1475-2743.2010.00271.x
Baumann K, Dignac M-F, Rumpel C, Bardoux G, Sarr A, Steffens M, Maron P-A (2013) Soil microbial diversity affects soil organic matter decomposition in a silty grassland soil. Biogeochemistry 114:201–212. https://doi.org/10.1007/s10533-012-9800-6
Biederbeck VO, Janzen HH, Campbell CA, Zentner RP (1994) Labile soil organic matter as influenced by cropping practices in an arid environment. Soil Biol Biochem 26:1647–1656. https://doi.org/10.1016/0038-0717(94)90317-4
Blair G, Lefroy R, Lisle L (1995) Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust J Agric Res 46:1459. https://doi.org/10.1071/AR9951459
Blanco-Canqui H, Schlegel AJ (2013) Implications of Inorganic Fertilization of Irrigated Corn on Soil Properties: Lessons Learned after 50 Years. J Environ Qual 42:861–871. https://doi.org/10.2134/jeq2012.0451
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize? J Hazard Mater 266:141–166. https://doi.org/10.1016/j.jhazmat.2013.12.018
Ding J, Jiang X, Ma M, Zhou B, Guan D, Zhao B, Zhou J, Cao F, Li L, Li J (2016) Effect of 35 years inorganic fertilizer and manure amendment on structure of bacterial and archaeal communities in black soil of northeast China. Appl Soil Ecol 105:187–195. https://doi.org/10.1016/j.apsoil.2016.04.010
Ding X, Han X, Liang Y, Qiao Y, Li L, Li N (2012) Changes in soil organic carbon pools after 10 years of continuous manuring combined with chemical fertilizer in a Mollisol in China. Soil Tillage Res 122:36–41. https://doi.org/10.1016/j.still.2012.02.002
Dong W-Y, Zhang X-Y, Dai X-Q, Fu X-L, Yang F-T, Liu X-Y, Sun X-M, Wen X-F, Schaeffer S (2014) Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl Soil Ecol 84:140–147. https://doi.org/10.1016/j.apsoil.2014.06.007
García-Delgado C, Barba-Vicente V, Marín-Benito JM, Mariano Igual J, Sánchez-Martín MJ, Sonia Rodríguez-Cruz M (2019) Influence of different agricultural management practices on soil microbial community over dissipation time of two herbicides. Sci Total Environ 646:1478–1488. https://doi.org/10.1016/j.scitotenv.2018.07.395
Gong W, Yan X, Wang J, Hu T, Gong Y (2009) Long-term manuring and fertilization effects on soil organic carbon pools under a wheat–maize cropping system in North China Plain. Plant Soil 314:67–76. https://doi.org/10.1007/s11104-008-9705-2
Gong X, Liu C, Li J, Luo Y, Yang Q, Zhang W, Yang P, Feng B (2019) Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Tillage Res 195:104355. https://doi.org/10.1016/j.still.2019.104355
Gu Y, Wang X, Yang T, Friman V, Geisen S, Wei Z, Xu Y, Jousset A, Shen Q (2020) Chemical structure predicts the effect of plant-derived low‐molecular weight compounds on soil microbiome structure and pathogen suppression. Funct Ecol 34:2158–2169. https://doi.org/10.1111/1365-2435.13624
Han J, Dong Y, Zhang M (2021) Chemical fertilizer reduction with organic fertilizer effectively improve soil fertility and microbial community from newly cultivated land in the Loess Plateau of China. Applied Soil Ecology 165:103966. https://doi.org/10.1016/j.apsoil.2021.103966
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9:1177–1194. https://doi.org/10.1038/ismej.2014.210
Huang R, Wang Y, Gao X, Liu J, Wang Z, Gao M (2020) Nitrous oxide emission and the related denitrifier community: A short-term response to organic manure substituting chemical fertilizer. Ecotoxicol Environ Saf 192:110291. https://doi.org/10.1016/j.ecoenv.2020.110291
