Abstract
Laboratory incubation experiments were conducted with uncoated urea or urea coated with dementholized oil (DMO), pitch (the mint oil discard), terpenes (the products of menthol mint oil), or dicyandiamide (DCD) to study the retardation of urea hydrolysis and nitrification in soil. Two levels (0.5 and 1 %) of coating were tested. Urea was applied at a rate of 200 mg kg−1 of dry weight of soil. The urea hydrolysis and nitrification processes were inhibited by all three natural products. All the three natural products viz., DMO, terpenes, and pitch significantly retarded urease activity of soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well established that the use of fertilizers is necessary for crop yield, but it can cause environmental problems such as increase of nitrate concentration in the groundwater, contribution to the formation of acid rain, ozone layer depletion due to release of nitrous oxides by denitrification, etc. Average estimates indicate that recovery of applied urea by kharif crops in India is 30–50%, but it can be as low as 20% in lowland rice because it can be lost through different processes (Katyal et al. 1985; Patra et al. 2000). By reducing these N losses in the field, it is possible to reduce rate of application and avoid N-pollution of the environment (Patra et al. 2002).
Fertilizer urea, when applied to soil, is hydrolysed by urease to NH4 + which is then oxidized to NO3 - which can be leached or denitrified. To ensure a continuous and optimal supply of N, and to decrease losses, chemicals that retard either urea hydrolysis, or nitrification or both have been extensively tested. In this context, slow-release urea forms such as sulfur-coated urea, lac-coated urea, polymer-coated urea, and urea super granules have been extensively investigated (Prasad et al. 1971; Prasad 1998); urease inhibitors retarding urea hydrolysis have been also studied (Gould et al. 1986). In order to improve nitrogen use efficiency of crops, several synthetic chemicals such as N-serve (nitrapyrin), DCD (dicynadiamide), CS2 (carbon disulphide), sodium chlorate, BHC (benzene hexachloride) etc. have been examined for inhibition of urea hydrolysis or nitrification or both in soils (Zaman et al. 2008). However, the use of many of these chemicals has been restricted to academic experimental studies because of high cost, lack of availability, and adverse effects on soil microflora (Purakayastha 1997).
Some natural products can inhibit nitrification such as Karanjin from Karanj (Pongamia glabra), tea waste containing tannins, and neem (Azadirachta indica; Prasad et al. 1971; Sahrawat and Parmar 1975; Sahrawat 1989). Another important discovery was the nitrification-inhibitory properties of Neem-cake-coated urea which has been used by Indian farmers (Prasad 1998).
A number of essential oils such as mint (Mentha sp.) oil find wide application as an antiseptic agent in pharmaceutical preparations. Chemical constituents such as menthol, carvone, thymol, terpene, pulegone present in mint oil and other essential oils have antimicrobial properties (Kiran and Patra 2002a, 2002b; Patra et al. 2001, 2002). Mentha spicata oil was as effective as DCD in retarding nitrification and increasing N use efficiency (Patra et al. 2002). During the last 4–5 years, several laboratory, greenhouse, and field experiments were conducted to evaluate the inhibition of nitrification and urea hydrolysis by such products (Patra et al. 2001, 2002, Kiran et al. 2003; Kiran and Patra 2003; Patra et al. 2006). Since many of the chemical inhibitors are expensive, not easily available and have significant adverse effect on beneficial soil microorganism, it is needed to find cheap natural products as urease and nitrification inhibitors (Prasad 1998).
The scope of the present investigation was to study the effects of natural essential oil by-products such as dementholized oil (DMO), and pitch (essential oil discard), and terpenes (the product of menthol mint oil) on nitrification and urease activity. The effects of these substances were compared with those of dicyandiamide (DCD). All these materials were used as urea-coating materials.
Materials and methods
Soil, inhibitors, and laboratory incubation
The soil (sandy loam, Entisol, Ustifluvent) used in this experiment has the following properties: pH 8.50, EC 0.40 dSm−1; organic C 4.60 g kg−1 soil; available N (alkaline permanganate extractable) 168 kg ha−1; available P (0.50 M NaHCO3 extractable) 12.8 kg ha−1; and available K (1 N NH4OAc extractable) 107 kg ha−1.
Prilled urea (46% N) was coated with the natural products or with DCD using castor (Ricinus communis) oil as the fixative material. Therefore, urea granules were first coated with castor oil (1.00%, v/w basis), air dried for 24 h and then coated with DMO, Pitch, terpenes, or DCD at two rates (0.50% and 1.00% on v/w basis).
Then, coated uncoated granules which contained 200 mg N kg−1 were used in the experiment which included ten treatments, four coating materials applied at two levels of coating (0.5 and 1.0%), uncoated urea and the control (no N and no coating material). The urea was applied at a rate of 200 mg kg−1 soil. The statistical design followed a completely randomized design (CRD) with three replicates.
The collected soils samples were mixed, homogenized, sieved (<2 mm), and pre-incubated in airtight containers at 5°C in the presence of moist soda-lime for 5 days (Chand et al. 2004). Soil (100 g) was placed in plastic containers and treated with urea granules or left untreated (control). The mixture soil urea was thoroughly mixed and incubated at 60% of maximum water holding capacity and at 25 ± 2°C for 3 weeks.
