Abstract
The losses of nitrogen (N) fertilizer applied to the soil microenvironment reduce its efficiency. Different strategies like the use of polymer coating and chemical nitrification inhibitors had been employed to reduce N losses. But these chemical nitrification inhibitors are very expensive. Thus, a study was conducted to investigate the effects of different concentrations of Parthenium extract, neem oil, and acidulated cow dung compost extract on N dynamics in the soil. Three concentrations of Parthenium extract (5, 10, and 15%) and neem oil (1, 2, and 3%) were coated on urea granules using a polymer as the binding agent. Three pH (2, 4, and 6 pH) based acidulated cow dung compost extracts were also coated on urea granules in the same pattern. These coated fertilizers and uncoated urea were applied in jars filled with soil (100 g per jar) at the rate of 1 g of fertilizer per jar. One treatment was kept as control (without fertilizer). Treatments along three replications were arranged according to the completely randomized design. Results depicted that all coating materials caused the release of N consistently from applied fertilizers compared to uncoated treatment. In addition, percent nitrified N was also reduced significantly in coated treatments in comparison to the uncoated urea and control treatments. However, the level of concentration effect was not obvious as lower concentrations of these extracts and oil also performed almost equal to that of higher concentrations in inhibiting nitrification process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The increasing population demands healthy food with more production of crops for which plant nutrients are vitally essential. Chemical-based inorganic fertilizers are of prime importance under soil management strategies to enhance the quality and quantity of agricultural outputs (Itelima et al. 2018). Nitrogen (N) plays an important role in plant growth and development as a prime mineral nutrient. Higher plants require large amounts of N for physiological and biochemical processes that lead to a higher quality of produce and increased yield. Low organic matter contents and several other factors are responsible for the deficiency of N in Pakistani soils. According to an estimate, 78–79% N is present in the atmosphere but it is in an inert gas. Thus, technical nutrient management practices are required (Leghari et al. 2016; Zhang et al. 2021). In modern agriculture, large amounts of N fertilizers are applied for higher production, but less than 50% of these applied fertilizers are utilized by the crops (Zhang et al. 2020). Recently, different strategies had been employed to enhance the efficiency of N fertilizers (Yaseen et al. 2021).
However quick hydrolysis of urea releases plant-available forms of N in the soil suddenly after exposure to moisture in the presence of the urease enzyme. Firstly, NH4+ is formed which is a plant available form and immobile in the soil (Lei et al. 2018). Excessive application of N fertilizer at an early stage of the plant induces low use efficiency of applied fertilizer because this excess fertilizer got lost via volatilization, nitrification, denitrification, and runoff (Jain and Abrol 2017; Anas et al. 2020). To reduce such losses, different strategies had been employed like the 4-R strategy (i.e., considering the right source, right rate, right time, and the right place for fertilizer application), split application of urea and controlled-release urea (Tao et al. 2018; Freedman 2018; Yaseen et al. 2016; Noor et al. 2017). Biodegradable polymer coated urea slows the release kinetics of available forms of N in the soil (Beig et al. 2020). Thus slow-release N fertilizers are very crucial to reduce the losses of N to the environment and groundwater. But N released in the form of NH4+ in the soil got converted to the nitrate form via nitrification process if not up-taken by the plants indicating nitrification as a less desirable process (Lasisi et al. 2019).
To overcome the nitrification losses from agricultural activities, different nitrification inhibitors had been used in recent years (Byrne et al. 2020). But commercial nitrification inhibitors are much more expensive for small farmers (Hatano et al. 2019). On the other hand, organic extracts and oils are much more cost-effective and good nitrification inhibitors in comparison to commercial nitrification inhibitors. As neem oil could reduce nitrification losses more effectively than the lower dose of dicyandiamide (Opoku et al. 2014). Parthenium hysterophorus is a well-known invasive weed with plenty of negative roles as agricultural, medicinal, and environmental hazards (Kaur et al. 2014). In addition to its other roles, its plant extract is a very good nitrification inhibitor (Mahmood et al. 2014). Kanchan and Jayachandra (1981) also showed that root and leaf extract of Parthenium hysterophorus effectively reduced the Nitrosomonas and Nitrobacter population in the soil resulting in inhibited nitrite production. Low pH also contributes to the inhibition of the nitrification process (Rose et al. 2020). Yet no study was conducted to coat such naturally occurring waste material extracts, especially Parthenium and cow dung extract to manage the nitrogen use efficiency. Thus based on these studies, it is clear that coating of organic extracts mixed with the polymer on urea could be beneficial for N management. In this way, an additional benefit of optimization of organic extracts will also be achieved. Thus, it was hypothesized that polymer mixed neem oil, Parthenium extract, and acidulated cow dung compost extract may reduce the nitrification losses of nitrogen in addition to the slow release of available N from urea fertilizer. So, in the present study, the effect of polymer mixed neem oil, Parthenium extract, and acidulated cow dung compost extract coated urea is compared with the uncoated urea for N release, NH4+, and NO3− concentrations in the soil and percent nitrified N at different time intervals.