Huang X, Liu L, Wen T, Zhu R, Zhang J, Cai Z (2015) Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f.sp. cubense infected soil during and after reductive soil disinfestation. Microbiol Res 181:33–42. https://doi.org/10.1016/j.micres.2015.08.004
Ishaq M, Ibrahim M, Lal R (2002) Tillage effects on soil properties at different levels of fertilizer application in Punjab, Pakistan. Soil Tillage Res 68:93–99. https://doi.org/10.1016/S0167-1987(02)00111-3
Ji L, Wu Z, You Z, Yi X, Ni K, Guo S, Ruan J (2018) Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric Ecosyst Environ 268:124–132. https://doi.org/10.1016/j.agee.2018.09.008
Jiang J, Wang Y-P, Yu M, Cao N, Yan J (2018) Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem Geol 501:86–94. https://doi.org/10.1016/j.chemgeo.2018.10.009
Jiang M, Li C, Gao W, Cai K, Tang Y, Cheng J (2022) Comparison of long-term effects of biochar application on soil organic carbon and its fractions in two ecological sites in karst regions. Geoderma Reg 28:e00477. https://doi.org/10.1016/j.geodrs.2021.e00477
Kautz T, López-Fando C, Ellmer F (2006) Abundance and biodiversity of soil microarthropods as influenced by different types of organic manure in a long-term field experiment in Central Spain. Appl Soil Ecol 33:278–285. https://doi.org/10.1016/j.apsoil.2005.10.003
Kim Y-N, Cho Y-S, Lee J-H, Seo H-R, Kim B-H, Lee D-B, Lee YB, Kim K-H (2022) Short-Term Responses of Soil Organic Carbon Pool and Crop Performance to Different Fertilizer Applications. Agronomy 12:1106. https://doi.org/10.3390/agronomy12051106
Lazcano C, Gómez-Brandón M, Revilla P, Domínguez J (2013) Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function: A field study with sweet corn. Biol Fertil Soils 49:723–733. https://doi.org/10.1007/s00374-012-0761-7
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68. https://doi.org/10.1038/nature16069
Lei J, Liang S-S, Tan Q-L, Hu X-C, Sun X-C, Zhao X-H (2019) NPK fertilization rates and reducing potential in the main Citrus producing regions of China. Plant Nutr Fert Sci 25:1504–1513. https://doi.org/10.11674/zwyf.18374
Li J, Wen Y, Li X, Li Y, Yang X, Lin Z, Song Z, Cooper JM, Zhao B (2018) Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil Tillage Res 175:281–290. https://doi.org/10.1016/j.still.2017.08.008
Li M, Zhang A, Wu H, Liu H, Lv J (2017a) Predicting potential release of dissolved organic matter from biochars derived from agricultural residues using fluorescence and ultraviolet absorbance. J Hazard Mater 334:86–92. https://doi.org/10.1016/j.jhazmat.2017.03.064
Li R, Tao R, Ling N, Chu G (2017b) Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res 167:30–38. https://doi.org/10.1016/j.still.2016.11.001
Li Z, Jiao Y, Yin J, Li D, Wang B, Zhang K, Zheng X, Hong Y, Zhang H, Xie C, Li Y, Duan Y, Hu Y, Zhu Z, Liu Y (2021) Productivity and quality of banana in response to chemical fertilizer reduction with bio-organic fertilizer: Insight into soil properties and microbial ecology. Agric Ecosyst Environ 322:107659. https://doi.org/10.1016/j.agee.2021.107659
Liang B, Lehmann J, Sohi SP, Thies JE, O’Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizão FJ (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213. https://doi.org/10.1016/j.orggeochem.2009.09.007