Analysis
Soil samples were removed at 1, 2, 3, 7, 14, and 21 days and extracted with 1 M Na2SO4–phenyl mercuric acetate (PMA) for urea-N, NH4 +-N and NO3 −-N analysis as described by Douglas and Bremner (1970), Yuen and Pollard (1952), and Sims and Jackson (1977), respectively.
The effect of the used products on urease activity was studied by incubating soil with urea (200 mg N kg−1 soil) coated with the four urease/nitrification inhibitors or with uncoated urea at 25 ± 2 °C for 4 h. The unhydrolysed urea was determined colorimetrically as described by Douglas and Bremner (1970).
Chromatographic (GC) analysis
By-products of dementholized oil (DMO) and terpenes were determined by gas chromatography using a Nucon make, microprocessor-controlled GLC model No. 5765 with a 10′ × 1/8″ FFAP column diameter filled with 80/100 chromosorb WAW, and a FID detector. The other conditions of the GLC were initial column temperature 100°C, final column temperature 220°C with a rise of 4°C/min. Injector and detector temperature was 225°C and the inlet pressure of the carrier gas N2 was 30 psi. Chromatography of pitch could not be done due to its coal-tar-like texture. The chemical composition of natural essential oil by-products is given in Table 1.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and least significant difference (LDS) were calculated by using T-method (Sakal and Rholf 1981).
Results and discussion
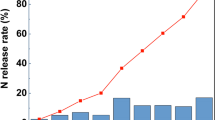
All used inhibitors significantly reduced the urea-N hydrolysis and their effect differed markedly with the two levels applied (Table 2). Most of the urea coated with different materials was hydrolysed in the first 2 days of incubation; only terpenes and pitch-coated urea-treated soil showed residual urea. Urea hydrolysis inhibition and, consequently, reduced ammonia volatilization by natural products like neem cake and mustard cake have been reported by Purakayastha (1997). Dicyandiamide did not inhibit urea hydrolysis.
All the coating materials significantly retarded the nitrification in soil in comparison to the uncoated urea (Table 2). Nitrification inhibition was more pronounced at 1.0% level of coating but the ranking order of the different forms changed with level of coating. At 0.5% level of coating, the ranking order was: Pitch > DCD > DMO > terpenes; whereas, at 1.0% level of coating the order was: DCD > DMO > Pitch > terpenes. Nitrification inhibition by the coating materials may be due to the sensitiveness of nitrifiers to these products as it has been shown for plant products rich in phenols and alkaloids, which act as antibacterial substances (Olsen and Reiners 1983). Nitrification, however, increased with all treatments by increasing in the incubation period.
The NH4 +-N concentration was higher in the dicyandiamide treated soils and in soils treated with other coating materials, than in the soil amended with uncoated urea. High accumulation of NH4 + in soil is not desirable as it makes the microsite soil pH high and the edaphic condition congenial for ammonia volatilization (Prakasa Rao and Puttanna 1987).
All three natural products significantly retarded urease activity at both the levels of coating; and the highest inhibition was at the highest level of coating (Table 3). Inhibition of urease activity at 0.5% ranked as: DMO > pitch > terpenes > DCD. Trends were different at 1.0% level of coating and it was in the decreasing order: terpenes > DMO > pitch > DCD. The phenolic compounds of tea waste can substantially retard N mineralization in soil by inhibiting microbial activity (Sivapalan and Fernando 1985); this inhibition may be responsible for the decrease in urease and nitrifying activity. The natural products were more inhibitory than DCD.
Thus, different coating materials used viz., pitch, dementholized oil, and terpenes are potential retardants of urease activity and nitrification unlike dicyandiamide which is a potential retardant of nitrification but has little effect on the urea hydrolysis. The compounds of the used by-product (Table 1) responsible for such retardation need further investigation. Among the three mint oil by-products used, pitch, being cheapest and having a semisolid consistency, is most effective to be used as a coating material.