2 Materials and Methods
A laboratory experiment was performed at Soil Fertility and Plant Nutrition Laboratory (SFPNL), Institute of Soil and Environmental Sciences (ISES), University of Agriculture, Faisalabad (UAF) to investigate the comparative effect of neem oil, Parthenium extract, and acidulated cow dung compost extract coated urea on nitrification potential and N release kinetics. Three replications of each treatment were designed according to the completely randomized design (CRD). The treatment plan was consisted of the following:
-
a)
Three concentrations of neem oil (1, 2, and 3%)
-
b)
Three concentrations of Parthenium extract (5, 10, and 15%)
-
c)
Three pH based cow dung extracts (2 pH, 4 pH, and 6 pH)
-
d)
Positive control (uncoated urea)
-
e)
Negative control (without fertilizer)
Urea coating with Parthenium extract, neem oil, and acidulated cow dung extracts was carried out in SFPNL, ISES, UAF. Neem oil was extracted by using a solvent extraction method (Puri 1999), while Parthenium extract was collected by following Anteneh and Mendesil method (Netsere and Mendesil 2011). A 10% Cow dung compost (with different pH) extract was collected from the Soil and Environmental Microbiology Laboratory, ISES, UAF. Oil and organic extracts were coated on urea granules with polymer as a binding agent. To avoid any contamination, controlled Laboratory conditions were maintained for all activities. Shade drying of coated urea was done and stored at room temperature (25 °C).
The experiment was conducted in the incubator by using disposable cups (250 cm3), and each cup was filled with 100 g soil after determining the physicochemical properties of the soil (Table 1). In all the cups, field capacity was maintained after saturation percentage measurement. Urea fertilizer was added in cups at the rate of 1 g in each cup (having 460 mg N). After the addition of soil, fertilizer, and water, the weight of cups was maintained at an interval of 24 h with distilled water. The whole experiment was carried out at 25 ± 1 °C and NH4+ and NO3− were measured after 20, 40, and 60 days of incubation through the indophenol blue method and phenoldisulphonic acid method, respectively (Keeney and Nelson 1989). Sum of NH4+ and NO3− was taken to determine cumulative nitrogen (N), while nitrate to ammonium ratio, percent nitrified N, and N release efficiency (Tong et al. 2018) were calculated by using the following formulas:
All collected data were analyzed according to completely randomized design (CRD) with factorial arrangements following Fisher’s analysis of variance (Steel et al. 1997). The mean comparison was done with the Tuckey HSD test.
3 Results
3.1 Ammonium Concentration in the Soil
Ammonium concentration in the soil was significantly controlled by all three types of extracts used for coating the urea fertilizer (Table 2). Uncoated urea gave the highest concentration of ammonium in the soil at the first interval that was significantly reduced at later intervals. While in the case of coated fertilizers, ammonium concentration showed an opposite trend as ammonium concentration was lower at the first interval, but this release was increased at later intervals. In the case of coated fertilizers, the release was maximum at the second interval in all types of coating materials, even differences were non-significant between organic amendments but in close view, Parthenium extract and acidulated cow dung extract showed slightly higher release than neem oil. At the third interval, release of ammonium was reduced in comparison to the second interval but still, release was significantly higher in coated fertilizers than uncoated fertilizer. Ammonium release was higher at a lower concentration of organic amendments but was reduced with an increase in the concentration. Ammonium release was only lower after concentration increased in the third interval.