Liang B, Ma C, Fan L, Wang Y, Yuan Y (2018) Soil amendment alters soil physicochemical properties and bacterial community structure of a replanted apple orchard. Microbiol Res 216:1–11. https://doi.org/10.1016/j.micres.2018.07.010
Liang Q, Chen H, Gong Y, Fan M, Yang H, Lal R, Kuzyakov Y (2012) Effects of 15 years of manure and inorganic fertilizers on soil organic carbon fractions in a wheat-maize system in the North China Plain. Nutr Cycl Agroecosystems 92:21–33. https://doi.org/10.1007/s10705-011-9469-6
Lin Y, Ye G, Kuzyakov Y, Liu D, Fan J, Ding W (2019) Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol Biochem 134:187–196. https://doi.org/10.1016/j.soilbio.2019.03.030
Liu Q, Pang Z, Yang Z, Nyumah F, Hu C, Lin W, Yuan Z (2021) Bio-fertilizer Affects Structural Dynamics, Function, and Network Patterns of the Sugarcane Rhizospheric Microbiota. Microb Ecol. https://doi.org/10.1007/s00248-021-01932-3
Liu W, Wang S, Lin P, Sun H, Hou J, Zuo Q, Huo R (2016) Response of CaCl2-extractable heavy metals, polychlorinated biphenyls, and microbial communities to biochar amendment in naturally contaminated soils. J Soils Sediments 16:476–485. https://doi.org/10.1007/s11368-015-1218-z
Lou Y, Wang J, Liang W (2011) Impacts of 22-year organic and inorganic N managements on soil organic C fractions in a maize field, northeast China. Catena 87:386–390. https://doi.org/10.1016/j.catena.2011.07.006
Manasa MRK, Katukuri NR, Nair D, Haojie SS, Yang Y, Guo Z, bo R (2020) Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J Environ Manage 269:110737. https://doi.org/10.1016/j.jenvman.2020.110737
Manjunath M, Kumar U, Yadava RB, Rai AB, Singh B (2018) Influence of organic and inorganic sources of nutrients on the functional diversity of microbial communities in the vegetable cropping system of the Indo-Gangetic plains. C R Biol 341:349–357. https://doi.org/10.1016/j.crvi.2018.05.002
Männistö MK, Kurhela E, Tiirola M, Häggblom MM (2013) Acidobacteria dominate the active bacterial communities of Arctic tundra with widely divergent winter-time snow accumulation and soil temperatures. FEMS Microbiol Ecol 84:47–59. https://doi.org/10.1111/1574-6941.12035
Mganga KZ, Razavi BS, Kuzyakov Y (2016) Land use affects soil biochemical properties in Mt. Kilimanjaro region CATENA 141:22–29. https://doi.org/10.1016/j.catena.2016.02.013
Moreira H, Pereira SIA, Vega A, Castro PML, Marques APGC (2020) Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J Environ Manage 257:109982. https://doi.org/10.1016/j.jenvman.2019.109982
Qiao C, Penton CR, Xiong W, Liu C, Wang R, Liu Z, Xu X, Li R, Shen Q (2019) Reshaping the rhizosphere microbiome by bio-organic amendment to enhance crop yield in a maize-cabbage rotation system. Appl Soil Ecol 142:136–146. https://doi.org/10.1016/j.apsoil.2019.04.014
Qiu S, Gao H, Zhu P, Hou Y, Zhao S, Rong X, Zhang Y, He P, Christie P, Zhou W (2016) Changes in soil carbon and nitrogen pools in a Mollisol after long-term fallow or application of chemical fertilizers, straw or manures. Soil Tillage Res 163:255–265. https://doi.org/10.1016/j.still.2016.07.002
Qiu Y-S, Wang X-N, Li P-F, Hou X-Q, Wang Y-L, Wu P-N, Huo W-B (2019) Different kinds of organic fertilizers and amounts on dryland soil fertility and corn yield in the current season. Soil Fert. Sci. China.182–189. https://doi.org/10.11838/sfsc.1673-6257.18498
