Abstract
Atrazine is one of the most used herbicides worldwide; however, consequences of its long-term agricultural use are still unknown. A laboratory study was performed to examine changes in microbial properties following ethylamino-15N-atrazine addition, at recommended agronomic dose, to five acidic soils from Galicia (NW Spain) showing different physico-chemical characteristics, as well as atrazine application history. Net N mineralization was observed in all soils, with nitrate being the predominant substance formed. The highest values were detected in soils with low atrazine application history. From 2% to 23% of the atrazine-15N was found in the soil inorganic-N pool, the highest values being detected after 9 weeks in soils with longer atrazine application history and lower indigenous soil N mineralization. The application of atrazine slightly reduced the amount of soil N mineralized and microbial biomass at short term. Soluble carbohydrates and β-glucosidase and urease activity decreased with incubation time, but were not significantly affected by the single application of atrazine. Microbial community structure changed as consequence of both soil type and incubation time, but no changes in the phospholipid fatty acid (PLFA) pattern were detected due to recent atrazine addition at normal doses. The saturated 17- to 20-carbon fatty acids had higher relative abundance in soils with a longer atrazine history and fungal biomass, as indicated by the PLFA 18:2ω6,9, decreased with the incubation time. The results suggested that the PLFA pattern and soil N dynamics can detect the long-term impact of repeated atrazine application to agricultural soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most agricultural soils in Western Europe are treated with herbicides at least once a year. Atrazine [2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine] is one of the most widely used herbicides for controlling broadleaf and grassy weeds in agriculture and forestry. Consequently, atrazine and its degradation products are the most frequently detected herbicide contaminants in surface and ground waters (Capriel et al. 1985; Herwig et al. 2001), and therefore, there is concern for negative effects of these compounds on both humans and the environment. Atrazine is subjected to abiotic and biotic degradation with reported half lives in soils ranging from a few days to several months depending on site conditions, application history, and agricultural practices (Erickson and Lee 1989; Assaf and Turco 1994; Koskinen and Clay 1998). In recent years, soils showing a potential for rapid mineralization of atrazine have been identified throughout the world (Barriuso and Houot 1996; Yassir et al. 1999). However, residues of both the parent compound and its degradation products have been detected in soils years after application (Schiavon 1988; Mahía et al. 2007). These findings are being taken into account by regulatory bodies in EU countries, leading to increased restrictions on the use of atrazine.
Microbial degradation is the dominant way of atrazine attenuation in soils (Abdelhafid et al. 2000a). There are several microorganisms that partially transform atrazine by N-dealkylation or dehalogenation reactions, but relatively few bacteria have been reported that completely mineralize the triazine ring (Kaufman and Kearney 1970; Mandelbaum et al. 1995; Bouquard et al. 1997). Degradation of pesticides is often slow because low supply and/or availability of the compound or essential nutrients. In the case of atrazine, oxidation of the alkali side chains provides the only source of C and energy available to microorganisms since the ring C atoms are fully oxidized and thus are not incorporated into microbial biomass (Cook 1987; Yanze-Kontchou and Gschwind 1994). Atrazine can be utilized as a sole C source by some mixed cultures and bacterial isolates (Behki and Khan 1986; Mandelbaum et al. 1993, 1995; Yanze-Kontchou and Gschwind 1994). Side-chain and ring-N atoms can be utilized as an N source by some microorganisms (Cook and Hütter 1981; Mandelbaum et al. 1995; Radosevich et al. 1995). Various organic and inorganic amendments have been shown to affect herbicide degradation. The addition of alternative N sources, resulting in a low C/N ratio, can inhibit atrazine mineralization (Cook and Hütter 1981; Alvey and Crowley 1995; Abdelhafid et al. 2000a, b) and the inhibitory effect increased with soil N mineralization rate; by the contrary, in soils with a high C/N ratio, atrazine mineralization may be enhanced as a result of limited soil N availability (Alvey and Crowley 1995; Topp et al. 1997).
The influence of s-triazines on soil microorganisms has been intensively investigated. These studies have shown that while some microbial groups can use an applied herbicide as a source of energy and nutrients, the herbicide may be toxic to other groups. The presence of these herbicides can modify the soil microbial communities thus altering the normal functioning of terrestrial ecosystems, which in turn might have implications for soil fertility and quality (Voets et al. 1974; Greaves 1982; Schaëfer 1993). In general, the impact of s-triazines on soil microbial communities has often been studied by conventional methods based on cultivation of soil microorganisms or on measurements of their microbial biomass and metabolic activities (Davies and Greaves 1981; Ghani et al. 1996; Sannino and Gianfreda 2001; Accinelli et al. 2001; Moreno et al. 2007; Hussain et al. 2009). To overcome the drawbacks of the culture-dependent methods, interest is currently focused on the use of molecular techniques, allowing the analysis of microorganisms in their natural habitats. In this context, phospholipid fatty acid (PLFA) pattern and DNA/RNA analysis have been successfully applied in the last decade to characterize soil microbial communities following atrazine addition (Ostrofsky et al. 2002; Rhine et al. 2003; Ross et al. 2006).