3.2 Nitrate Concentration in the Soil
The uncoated urea showed maximum nitrate concentration at the first interval that was lowered at latter intervals but still higher in comparison to the coated treatments. In coated fertilizer treated jars, nitrate concentration was significantly lower in comparison to uncoated urea. At latter intervals, the concentration of nitrate was slightly increased in coated treatments but still lower in comparison to uncoated urea showing the inhibition of the nitrification process in the soil. Among concentrations of organic amendments, lower concentrations showed more reliable results. Among the type of organic amendments, acidulated cow dung extract showed slightly higher nitrate concentration in the soil, while other amendments showed slightly lower concentrations (Table 3).
3.3 Cumulative N Concentration in the Soil
Table 4 showed that the concentration of cumulative N was significantly lower in the coated treatments in comparison to the uncoated treatment at the first interval. While the release was increased with time in coated treatments but decreased in uncoated treatment. All organic amendments performed almost equally in N release, though acidulated cow dung extract coated urea showed a slight increase in the N release. At the last interval, N release was decreased in all treatments but the decrease in the release was significantly more in uncoated treatment than coated treatments. It was also observed that lower concentrations of organic extracts gave more trustworthy results as cumulative N release was lower at the first interval but increased at the second interval and this release was more in comparison to higher concentrations, even at third interval release was higher with lower concentrations.
3.4 Nitrate to Ammonium Ratio
Nitrate to ammonium ratios given in Fig. 1 depicted that nitrate to ammonium ratio was higher in both control treatments. While in organic extracts and oil-coated fertilizer treatments, nitrate to ammonium ratios were significantly lower in comparison to treatments without extracts. Though at all intervals nitrate to ammonium ratio was higher in uncoated urea treatment, the highest ratio was observed at the second interval. In coated treatments, nitrate to ammonium ratios were higher at the first and last interval in comparison to second intervals. Among extracts, non-significant results were seen between Parthenium extract, neem oil, and acidulated cow dung extract, though acidulated cow dung showed a slightly higher ratio.
Nitrate to ammonium ratios for treatments at 20, 40, and 60 days interval. Negative control, no fertilizer; Positive control, uncoated urea; PE, Parthenium extract; NO, neem oil; ACE, acidulated cow dung compost; Means not sharing the same letters differ significantly (at 5% probability) by Tukey HSD test
3.5 Percent Nitrified N (%)
Figure 2 illustrated the percent nitrified N for all treatments at all intervals. These results clearly stated that the nitrification process was significantly reduced with the use of organic extracts coated urea in comparison to treatments not receiving extracts. Though all the extracts performed significantly to lower the nitrification process, the results of Parthenium extract were much better. Among the concentrations of these extracts, all concentrations showed significant results, even lower concentrations also performed equally to that of higher concentrations.
Percent nitrified N (%) for treatments at 20, 40, and 60 days interval. Negative control, no fertilizer; Positive control, uncoated urea; PE, Parthenium extract; NO, neem oil; ACE, acidulated cow dung compost; Means not sharing the same letters differ significantly (at 5% probability) by Tukey HSD test
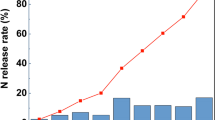
3.6 N release Efficiency (%)
N release efficiency was highest at the first interval for uncoated urea but it was reduced significantly at latter intervals and even at 60 days interval, its release efficiency was just 12% (Fig. 3). In the case of coated treatments, release efficiency was lower at the first interval but at the second interval, it was increased significantly. At the third interval, a slight decline was seen in N release efficiency by the coated treatments but the release was still much higher than uncoated treatment. Among different types of coating materials, N release efficiency was slightly higher in Parthenium extract coated urea than other coating materials.
4 Discussion
The use of chemical fertilizers is a basic commodity in today’s agricultural systems (Yan et al. 2020). Specifically, nitrogen (N) fertilizers are much important as nitrogen is an essential macronutrient for all plants (Li et al. 2021). But with the addition of N fertilizer in the soil, several problems originate, as much of the applied N is lost via nitrification and subsequent denitrification processes (Belete et al. 2018; Van Eerd et al. 2017). It is stated in many studies that applied N undergoes several interactions with loss pathways, chemical reactions, soil microbes, and plant roots (Castellano-Hinojosa et al. 2019; Abbas et al. 2020). On oxidation, by the nitrifying bacteria, ammonium first converts to nitrite then to nitrate in two consecutive steps. During this process, nitrogen might be lost as nitrous oxide or nitrite form (Zhu et al. 2021). The final product of this reaction is NO3− which might also be lost via nitrate leaching or denitrification process resulting in groundwater, surface water, and atmospheric contamination (Wang et al. 2019). Thus, it is very crucial to control the nitrogen dynamics in the soil. A laboratory experiment was conducted at SFPN, ISES, UAF to check the effect of polymer mixed organic extracts and oil-coated urea on the N release efficiency, percent nitrified N, and respective ammonium and nitrate concentrations in the soil.