Ren J, Liu X, Yang W, Yang X, Li W, Xia Q, Li J, Gao Z, Yang Z (2021) Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J Environ Manage 299:113650. https://doi.org/10.1016/j.jenvman.2021.113650
Sainepo BM, Gachene CK, Karuma A (2018) Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag 13:4. https://doi.org/10.1186/s13021-018-0091-7
Sarathchandra SU, Ghani A, Yeates GW, Burch G, Cox NR (2001) Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol Biochem 33:953–964. https://doi.org/10.1016/S0038-0717(00)00245-5
Savci S (2012) An Agricultural Pollutant: Chemical Fertilizer. Int J Environ Sci Dev 73–80. https://doi.org/10.7763/IJESD.2012.V3.191
Shen Z, Wang D, Ruan Y, Xue C, Zhang J, Li R, Shen Q (2014) Deep 16S rRNA Pyrosequencing Reveals a Bacterial Community Associated with Banana Fusarium Wilt Disease Suppression Induced by Bio-Organic Fertilizer Application. PLoS ONE 9:e98420. https://doi.org/10.1371/journal.pone.0098420
Siciliano SD, Palmer AS, Winsley T, Lamb E, Bissett A, Brown MV, van Dorst J, Ji M, Ferrari BC, Grogan P, Chu H, Snape I (2014) Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol Biochem 78:10–20. https://doi.org/10.1016/j.soilbio.2014.07.005
Sodhi GPS, Beri V, Benbi DK (2009) Using Carbon Management Index to Assess the Impact of Compost Application on Changes in Soil Carbon after Ten Years of Rice–Wheat Cropping. Commun Soil Sci Plant Anal 40:3491–3502. https://doi.org/10.1080/00103620903326024
Sun R, Guo X, Wang D, Chu H (2015) Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl Soil Ecol 95:171–178. https://doi.org/10.1016/j.apsoil.2015.06.010
Trivedi P, Anderson IC, Singh BK (2013) Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 21:641–651. https://doi.org/10.1016/j.tim.2013.09.005
Varvel GE, Wilhelm WW (2011) No-tillage increases soil profile carbon and nitrogen under long-term rainfed cropping systems. Soil Tillage Res 114:28–36. https://doi.org/10.1016/j.still.2011.03.005
Wang L, Li J, Yang F, Raza EY, Huang W, Shen Q (2017) Application of Bioorganic Fertilizer Significantly Increased Apple Yields and Shaped Bacterial Community Structure in Orchard Soil. Microb Ecol 73:404–416. https://doi.org/10.1007/s00248-016-0849-y
Wang WJ, Dalal RC, Moody PW, Smith CJ (2003) Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol Biochem 35:273–284. https://doi.org/10.1016/S0038-0717(02)00274-2
Wang X, Zabowski D (1998) Nutrient composition of Douglas-fir rhizosphere and bulk soil solutions. In: Box JE (ed) Root Demographics and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems. Springer Netherlands, Dordrecht, pp 89–103. https://doi.org/10.1007/978-94-011-5270-9_7
Wu C, Shi L, Xue S, Li W, Jiang X, Rajendran M, Qian Z (2019) Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci Total Environ 647:1158–1168. https://doi.org/10.1016/j.scitotenv.2018.08.087
Wu H, Yang X, Fan J, Miao W, Ling N, Xu Y, Huang Q, Shen Q (2009) Suppression of Fusarium wilt of watermelon by a bio-organic fertilizer containing combinations of antagonistic microorganisms. Biocontrol 54:287–300. https://doi.org/10.1007/s10526-008-9168-7
Wu Z, Hao Z, Sun Y, Guo L, Huang L, Zeng Y, Wang Y, Yang L, Chen B (2016) Comparison on the structure and function of the rhizosphere microbial community between healthy and root-rot Panax notoginseng. Appl Soil Ecol 107:99–107. https://doi.org/10.1016/j.apsoil.2016.05.017