References
Chand S, Anwar M, Patra DD, Khanuja SPS (2004) Effect of mint distillation waste on soil microbial biomass in a mint-mustard cropping sequence. Commun Soil Sci Plant Anal 35:243–254. doi:10.1081/CSS-120027647
Douglas LA, Bremner JM (1970) Extraction and colorimetric determination of urea in soils. Proc- Soil Sci Soc Am 34:859–862
Gould WD, Hagedorn C, McCready RGL (1986) Urea transformations and fertilizers efficiency in soil. Adv Agron 40:209–238. doi:10.1016/S0065-2113(08)60283-7
Katyal JC, Singh B, Sharma VK, Craswell ET (1985) Efficiency of some modified urea fertilizers in wet land rice grown on permeable soil. Fert Res 8:137–146. doi:10.1007/BF01048897
Kiran U, Patra DD (2002a) Influence of natural essential oils and synthetic nitrification inhibitor on crop yield and nitrogen use efficiency in mint (Mentha arvensis) - mustard (Brassica juncea) cropping sequence. J Indian Soc Soil Sci 50:64–69
Kiran U, Patra DD (2002b) Augmenting yield and urea-N utilization efficiency in wheat (Triticum aestivum) through use of natural essential oils and dicyandiamide coated urea in light textured soils of Central Uttar Pradesh, India. Commun Soil Sci Plant Anal 33:1375–1388. doi:10.1081/CSS-120004287
Kiran U, Patra DD (2003) Medicinal and aromatic plant material as nitrification inhibitors for augmenting yield and nitrogen uptake of Japanese mint (Mentha arvensis L. Var. Piperascens). Bioresour Technol 86:267–276. doi:10.1016/S0960-8524(02)00143-8
Kiran U, Patra DD, Kumar S (2003) Effect of Artemisia annua and Mentha spicata oil as natural nitrification inhibitors vis-a-via dicynadiamide (DCD) in regulating nitrogen availability, N uptake and yield of Japanese mint (Mentha arvensis) in sandy loam soils in central Indian subtropical conditions. Agric Ecosyst Environ 94:237–245. doi:10.1016/S0167-8809(02)00027-0
Olsen PK, Reiners WA (1983) Nitrification in subalpine Balsam Fir soils: tests for inhibitory factors. Soil Biol Biochem 15:413–418. doi:10.1016/0038-0717(83)90005-6
Patra DD, Anwar M, Chand S (2000) Integrated nutrient management and waste recycling for restoring soil fertility and productivity in Japanese mint (Mentha arvensis) and mustard (Brassica juncea) sequence in Uttar Pradesh. Agric Ecosyst Environ 80:267–275. doi:10.1016/S0167-8809(00)00151-1
Patra DD, Anwar M, Chand S, Chattopadhyay A, Prasad A, Pandey P, Kumar A, Singh S, Srivastava RK, Krishna A, Singh V, Tomar VKS, Bansal RP, Singh AK, Singh K, Bahl JR, Kumar S (2001) Use on mint essential oil as an agrichemical: control of N- loss in crop fields by using mint essential oil coated urea as fertilizer. Curr Sci 81(12):1526–1528
Patra DD, Anwar M, Chand S, Kiran U, Rajput DK, Kumar S (2002) Nimin and Mentha spicata oil as nitrification inhibitors for optimum yield of Japanese mint (Mentha arvensis). Commun Soil Sci Plant Anal 33:451–460. doi:10.1081/CSS-120002756
Patra DD, Kiran U, Pandey P (2006) Urease and nitrification retardation properties in natural essential oils and their by-products. Commun Soil Sci Plant Anal 37:1663–1673. doi:10.1080/00103620600710306
Prakasa Rao EVS, Puttanna K (1987) Nitrification and ammonia volatilization losses from urea and dicyandiamide-treated urea in a sandy loam soil. Plant Soil 97:201–206. doi:10.1007/BF02374942
Prasad R (1998) Fertilizer urea, food security, health and the environment. Curr Sci 75:677–683
Prasad R, Rajale GB, Lakhdive BA (1971) Nitrification retarders and slow release nitrogenous fertilizer. Adv Agron 23:337–405. doi:10.1016/S0065-2113(08)60156-X
Purakayastha TJ (1997) Evaluation of some modified urea fertilizers applied to rice. Fertil News 42:53–56
Sahrawat KL (1989) Effect of nitrification inhibitors on nitrogen transformations, other than nitrification in soil. Adv Agron 42:279–309. doi:10.1016/S0065-2113(08)60527-1
Sahrawat KL, Parmar BS (1975) Alcohol extract of ‘Neem’ (Azadirachta indica L.). seed as nitrification inhibitor. J Indian Soc Soil Sci 23:131–134
Sims JR, Jackson GD (1977) Rapid analysis of soil nitrate with chromotropic acid. Proc- Soil Sci Soc Am 35:603–606
Sakal RR, Rholf FJ (1981). In Biometry: The Principles and Practices of Statistics in Biological Research 2nd Edition, Freeman, New York 1981.
Sivapalan K, Fernando V (1985) N-mineralization in polyphenol rich plant residues and their effect on nitrification of applied ammonium sulphate. Soil Biol Biochem 17:547–551. doi:10.1016/0038-0717(85)90023-9
Yuen SH, Pollard AG (1952) The determination of nitrogen in agricultural materials by the Nessler's reagent: preparation of the reagent. J Sci Food Agric 3:441–447. doi:10.1002/jsfa.2740031002
Zaman M, Ngujen ML, Blennerharsett Quin BF (2008) Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol Fertil Soils 44:693–705. doi:10.1007/s00374-007-0252-4
Acknowledgments
The authors are thankful to the Director, Central Institute of Medicinal and Aromatic Plants (CIMAP), Lucknow, India, for providing necessary facilities and encouragement during the course of investigation and Indian Council of Agricultural Research, New Delhi, for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patra, D.D., Kiran, U., Chand, S. et al. Use of urea coated with natural products to inhibit urea hydrolysis and nitrification in soil. Biol Fertil Soils 45, 617–621 (2009). https://doi.org/10.1007/s00374-009-0372-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0372-0