Results depicted that the ammonium concentration was much higher in comparison to nitrate concentration in all treatments at all intervals possibly due to the presence of amide N in urea fertilizer. Each urea molecule contains two amide groups; these amide groups could easily be converted to ammonium form on hydrolysis of urea. While conversion to nitrate requires more time to stay in the environment, nitrate formation requires ammonium to be formed first for the process of nitrification. Our results are in line with the findings of Sigurdarson et al. (2018) who reported the conversion of urea to ammonia and carbonic acid upon hydrolysis. That ammonia is further converted to ammonium, as ammonia is an unstable product.
Furthermore, our outcomes on nitrate to ammonium ratio and percent nitrified N demonstrated the reduced nitrification process in the organic extracts and oil-coated urea treatments as compared to the uncoated urea. The reduction in nitrification might be due to the nitrification inhibiting compound present in these organic amendments (Wu et al. 2017; Plaimart et al. 2021), as Parthenium extract contain parthenin, phenolics, and terpenes that are having nitrification inhibiting potential (Chandrasena and Narayana 2018). Similarly, neem oil contains melacin to inhibit the nitrification process (Savich and Novik 2021). Anyhow, acidulated cow dung compost extract at low pH also reduced percent nitrified N that could be due to its low pH character, as low pH is itself a nitrification inhibitor. With a decrease in pH, nitrification reduces and even ceases below pH 6 (Fumasoli et al. 2017). It was observed in the present study that low concentrations of these extracts and oil reduced nitrified N percentage in consistence with that of higher concentrations. Thus, 5% Parthenium extract, 1% neem oil, and 2 pH acidulated cow dung compost extract were found sufficient for this purpose in consistence with the findings of Kumar et al. (2011) who reported a decline in net nitrification and enhanced nitrogen use efficiency in lowland irrigated rice with lower concentration of neem oil. They reported that 500 mg kg−1 neem oil was found sufficient for coating on urea granules to reduce nitrification process and to enhance nitrogen use efficiency of rice.
Our findings showed that all the coating materials significantly reduced the N release efficiency by lowering the release of ammonium, nitrate, and cumulative N in the soil in comparison to uncoated urea at earlier intervals. Though an increase in release efficiency was seen at latter intervals from coated urea, that was not much higher, while in uncoated urea the release of N was significantly lowered at latter intervals than the first interval. That trend could be due to the reason that coating material acts as a physical barrier against the counter-ions and water molecules allowing only a limited amount to be passed through very small pores on its surface. This character of coating material usually slows the process of nutrient release from the fertilizer granule. In a similar study, Trinh et al. (2015) established a model to state the release kinetics of N from coated urea which clearly described that the release of N was lower in coated fertilizer than uncoated.
Overall, all coating materials behaved almost equally in controlling the release of plant-available N in consistence with the crops N demand. As early seedlings require a small amount of available N but at latter plant stages, the established plants require more N. Antimicrobial characteristics of the extracts and oil might lower the degradation process of polymer coated on the fertilizer granule to control the N release. So, this controlled release might be a consequence of this trait. In line with our findings, Shivay et al. (2016) reported that sulfur coated urea slowed the N release due to its antimicrobial properties. Among organic extracts and oil, Parthenium extract performed relatively better than all others. It might be due to the allelochemicals present in Parthenium extract that could act as inhibitors for nearby plants and microbial populations. Thus, a prominence concerning nitrification inhibition and controlled release of N than other organic amendments would be a consequence of these allelochemicals. In a similar manner, Shafiq et al. (2020) reported allelopathic effects of Parthenium. Thus, based on the above discussion, it is clear that using polymer mixed Parthenium extract, neem oil, and acidulated cow dung compost extract for coating on urea could helpfully enhance the nitrogen use efficiency in addition to the eradication of these waste materials in a positive way.
5 Conclusion
Based on our findings we concluded the organic extracts coated fertilizers have the potential to control and release N effectively, especially the Parthenium extract, neem oil, and acidulated cow dung compost extract. Our results provided new insight on urea coating by these extracts that could reduce nitrification chances and supply ammonium consistently which is essential for field crop demand. Future research should focus on clarifying the mechanism of biochemical interaction, assessing the contribution of respective microbial populations to the long-term nitrogen in the soil.