Xiang X, Liu J, Zhang J, Li D, Xu C, Kuzyakov Y (2020) Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Tillage Res 196:104491. https://doi.org/10.1016/j.still.2019.104491
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8. https://doi.org/10.1016/j.ejsobi.2016.02.004
Xun W, Huang T, Zhao J, Ran W, Wang B, Shen Q, Zhang R (2015) Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities. Soil Biol Biochem 90:10–18. https://doi.org/10.1016/j.soilbio.2015.07.018
Yang X, Feng Y, Zhang X, Sun M, Qiao D, Li J, Li X (2020) Mineral soil conditioner requirement and ability to adjust soil acidity. Sci Rep 10:18207. https://doi.org/10.1038/s41598-020-75192-5
Yang X, Ren W, Sun B, Zhang S (2012) Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 177–178:49–56. https://doi.org/10.1016/j.geoderma.2012.01.033
Yang Y, Wu J, Zhao S, Gao C, Pan X, Tang DWS, van der Ploeg M (2021) Effects of long-term super absorbent polymer and organic manure on soil structure and organic carbon distribution in different soil layers. Soil Tillage Res 206:104781. https://doi.org/10.1016/j.still.2020.104781
Yang Y-H, Chen D-M, Jin Y, Wang H-B, Duan Y-Q, Guo X-K, He H-B, Lin W-X (2011) Effect of Different Fertilizers on Functional Diversity of Microbial Flora in Rhizospheric Soil Under Tobacco Monoculture. Acta Agron Sin 37:105–111. https://doi.org/10.1016/S1875-2780(11)60003-5
Ye L, Zhao X, Bao E, Li J, Zou Z, Cao K (2020) Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci Rep 10:177. https://doi.org/10.1038/s41598-019-56954-2
Yu G, Fang H, Gao L, Zhang W (2006) Soil organic carbon budget and fertility variation of black soils in Northeast China. Ecol Res 21:855–867. https://doi.org/10.1007/s11284-006-0033-9
Yu M, Meng J, Yu L, Su W, Afzal M, Li Y, Brookes PC, Redmile-Gordon M, Luo Y, Xu J (2019) Changes in nitrogen related functional genes along soil pH, C and nutrient gradients in the charosphere. Sci Total Environ 650:626–632. https://doi.org/10.1016/j.scitotenv.2018.08.372
Zhang Y, Li Y, Liu Y, Huang X, Zhang W, Jiang T (2021) Responses of Soil Labile Organic Carbon and Carbon Management Index to Different Long-Term Fertilization Treatments in a Typical Yellow Soil Region. Eurasian Soil Sci 54:605–618. https://doi.org/10.1134/S1064229321040189
Zhao Q, Classen AT, Wang W-W, Zhao X-R, Mao B, Zeng D-H (2017) Asymmetric effects of litter removal and litter addition on the structure and function of soil microbial communities in a managed pine forest. Plant Soil 414:81–93. https://doi.org/10.1007/s11104-016-3115-7
Zhu L-X, Cao M-M, Sang C-C, Chen R-B, Xu S-W, Li L-L, Liu T-X (2022) Effects of Bio-Fertilizer Partially Substituting Chemical Fertilizer on Soil Fertility and Enzyme Activity in Maize Field. J Sichuan Agri Uni 40:67–72. https://doi.org/10.16036/j.issn.1000-2650.202107017
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 41977001).
Author information
Authors and Affiliations
Contributions
Qiongyao Yang: Experiment, Software, Data curation, Writing – original draft. Mingkui Zhang: Writing – review & editing, Supervision.
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Tida Ge.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Zhang, M. Effect of bio-organic fertilizers partially substituting chemical fertilizers on labile organic carbon and bacterial community of citrus orchard soils. Plant Soil 483, 255–272 (2023). https://doi.org/10.1007/s11104-022-05735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05735-4