Most atrazine studies focused on short-term application of the herbicide at large concentrations; although it is well known that under field conditions, the herbicide is often applied annually at low dose for many successive years in the same soil. The concentration of atrazine in soils is an important factor which affects biodegradation and the microbial response (Dzantor and Felsot 1991; Gan et al. 1996). In Galicia (NW Spain), there is a widespread use of atrazine in agricultural soils and a high potential risk of surface and groundwater contamination due to abundant precipitations and sandy textured soils. However, information concerning the impact of s-triazine compounds on microorganisms of these acidic soils with relatively high organic matter content is scarce. The purpose of this research was to evaluate the microbial response to atrazine at recommended agronomic dose in soils from the temperate humid zone with different histories of atrazine application and contrasting physico-chemical properties. PLFA analysis and several biochemical properties (N mineralization, urease activity, β-glucosidase activity, soluble carbohydrates) were used to characterize the soil microbial communities. Thus, the microbial variables included biomass, activity and community structure measurements, as well as enzyme activities connected to the C- and N-cycles. The kinetics of atrazine degradation was also examined. This is one of the few studies that combine microbial community changes induced by both recent and repeated herbicide application at field doses and herbicide dissipation.
Material and methods
Soils
Five contrasting agricultural soils (P, E, M, G, and C), developed over different parent material and with a history of 10–40 years of annual maize cropping and atrazine application, located in the temperate humid zone (Galicia, NW Spain) were used (Table 1). It should be noticed that only four soil types were considered since P and E samples were collected in the same agricultural experimental area from plots with different previous exposure to herbicide. Soil subsamples, collected randomly in spring 2005 before maize planting, from the top 15 cm of the A horizon, were mixed, sieved (< 2 mm), and thoroughly homogenized.
Experiment conditions
Ethylamino-chain-15N-labeled-atrazine (99 atom% 15N in excess, purity >98.5) was purchased from Isotec (USA). Fresh soils (aliquots equivalent to 50 g of dry weight) were incubated under controlled conditions (28°C, 85% of water holding capacity, darkness). The soils were amended with atrazine at the recommended agronomic dose (8 ml of an atrazine solution of 30 mg L−1 to give a final concentration of 5 mg herbicide per kilogram soil). The same volume of distilled water was added to the unamended control soils. Biochemical properties and community structure were measured after 3, 6, 9, and 12 weeks of incubation. Sampling was destructive with three replicates used for each treatment-time combination, resulting in a total of 24 replicates of each soil (12 untreated and 12 treated with atrazine).
Biochemical properties and community structure
Inorganic N (NH +4 and NO −3 ) was analyzed with an extraction–diffusion method at room temperature, the evolved NH3 being trapped in 10 ml of 0.005 M H2SO4 and measured by back titration with 0.01 M NaOH (Couto-Vázquez and González-Prieto 2006). The resulting (NH4)2SO4 solutions were then acidified with 1 ml 0.005 M H2SO4 and evaporated to obtain (NH4)2SO4 salts, whose 15N/14N ratio was measured in an elemental analyzer (EA) coupled on-line with an isotopic ratio mass spectrometer (Finnigan Mat, delta C, Bremen, Germany). Traceability of results was verified by including, in each series of ten analyses, an isotopic certified standard (ammonium sulfate IAEA-305a and IAEA-305b, alternately). For each soil and sampling date: (a) net soil N mineralization was calculated as the variation in N content (total inorganic N, NH +4 -N, NO −3 -N) during the incubation subtracting the initial N content, and (b) the soil inorganic N content and its atom% 15N in excess (compared with the natural abundance in the corresponding control soil not added with atrazine) were used to calculate the net N mineralization rate of the added 15N-labeled atrazine. All samples were analyzed in duplicate.
The β-glucosidase activity was measured following the procedure of Eivazi and Tabatabai (1988), which determines the released p-nitrophenol after incubation of the soil with p-nitrophenyl glucoside solution for 3 h at 37°C. Urease activity was estimated by incubating soil samples with an aqueous urea solution and extracting the NH +4 with 1 M KCl and 0.01 M HCl followed by colorimetric NH +4 determination by a modified indophenol reaction (Kandeler and Gerber 1988). Soluble carbohydrates content was estimated colorimetrically by the anthrone method after extraction with distilled water (1:10 w/v) at 80°C for 24 h (Puget et al. 1999).