6 Data Availability Statement
All data of this manuscript is available from the corresponding author on request.
References
Abbas Q, Yousaf B, Ali MU, Munir MAM, El-Naggar A, Rinklebe J, Naushad M (2020) Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: a review. Environ Int 138:105646. https://doi.org/10.1016/j.envint.2020.105646
Anas M, Liao F, Verma KK, Sarwar MA, Mahmood A, Chen ZL, Li Q, Zeng XP, Liu Y, Li YR (2020) Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol Res 53:1–20. https://doi.org/10.1186/s40659-020-00312-4
Beig B, Niazi MBK, Jahan Z, Kakar SJ, Shah GA, Shahid M, Zia M, Haq MU, Rashid MI (2020) Biodegradable polymer coated granular urea slows down N release kinetics and improves spinach productivity. Polymers 12:2623. https://doi.org/10.3390/polym12112623
Belete F, Dechassa N, Molla A, Tana T (2018) Effect of nitrogen fertilizer rates on grain yield and nitrogen uptake and use efficiency of bread wheat (Triticum aestivum L.) varieties on the Vertisols of central highlands of Ethiopia. Agric Food Sec 7:1–12. https://doi.org/10.1186/s40066-018-0231-z
Byrne MP, Tobin JT, Forrestal PJ, Danaher M, Nkwonta CG, Richards K, Cummins E, Hogan SA, O’Callaghan TF (2020) Urease and nitrification inhibitors—as mitigation tools for greenhouse gas emissions in sustainable dairy systems: a review. Sustainability 12:6018. https://doi.org/10.3390/su12156018
Castellano-Hinojosa A, González-López J, Vallejo A, Bedmar EJ (2019) Linking ammonia volatilization with moisture content and abundance of nitrification and denitrification genes in N-fertilized soils. In ‘Microbial Probiotics for Agricultural Systems’. Springer, Cham, pp 29–43. https://doi.org/10.1007/978-3-030-17597-9_3
Chandrasena N, Narayana A (2018) Parthenium weed: uses and Parthenium Weed. Biol Ecol Manag 7:190
Freedman B (2018) ~ Flows and cycles of nutrients. In ‘Environmental Science’. (Dalhousie Libraries)
Fumasoli A, Bürgmann H, Weissbrodt DG, Wells GF, Beck K, Mohn J, Morgenroth E, Udert KM (2017) Growth of Nitrosococcus-related ammonia oxidizing bacteria coincides with extremely low pH values in wastewater with high ammonia content. Environ Sci Technol 51:6857–6866. https://doi.org/10.1021/acs.est.7b00392
Hatano S, Fujita Y, Nagumo Y, Ohtake N, Sueyoshi K, Takahashi Y, Sato T, Tanabata S, Higuchi K, Saito A, Ohyama T (2019) Effect of the nitrification inhibitor 3, 4 dimethylpyrazole phosphate on the deep placement of nitrogen fertilizers for soybean cultivation. Int J Agron 2019:9724214. https://doi.org/10.1155/2019/9724214
Itelima JU, Bang WJ, Onyimba IA, Sila MD, Egbere OJ (2018) Bio-fertilizers as key player in enhancing soil fertility and crop productivity: a review. Direct Res J Agric Food Sci 6:73–83. http://hdl.handle.net/123456789/1999
Jain V, Abrol YP (2017) Plant nitrogen use efficiency. In ‘The Indian Nitrogen Assessment’. Elsevier, pp 163–173. https://doi.org/10.1016/B978-0-12-811836-8.00011-2
Kanchan S, Jayachandra (1981) Effect of Parthenium hysterophorus on nitrogen-fixing and nitrifying bacteria. Can J Bot 59(2):199–202. https://doi.org/10.1139/b81-030
Kaur M, Aggarwal NK, Kumar V, Dhiman R (2014) Effects and Management of Parthenium hysterophorus: a weed of global significance. Int Sch Res Notices 2014:368647. https://doi.org/10.1155/2014/368647
Keeney DR, Nelson DW (1989) Nitrogen inorganic forms. In ‘Methods of soil analysis part 2. Chemical and microbiological properties. Madison (WI): Soil Science Society of America and American Society of Agronomy’. pp 643–698
Kumar D, Devakumar C, Kumar R, Panneerselvam P, Das A, Shivay YS (2011) Relative efficiency of prilled urea coated with major neem (Azadirachta indica A. Juss) oil components in lowland irrigated rice of the Indo-Gangetic plains. Arch Agron Soil Sci 57:61–74. https://doi.org/10.1080/03650340903223144