The microbial community structure was determined by PLFA analysis using the procedure and nomenclature described by Frostegård et al. (1993). The total microbial biomass was estimated as the sum of total PLFAs (totPLFAs). The sum of the PLFAs considered to be predominantly of bacterial origin was used as an index of the bacterial biomass (bactPLFAs), and the quantity of the PLFA 18:2ω6,9 was used as an indicator of the fungal biomass (fungPLFAs) (Frostegård and Bååth 1996). The PLFAS i14:0, i15:0, i16:0, and 10Me18:0 are predominantly found in gram-positive (G+) bacteria and the PLFAs cy17:0, cy19:0, 16:1 ω7c, and 18:1ω7 characterize gram-negative (G−) bacteria (Zelles 1999).
Statistical analysis
All results were obtained by triplicate determinations and were expressed on the basis of oven-dry (105°C) weight of soil. The data were analyzed by a three-way analysis of variance (ANOVA) to determine the percentage of the variation attributable to the factors soil type, incubation time, and single atrazine addition. For each incubation time, the effect of atrazine addition was examined by the standard unpaired t test comparing the data of some parameters analyzed in the untreated and atrazine-treated samples of the same soil (n = 3) and by the paired t test comparing the untreated and atrazine-treated samples of five soils (n = 5). For each variable, the standard error of the difference between means was calculated by one-way ANOVA. Data corresponding to the concentrations of all the individual PLFAs, expressed in mole percent and logarithmically transformed, were subjected to principal component analysis (PCA) to elucidate the main differences in the PLFA patterns. All statistical analyses were made using SPSS 15.0 statistical package.
Results
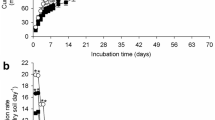
Net N mineralization after 12 weeks of aerobic incubation varied from 24 to 112 mg kg−1 soil and from 19 to 135 mg kg−1 soil in the untreated and atrazine-treated samples, respectively (Fig. 1). The net N mineralization values were significantly affected by soil type, incubation time, and atrazine treatment; the interaction between these factors also being significant (Table 2). The soil type and incubation time explained most of the variance (47% and 36%, respectively), whereas atrazine treatment account for only 1.8% of variance, and the interaction between these factors explained a further 9%. Nitrogen mineralization increased significantly with the incubation time. During the whole incubation time, M soil had the lowest N mineralization capacity, followed by P and E soils with similar values and G and C soils that exhibited the highest N mineralization values. Similar results were obtained for the fraction of total soil N that was mineralized during the incubation, with values following the order: M (−1.05% to 2.22% of total N) < E = P (0.04% to 3.30% of total N) < G (1.1% to 4.83% of total N) < C (1.44% to 6.0% of total N).
For all soils and most incubation times, the net mineralized N in atrazine-treated samples was slightly lower than in the corresponding untreated samples, although differences were not statistically significant at some incubation times. Thus, the application of 5 mg atrazine per kilogram soil resulted in an N mineralization reduction of 1.6–34 mg N kg−1, the highest values being detected after 3 or 6 weeks of incubation. However, significant increases (unpaired t test, P < 0.005) in N mineralization, ranging from 25 to 43 mg N kg−1, were also observed as consequence of atrazine addition in G and C soils at 9–12 weeks of incubation. Thus, compared with those of the respective untreated soils, the inorganic-N pool of all atrazine-treated soils was significantly reduced at 3 and 6 weeks of incubation (paired Student's t test, P < 0.005), while an inconsistent and non-significant atrazine effect was observed after both 9 and 12 weeks of incubation.
Since atrazine was labeled in the 15N-ethylamine-side chain, the N mineralization data also provided information on atrazine dynamics in these agricultural soils, as well as on its contribution to the soil N labile pool. 15NHCH2CH3 and 15NH3 are released in the intermediate processes of the 15N-ethylamino-atrazine degradation pathway; thus the presence of NH +4 -15N and NO -3 -15N in the KCl extracts of atrazine-added soils showed that ethylamine side chain of atrazine had been degraded by soil microorganisms. Figure 2 shows the percentage of added 15N-ethylamine-atrazine found in the soil inorganic-N pool at each incubation time. After 3 weeks of incubation in P, E, G, and M soils, the 15N mineralization values ranged from 5% to 19%, then increased until maximum values at 9 weeks (8–23%), and finally, they decreased significantly during the last 3 weeks of incubation. In contrast, 15N mineralization values increased progressively in C soil from 3 weeks (2%) until the end of the incubation (10%). In all soils and incubation times, less than 0.7% of the total N mineralized was derived from 15N atrazine. The atrazine degradation rate followed the order soil M > P > E > G > C. A significant negative relationship between net N soil mineralized and 15N-atrazine mineralized was observed at all incubation times (3 weeks, r = −0.931, P < 0.005; 6 weeks, r = −0.866, P < 0.0005; 9 weeks, r = −0.749, P < 0.002; 12 weeks, r = −0.864, P < 0.005).
The enzyme activities and soluble carbohydrate concentrations are shown in Fig. 3. Average values considering all incubation times and both untreated and atrazine-treated samples of five different soils were 114 ± 53 μg p-nitrophenol per gram soil per hour, 19 ± 12 μg N-NH +4 per gram soil per hour, and 47 ± 25 μg C per gram soil, for β-glucosidase, urease, and soluble carbohydrates. The atrazine treatment hardly affected these variables, whereas incubation time, explaining 18–64% of variance, and soil type, accounting for 30–75% of variance, had a pronounced influence on these properties, the interaction between these factors also being significant (Table 2). There was a significant reduction (P < 0.05) of these variables between 3 and 6 weeks of incubation and then values hardly changed (enzyme activities) or slightly tend to recover (soluble carbohydrates) until the end of the incubation (Fig. 3). In general, during the whole incubation, G, E, and M soils exhibited the highest values and C and P soils the lowest. When the incubation time was excluded and the data were analyzed independently for each incubation time, these biochemical parameters were not significantly different in untreated and atrazine-treated samples (paired Student's t test, P > 0.05 at 3, 6, 9, and 12 weeks).
The microbial biomass, estimated as totPLFAs, ranged from 1.3 to 3.6 μmol g−1 organic C; the highest values found in G and C soils, followed by M, P, and E soils (Table 3). The amounts of PLFAs that were chosen to represent bacteria and fungi PLFAs varied between 440 and 1,410 and 17 and 72 nmol g−1 organic C, respectively and comprised 29–40 and 1–2.4 mol% of the total amount of PLFAs. The amount of PLFAs representative of G+ bacteria and G− bacteria ranged from 220 to 590 nmol g−1 organic C and 120 to 420 nmol g−1 organic C, respectively, and comprised 15–21 and 7–18 mol% of the total amount of PLFAs. The ANOVA indicated that the total biomass and the biomass of specific groups were significantly affected by soil type and incubation time and the interaction between these factors was also significant (Table 2). In general, soil type explained most of variance (32–50%) and the incubation time accounted for a 14–21%; however, for fungPLFA values, the importance of incubation time increased notably (45% of variance) as compared with the soil effect (8% of variance). The recent atrazine addition only showed a significative influence on total and G− bacteria, explaining 1–4% of variance.
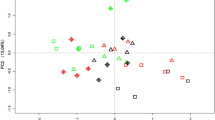
The PCA performed with the whole data set showed that the main differences in the PLFA pattern were due to soil type (Fig. 4a). The first component, differentiating between C and G soils (amphibolites and sediments, last 8–10 years of annual atrazine application), E soil (granite, 30 years of atrazine application, last 10 years without application) and M and P soils (acid schist and granite, last 20–40 years of annual atrazine application history), explained 34% of variation, while the third component, mainly differentiating samples according to the incubation time, only explained 11% of the variation. The second component, explaining 13% of variance, was not correlated to any of the factors studied. The C and G soils (having positive values along PC1) were mainly characterized by high concentrations of 14 to −16 carbon-saturated fatty acids (i16:0, a15:0, i15:0, 14:0) and monounsaturated fatty acid 16:1ω7c and 16:1ω5, while the M and P soils (with negative values along PC1) were characterized by high concentrations of the saturated 17 to 20 carbon fatty acids (Fig. 4b). The PLFAs decreasing with the incubation time (with positive values along PC3) were mainly of fungal (18:2ω6,9, 18:1ω9) and bacterial (18:1ω7, i14:0) origin. The distribution of samples in PCA also indicated that for the same incubation time, differences among untreated and recently atrazine-added samples were detected; however, a non-consistent pattern was observed for the five studied soils. Furthermore, changes produced were negligible compared to those provoked by soil type and incubation time.
Score and loading plots from principal component analysis performed on the PLFAs of the samples untreated (open symbols) and treated (filled symbols) with atrazine from the studied soils (P, E, M, G, and C) at different incubation times. Factors 1 and 3 accounted for 34% and 11% of the variance, respectively
Discussion
Most biochemical properties analyzed were significantly affected by soil type and incubation time after atrazine addition, and not significantly or only slightly affected by the recent atrazine addition at normal dose. A similar pattern concerning both recent herbicide and incubation effects was observed independently of soil studied and parameter measured. Thus, except few cases (see below) the biochemical parameters were similar in the two different treatments (untreated and treated with 5 mg atrazine per kilogram soil), indicating that atrazine at normal doses had little effect on the soil microorganisms.