Lasisi AA, Akinremi OO, Kumaragamage D (2019) Efficacy of a new N-(n-butyl) thiophosphorictriamide formulation in reducing ammonia volatilization from urea-based fertilizers. Can J Soil Sci 99:395–405. https://doi.org/10.1139/cjss-2018-0072
Leghari SJ, Wahocho NA, Laghari GM, HafeezLaghari A, MustafaBhabhan G, HussainTalpur K, Bhutto TA, Wahocho SA, Lashari AA (2016) Role of nitrogen for plant growth and development: a review. Adv Environ Biol 10:209–219
Lei T, Gu Q, Guo X, Ma J, Zhang Y, Sun X (2018) Urease activity and urea hydrolysis rate under coupling effects of moisture content, temperature, and nitrogen application rate. Int J Agric Biol Eng 11:132–138. https://doi.org/10.25165/j.ijabe.20181102.3784
Li HF, An SD, Zhang L, Peng H, Ma W, Meng X, Ye HM (2021) Urea fertilizer with precisely regulable slow-release performance by complexing with random copolyester. J Environ Chem Eng 9:105120. https://doi.org/10.1016/j.jece.2021.105120
Mahmood R, Shahid AA, Usmani A, Haider MS, Ali S (2014) Nitrification inhibition potential of various leaf extracts. Philipp Agric Sci 97:287–293
Netsere A, Mendesil E (2011) Allelopathic effects of Parthenium hysterophorus L. aqueous extracts on soybean (Glycine max L.) and haricot bean (Phaseolus vulgaris L.) seed germination, shoot and root growth and dry matter production. J Appl Bot Food Qual 84:219–222
Noor S, Yaseen M, Naveed M, Ahmad R (2017) Use of controlled release phosphatic fertilizer to improve growth, yield and phosphorus use efficiency of wheat crop. Pak J Agric Sci 54:541–547. https://doi.org/10.21162/PAKJAS/18.6533
Opoku A, Chaves B, De Neve S (2014) Neem seed oil: a potent nitrification inhibitor to control nitrate leaching after incorporation of crop residues. Biol Agric Hortic 30:145–152. https://doi.org/10.1080/01448765.2014.885394
Plaimart J, Acharya K, Mrozik W, Davenport RJ, Vinitnantharat S, Werner D (2021) Coconut husk biochar amendment enhances nutrient retention by suppressing nitrification in agricultural soil following anaerobic digestate application. Environ Pollut 268:115684. https://doi.org/10.1016/j.envpol.2020.115684
Puri HS (1999) Neem-the divine tree. Harwood academic publishers, Amsterdam
Rose TJ, Kearney LJ, Van Zwieten L, Rose MT (2020) Low pH of a high carbon gleysol contributes to nitrification inhibition resulting in low N2O soil emissions and limited effectiveness of nitrification inhibitors. Soil Syst 4:75. https://doi.org/10.3390/soilsystems4040075
Savich V, Novik G (2021) Waste biodegradation and utilization by Pseudomonas species. J Microbiol Biotechnol Food Sci 2021:851–857. https://doi.org/10.15414/jmbfs.2016.6.2.851-857
Shafiq F, Irfan S, Shakir SK (2020) Comparative allelopathic effects of different parts of Parthenium hysterophorus L. on seed germination and biomasses of Cicer arietinum L. J Stress Physiol Biochem 16:64–75
Shivay YS, Pooniya V, Prasad R, Pal M, Bansal R (2016) Sulphur-coated urea as a source of sulphur and an enhanced efficiency of nitrogen fertilizer for spring wheat. Cereal Res Commun 44:513–523. https://doi.org/10.1556/0806.44.2016.002
Sigurdarson JJ, Svane S, Karring H (2018) The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev Environ Sci Biotechnol 17(2):241–258. https://doi.org/10.1007/s11157-018-9466-1
Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics, 2nd edn. McGraw Hill Inc., New York, USA
Tao H, Morris TF, Kyveryga P, McGuire J (2018) Factors affecting nitrogen availability and variability in cornfields. Agron J 110:1974–1986. https://doi.org/10.2134/agronj2017.11.0631