The data of the present experiment clearly showed that atrazine, if used as recommended, does not lead to significant effects on urease and β-glucosidase activities and consequently suggest that the herbicide does not cause short-time effects on soil microbial activity. In most cases, little effect of atrazine application at field rates on several enzymes activities have also been reported in literature (Voets et al. 1974; Gianfreda et al. 1994; Sannino and Gianfreda 2001). Under laboratory conditions, Perucci et al. (2000) found that herbicide addition to the soil at field rate did not affect phosphatase activity, but this was greatly enhanced by applying the herbicide at ten times the field rate. Kruglow et al. (1974) reported that urease was unaffected by atrazine application at 3 kg ha−1 for up to 3 years and Davies and Greaves (1981) showed that the effects of 2–9 kg atrazine per hectare on dehydrogenase, phosphatase, and urease activities were not statistically significant. Recently, Moreno et al. (2007) found that high concentrations of atrazine added to soil (250–1,000 mg kg−1) increased notably the hydrolytic activity of urease after 45 days of incubation with the herbicide. This could, however, be due to the high application rate with atrazine functioning as substrate for the soil microorganisms. In agreement with these laboratory studies, no effect and sometimes inconsistent small stimulatory or adverse effects of atrazine application at agronomic doses on urease and β-glucosidase activities were also observed under field conditions (Mahía et al. 2007). The results supported that it is difficult to understand the impact of pesticides on soil enzyme activities because there are direct and indirect effects (Schaëfer 1993; Nannipieri 1994).
Since short-term (3–6 weeks) N mineralization values were significantly reduced by herbicide application, the results indicate that measurements of net N mineralization is a more sensitive indicator of atrazine impact on these agricultural soil ecosystems than β-glucosidase and urease activity assays were. Pesticides can both stimulate and inhibit N mineralization (Chen et al. 2001; Haney et al. 2002). We found no consistent effects of the atrazine addition on N mineralization values after a longer incubation time (9–12 weeks), indicating only a temporal and non-persistent influence of a single application of herbicide at normal doses. Likewise, Hart and Brookes (1997) also showed that pesticides at the recommended rates had no long-term harmful effects on soil microbial activity as assessed by N mineralization.
The presence of isotopically labeled inorganic N in the KCl extracts clearly demonstrated that the side-chain atrazine was utilized/mineralized by atrazine-degrading microorganisms, particularly in soils with low N availability. This is in agreement with many studies showing that N status may alter microbial processes and the use of added N sources and thus influence rates of herbicide degradation (Cook and Hütter 1981; Entry et al. 1993; Mandelbaum et al. 1995; Radosevich et al. 1995; Bichat et al. 1999). The significant negative relationship found between net soil N mineralization and 15N-atrazine mineralized is consistent with results showing lower atrazine mineralization rates when soil N availability increased as a consequence of organic and inorganic amendments (Alvey and Crowley 1995; Abdelhafid et al. 2000b; Haney et al. 2002) and higher percentages of atrazine mineralized when soil N availability decreased (Bichat et al. 1999; Abdelhafid et al. 2000b).
Atrazine application history appeared also to be a factor which determined the mineralization of the atrazine side chain of these soils (Table 1 and Figs. 1 and 2), with increasing mineralization with time of atrazine application. Rousseaux et al. (2001), in a 131-day incubation study with 12 soils, clearly grouped the soils according to their ability to mineralize atrazine; a first group included soils with a long atrazine application history that showed enhanced degradation of side-chain atrazine (52–61% of that initially added) and a second group included soils without adaptation to atrazine addition with lower degradation values (19–38% of that initially added). Yassir et al. (1998) reported that after a 60-day incubation period with labeled atrazine, the cumulative degradation never exceeded 10% of the initial amount added for the 14C-ethylamine-atrazine and 3% for the 14C-isopropylamine-atrazine in a soil with no history of atrazine treatment. It is well known that dynamics of C cycle differs notably from that of N cycle; therefore, although the values obtained in the present study (15N-ethylamino-atrazine) cannot be directly compared with those obtained by Yassir et al. (1998) (14C-ethylamine-atrazine), similar results showing a marked influence of previous exposure to atrazine on kinetics of atrazine degradation were clearly observed independently of the label of the atrazine compound used in our two experiments. The results obtained here are also consistent with other studies showing that previous exposure of atrazine is a determinant factor governing atrazine biodegradation in agricultural soils from the temperate humid zone (Mahía and Díaz-Raviña 2007; Mahía et al. 2008a, b).
Thus, atrazine degradation and 15N mineralization in the soils studied here could be explained by a combination of N availability and history of atrazine application. The P and M soils (20–40 years history of atrazine application) probably had a very active community of atrazine degraders due to repeated annual atrazine application. Despite of shorter atrazine history, the M soil exhibited higher percentage atrazine degradation than the P soil, probably due to the lower soil N levels. The E soil, with the highest initial inorganic N levels and intermediate net N mineralized values, had been treated with atrazine during 30 years, but had not received the herbicide in the last 10 years; therefore, its capacity to degrade atrazine could have considerably decreased compared to soils with recent applications. The G and C soils exhibited the lower percentages of 15N-ethylamine-atrazine degradation, which can be explained by a shorter atrazine application history (8–10 year) and high soil inorganic N levels.
PLFA analysis indicated that the range of values obtained for total biomass and the biomass of specific groups were in the reported range for agricultural soils (Frostegård and Bååth 1996) and were mainly determined by the soil type and incubation time. As occurred for N mineralization, a minor negative and significant atrazine effect was detected on most biomass parameters during the first 3 weeks of incubation, whereas no effect was observed over the rest of incubation period. Thus, also the PLFA pattern was little affected by recent atrazine addition at normal rates.
The PLFAs 18:2ω6,9 and 18:1ω9, characteristic of fungi, and 18:1ω7, i14:0, characteristic of bacteria, displayed the largest decreases in concentration with the incubation time. Prolonged incubation of soils often results in a decrease in fungal PLFAs (Frostegård et al. 1996; Bååth et al. 2004), although these studies were made in forest soils. The decrease in fungal PLFAs was then ascribed to ectomycorrhizal fungi dying off when deprived of their plant host. In the present study, agricultural soils were used, and thus, no ectomycorrhiza were present. Instead, the soil incubation could have altered the competitive strength between fungi and bacteria. However, since the effect of incubation time was found in a separate principal component from that due to soil/atrazine application, the incubation time did not confound the study of atrazine effects.
The PCA analysis clearly showed that the main differences in the community structure were due to soil type and to a lesser extent to incubation time, whereas no changes were detected as consequence of recent atrazine application. The fact that individual PLFAs along PC1 in Fig. 4a discriminated between soils with lower (C and G), intermediate (E) and higher (M and P) application history and between samples of same soil with different previous exposure to herbicide (P and E) could suggest that atrazine application history rather than soil type is determinant for soil microbial composition of the 0–15 cm layer of these agricultural soils under continuous maize cultivation. Effects of atrazine application on the microbial community composition have earlier been found (Chang et al. 2001; Rhine et al. 2003; Seghers et al. 2003; Ross et al. 2006). The results of Rhine et al. (2003) indicated that repeated exposure to atrazine provided sufficient selective pressure to cause shifts in fatty acid composition of the soil microbial communities, but specific groups of microorganisms affected were not identified. Likewise, Seghers et al. (2003) indicated that the long-term use of the atrazine (20 years) at a normal agronomic dose resulted in an altered soil microbial community, in particular for the methanotrophic bacteria, characterized by the group-specific polymerase chain reaction-denaturing gradient gel electrophoresis. Chang et al. (2001) and Ross et al. (2006) also found differences in bacterial community structure determined by PCR-DGGE technique, in short-term laboratory experiments at high concentrations of atrazine. It should be noticed, however, that changes observed under field conditions were much less marked than those under laboratory conditions, which can be due to the low atrazine doses, time passed after application, and the influence of other factors (environmental conditions, soil properties, management practices, plant, etc.) that confounded the atrazine effect. In our case, a residual herbicide effect on PLFA pattern seem to be detected as consequence of previous repeated annual application of herbicide under field conditions, indicating that microbial communities structure measured by the PLFA pattern can be a useful tool to detect the long-term impact of atrazine application to agricultural soils.
Conclusion
It can be concluded that atrazine not only affect the target organisms (weeds) but also the microbial community present in these agricultural soils from temperate humid zone with 8–40 years of annual herbicide application history. However, recently added atrazine at agronomic dose did not substantially alter most soil biochemical properties analyzed (β-glucosidase, urease, soluble carbohydrates) and only slightly decreased soil N mineralization and microbial biomass values. The soil PLFA pattern indicated that the response was affected by previous exposure to atrazine, which seems to indicate that atrazine should be applied with precaution since repeated herbicide application during 8–40 year can induce changes in microbial community structure. Our results also showed that soil N dynamics can be influenced by annual and repeated application of atrazine. Future work should be concentrate on the mechanisms of the induced microbial changes as consequence of recent and repeated herbicide addition, as well as how rapid the effects will disappear after herbicide application ceases.