Tong X, He X, Duan H, Han L, Huang G (2018) Evaluation of controlled release urea on the dynamics of nitrate, ammonium, and its nitrogen release in black soils of northeast China. Int J Environ Res Public Health 15:119. https://doi.org/10.3390/ijerph15010119
Trinh TH, Kushaari K, Shuib AS, Ismail L, Azeem B (2015) Modelling the release of nitrogen from controlled release fertiliser: constant and decay release. Biosyst Eng 130:34–42. https://doi.org/10.1016/j.biosystemseng.2014.12.004
Van Eerd LL, Turnbull JJD, Bakker CJ, Vyn RJ, McKeown AW, Westerveld SM (2017) Comparing soluble to controlled-release nitrogen fertilizers: storage cabbage yield, profit margins, and N use efficiency. Canadian J Plant Sci 98:815–829. https://doi.org/10.1139/cjps-2017-0277
Wang Y, Ying H, Yin Y, Zheng H, Cui Z (2019) Estimating soil nitrate leaching of nitrogen fertilizer from global meta-analysis. Sci Total Environ 657:96–102. https://doi.org/10.1016/j.scitotenv.2018.12.029
Wu D, Senbayram M, Well R, Brüggemann N, Pfeiffer B, Loick N, Stempfhuber B, Dittert K, Bol R (2017) Nitrification inhibitors mitigate N2O emissions more effectively under straw-induced conditions favoring denitrification. Soil Biol Biochem 104:197–207. https://doi.org/10.1016/j.soilbio.2016.10.022
Yan S, Wu Y, Fan J, Zhang F, Zheng J, Qiang S, Guo J, Xiang Y, Zuo H, Wu L (2020) Dynamic change and accumulation of grain macronutrient (N, P and K) concentrations in winter wheat under different drip fertigation regimes. Field Crops Res 250:107767. https://doi.org/10.1016/j.fcr.2020.107767
Yaseen M, Ahmad A, Naveed M, Ali MA, Shah SSH, Hasnain M, Ali HM, Siddiqui MH, Salem MZ, Mustafa A (2021) Subsurface-applied coated nitrogen fertilizer enhanced wheat production by improving nutrient-use efficiency with less ammonia volatilization. Agron 11:2396. https://doi.org/10.3390/agronomy11122396
Yaseen M, Aziz MZ, Abbas T (2016) Phosphorus release pattern from polymer coated diammonium phosphate: effect of coating concentrations, layers and soil moisture. Conference on Sustainable Crop and Animal Production System, 8–10, December, 2016, the University of Haripur, Pakistan
Zhang W, Yu C, Wang X, Hai L (2020) Increased abundance of nitrogen transforming bacteria by higher C/N ratio reduces the total losses of N and C in chicken manure and corn stover mix composting. Bioresour Technol 297:122410. https://doi.org/10.1016/j.biortech.2019.122410
Zhang R, Yue Z, Chen X, Wang Y, Zhou Y, Xu W, Huang R (2021) Foliar applications of urea and melatonin to alleviate waterlogging stress on photosynthesis and antioxidant metabolism in sorghum seedlings. Plant Growth Regul, pp 1–10. https://doi.org/10.1007/s10725-021-00705-9
Zhu W, Van Tendeloo M, Xie Y, Timmer MJ, Peng L, Vlaeminck SE (2021) Storage without nitrite or nitrate enables the long-term preservation of full-scale partial nitritation/anammox sludge. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151330
Acknowledgements
I want to acknowledge the lab staff of Soil Fertility and Plant Nutrition Lab and Soil and Environmental Microbiology Lab for their kind support during the conduction of the trial. I also want to acknowledge my supervisor for his guidance and support. I am thankful to the Higher Education Commission (HEC) Pakistan for their research support initiative under the Indigenous PhD fellowship.
Funding
The study and its the research activities were funded by the Higher Education Commission (HEC) Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, A., Yaseen, M., Asghar, H.N. et al. Comparative Effect of Various Organic Extracts Coated Urea Fertilizer on the Release Pattern of Ammonium and Nitrate in the Soil at Different Time Intervals. J Soil Sci Plant Nutr 22, 2603–2611 (2022). https://doi.org/10.1007/s42729-022-00830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00830-y