References
Abdelhafid R, Houot S, Barriuso E (2000a) Dependence of atrazine degradation on C and N availability in adapted and non-adapted soils. Soil Biol Biochem 32:389–401
Abdelhafid R, Houot S, Barriuso E (2000b) How increasing availabilities of carbon and nitrogen affect atrazine behaviour in soils. Biol Fertil Soils 30:333–340
Accinelli C, Giovanni D, Vicari A, Catizone P (2001) Atrazine and metolachlor degradation in subsoils. Biol Fertil Soils 33:495–500
Alvey S, Crowley DE (1995) Influence of organic amendments on biodegradation of atrazine as a nitrogen source. J Environ Qual 24:1156–1162
Assaf NA, Turco RF (1994) Influence of carbon and nitrogen application on the mineralization of atrazine and its metabolites in soil. Pest Sci 41:41–47
Bååth E, Nilsson LO, Göransson H, Wallander H (2004) Can the extent of degradation of soil fungal mycelium during soil incubation be use to estimate ectomycorrizal biomass in soil? Soil Biol Biochem 36:2105–2109
Barriuso E, Houot S (1996) Rapid mineralization of the s-triazine ring of atrazine in soils in relation to soil management. Soil Biol Biochem 28:1341–1348
Behki RM, Khan SU (1986) Degradation of atrazine by Pseudomonas: N-dealkylation and dehalogenation of atrazine and its metabolites. J Agr Food Chem 34:746–749
Bichat F, Sims GK, Mulvaney RL (1999) Microbial utilization of heterocyclic nitrogen from atrazine. Soil Sci Soc Am J 63:100–110
Bouquard C, Ouzzani J, Prome JC, Michael-Briand Y, Plesiat P (1997) Dechlorination of atrazine by a Rhizobium sp. isolate. Appl Environ Microbiol 63:862–866
Capriel P, Haisch A, Khan SU (1985) Distribution and nature of bound (nonextractable) residues of atrazine in a mineral soil nine years after herbicide application. J Agr Food Chem 33:567–569
Chang YJ, Hussain A, Stephen JR, Mullen MD, White DC, Peacock A (2001) Impact of herbicides on the abundance and structure of indigenous beta-subgroup ammonia-oxidizer communities in soil microcosms. Environ Toxicol Chem 20:2462–2468
Chen SK, Edwards CA, Subler S (2001) Effects of the fungicides benomyl, captan and chlorothalonil on soil microbial activity and nitrogen dynamics in laboratory incubations. Soil Biol Biochem 33:1971–1980
Cook AM (1987) Biodegradation of s-triazine xenobiotics. FEMS Microbiol Rev 46:93–116
Cook SM, Hütter R (1981) S-triazines as nitrogen sources for bacteria. J Agr Food Chem 29:1135–1143
Couto-Vázquez A, González-Prieto SJ (2006) Short- and medium-term effects of three fire fighting chemicals on the properties of a burnt soil. Sci Total Environ 371:353–361
Davies H, Greaves MP (1981) Effects of some herbicides on soil enzyme activities. Weed Res 21:205–209
Dzantor EK, Felsot AS (1991) Microbial responses to large concentrations of herbicides in soil. Environ Toxicol Chem 10:649–655
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606
Entry JA, Mattson KG, Emmingham WH (1993) The influence of N on atrazine and 2,4-dichlorophenoxyactetic acid mineralization in grassland soils. Biol Fertil Soils 16:179–182
Erickson LE, Lee KH (1989) Degradation of atrazine and related s-triazines. Crit Rev Environ Contr 19:1–14
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Frostegård A, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed soils as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Frostegård A, Tunlid A, Bååth E (1996) Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metal. Soil Biol Biochem 28:55–63
Gan J, Becker RL, Koskinen WC, Buhler DD (1996) Degradation of atrazine in two soils as a function of concentration. J Environ Qual 25:1064–1072
Ghani A, Wardle DA, Rahman A, Lauren DR (1996) Interaction between 14C-labelled atrazine and the soil microbial biomass in relation to herbicide degradation. Biol Fertil Soils 21:17–22
Gianfreda L, Sannino F, Ortega N, Nannipieri P (1994) Activity of free and immobilized urease in soil: effects of pesticides. Soil Biol Biochem 26:77–784
Greaves MP (1982) Effects of pesticides on soil microorganisms. In: Burns RG, Slater JH (eds) Experimental Microbial Ecology. Blackwell, Oxford, pp 613–630
Haney RL, Senseman SA, Krutz LJ, Hons FM (2002) Soil carbon and nitrogen mineralization as affected by atrazine and glyphosate. Biol Fertil Soils 35:35–40
Hart MR, Brookes PC (1997) Soil microbial biomass and mineralisation of soil organic matter after 19 years of cumulative field applications of pesticides. Soil Biol Biochem 28:1641–1649
Herwig U, Klumpp E, Narres H-D, Schwuger MJ (2001) Physicochemical interactions between atrazine and clay minerals. Appl Clay Sci 18:211–222
Hussain S, Siddique T, Saleem M, Arshad M, Khalid A (2009) Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv Agron 22:159–200
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kaufman DD, Kearney PC (1970) Microbial degradation of s-triazine herbicides. Res Rev 32:235–265
Koskinen WC, Clay SA (1998) Factors affecting atrazine rate in North Central U.S. Soils. Rev Environ Contam Toxicol 151:117–165
Kruglow JW, Gersz NB, Piercewa AN, Bay-Bienko NW, Michajlowa EI (1974) The effect of long-term herbicide application on microflora and some biochemical processes in soil. Roczniki Gleboznawcze 26:159–164
Mahía J, Díaz-Raviña M (2007) Atrazine degradation and residues distribution in two acid soils from temperate humid zone. J Environ Qual 36:826–831
Mahía J, Martín A, Carballas T, Díaz-Raviña M (2007) Atrazine degradation and enzyme activities in an agricultural soil under two tillage systems. Sci Total Environ 378:187–194
Mahía J, Cabaneiro A, Carballas T, Díaz-Raviña M (2008a) Microbial biomass and C mineralization in agricultural soil as affected by atrazine addition. Biol Fertil Soils 45:99–105
Mahía J, Martín A, Díaz-Raviña M (2008b) Extractable atrazine and its metabolites in agricultural soils from temperate humid zone. Environ Geochem Health 30:147–152
Mandelbaum RT, Wackett LP, Allan DL (1993) Mineralization of the s-triazine ring of atrazine by stable bacterial mixed cultures. Appl Environ Microbiol 69:1695–1701
Mandelbaum RT, Allan DL, Wackett LP (1995) Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl Environ Microbiol 61:1451–1457
Moreno JL, Aliaga A, Navarro S, Hernández T, García C (2007) Effects of atrazine on microbial activity in semiarid soil. Appl Soil Ecol 35:120–127
Nannipieri P (1994) The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In: Pankurst CE, Doube BM, Gupta VVSR, Grace PR (eds) Soil Biota: Management in Sustainable Farming Systems. CSIRO, Melbourne East, pp 238–244
Ostrofsky EB, Robinson JB, Traina SJ, Tuovinen OH (2002) Analysis of atrazine-degrading microbial communities in soils using most-probable-number enumeration, DNA hybridization, and inhibitors. Soil Biol Biochem 34:1449–1459
Perucci C, Dummontet S, Mazzatura A (2000) Effects of organic amendment and herbicide treatment on soil microbial biomass. Biol Fertil Soils 32:17–23
Puget P, Angers DA, Chenu C (1999) Nature of carbohydrates associated with water-stable aggregates of two cultivated soils. Soil Biol Biochem 31:55–63
Radosevich M, Traina SJ, Hao L, Tuovinen OH (1995) Degradation and mineralization of atrazine by a soil bacterial isolate. Appl Environ Microbiol 61:297–302
Rhine ED, Fuhrmann JJ, Radosevich M (2003) Microbial community responses to atrazine exposure and nutrient availability: linking degradation capacity to community structure. Microbial Ecol 46:145–160
Ross M, Goberna M, Moreno JL, Hernández T, García C, Insam H, Pascual JA (2006) Molecular and physiological bacterial diversity of a semiarid soil contaminated with different levels of formulated atrazine. Appl Soil Ecol 34:93–102
Rousseaux S, Hartmann A, Soulas G (2001) Isolation and characterisation of new Gram-negative and Gram-positive atrazine degrading bacteria from different French soils. FEMS Microbiol Ecol 36:211–222
Sannino F, Gianfreda L (2001) Pesticide influence on soil enzymatic activities. Chemosphere 45:417–425
Schaëfer A (1993) Pesticide effects on enzyme activities in the soil ecosystem. In: Bollag JM, Stotzky G (eds) Soil Biochemistry Vol. 8. Marcel Dekker, New York, pp 273–371
Schiavon M (1988) Studies of the leaching of atrazine, of its chlorinated derivatives, and of hydroxyatrazine from soil using 14C ring-labelled compounds under outdoor conditions. Ecotoxicol Environ Saf 15:46–54
Seghers D, Verthé K, Reheul D, Bulcke R, Siciliano SD, Vertraete W, Top EM (2003) Effect of long-term herbicide applications on the bacterial community structure and function in an agricultural soil. FEMS Microbiol Ecol 46:139–146
Topp E, Vallaeys T, Soulas G (1997) Pesticides: microbial degradation and effects on soil microorganisms. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern Soil Microbiology. Marcel Dekker, New York, pp 547–575
Voets JP, Meerschman P, Verstraete W (1974) Soil microbiological and biochemical effects of long-term atrazine applications. Soil Biol Biochem 6:149–152
Yanze-Kontchou C, Gschwind N (1994) Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl Environ Microbiol 60:4297–4302
Yassir A, Rie C, Soulas G (1998) Microbial N-Dealkylation of atrazine: effect of exogenous organic substrates and behaviour of the soil microflora. Pest Sci 54:75–82
Yassir A, Lagacherie B, Houot S, Soulas G (1999) Microbial aspects of atrazine biodegradation in relation to history of soil treatment. Pest Sci 55:799–809
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Acknowledgments
The authors thank Marcos Godoy Iglesias for the technical assistance. This study was supported by the Secretaría Xeral de Investigación e Desenvolvemento da Xunta de Galicia (Project PGIDT01PXI40002PR) and by Spanish Ministerio de Ciencia y Tecnología (Project AGL2005-05726-C02-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahía, J., González-Prieto, S.J., Martín, A. et al. Biochemical properties and microbial community structure of five different soils after atrazine addition. Biol Fertil Soils 47, 577–589 (2011). https://doi.org/10.1007/s00374-011-0569-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0569-x