Abstract
Harvesting fallen needles (straw) in loblolly pine (Pinus taeda L.) plantations provides forest owners with a substantial source of income, but this practice and the type of fertilizer used to replenish nutrients removed with straw harvests may alter soil microbiological and physical properties. This study was conducted to explore the influence of annual straw harvesting, fertilization, and fertilizer source (inorganic vs. broiler poultry litter) in a loblolly pine plantation in the mid-south USA on: (1) soil microbial biomass C, (2) soil dehydrogenase activity, and (3) key soil physical properties (soil strength, bulk density, porosity, aeration, soil moisture content, organic matter, and available water holding capacity). All treatments that included straw harvesting increased bulk density and reduced soil porosity. Annual straw harvesting conducted with annual fertilization of inorganic nitrogen and phosphorus fertilization was associated with the most pronounced increases in soil strength and reductions in organic matter, available water holding capacity, microbial biomass C, and dehydrogenase activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Harvesting of fallen needles (straw) in the abundant southern pine forests of the southeastern USA for use as mulch can improve profitability of plantation management by providing a substantial mid-rotation source of income (Lopez-Zamora et al. 2001). Pine straw mulch has emerged as a substantial commercial product for horticultural crops and landscaping in urban and suburban areas (Duryea and Edwards 1989). Adding straw harvesting to conventional timber management regimes has been shown to markedly increase profits, with straw revenue potentially exceeding that of traditional forest products (Roise et al. 1991; Haywood et al. 1998). Depending on market and plantation conditions, net revenues of straw harvesting range from $172 to $427 ha−1 year−1 in the southeastern USA (Haywood et al. 1998).
Soil organic matter removals inherent with straw harvesting may be detrimental to soil biological properties that serve as indicators of ecological sustainability. Fallen pine straw is the predominant organic matter in the soil organic horizon of pine forests, and it is the major reservoir of carbon used by soil microbes as energy substrates and carbon sources in the synthesis of new cells (Pritchett and Fisher 1987; Wagner and Wolf 1999). Soil microbial biomass and activity are highly sensitive to changes in soil organic matter and are thus used as indicators of soil quality and sustainability (Powlson and Brookes 1987; Fauci and Dick 1994; Harris 2003). Removal of the forest floor (a mixture of leaves and woody debris) decreased soil microbial biomass carbon (C mic) due to reduced substrate availability in a study simulating organic matter removals associated with tree harvesting and site preparation in a boreal forest (Tan et al. 2005). Straw raking may have a more pronounced impact on soil microbial biomass in pine plantations of the southeastern USA due to its relatively warmer and wetter climate, but such information is lacking.
Activities other than soil organic matter removal associated with straw harvesting may also impact soil biological properties. Understory biomass is typically suppressed in straw harvesting management regimes to improve straw quality by eliminating woody and herbaceous debris (Mills and Robertson 1991). Since this understory vegetation can provide rhizodeposition (Gallardo and Schlesinger 1994; Donegan et al. 2001; Högberg et al. 2001), its suppression may thus reduce microbial biomass and activity. Significant declines in microbial biomass have been found in response to vegetation suppression in forests (Busse et al. 1996, 2006), but a lack of changes in microbial communities has been reported also (Busse et al. 2001). Busse et al (2001) attributed the absence of changes in microbial biomass in response to vegetation suppression to the presence of relatively weathered, nutrient-rich soils and the warm climate of California in their study that fostered rapid equilibrium of C inputs and outputs. Such conditions may influence treatment responses in the southeastern USA as well. Biochemical transformations of organic matter conducted by soil microbes are essential for fulfilling most of the nutrient requirements of trees (Diaz-Raviña et al. 1993; Gallardo and Schlesinger 1994; Blazier et al. 2005). Thus, periodic fertilization has been recommended to remedy nutrient removals that can occur with straw harvesting (Haywood et al. 1998; Lopez-Zamora et al. 2001). Fertilizing annually raked stands has been shown to maintain (Haywood et al. 1995) or increase longleaf pine (Pinus palustris Mill.) straw production (Dickens 1999) over that of unfertilized raked stands, presumably due to improving soil nutrition. However, fertilization of a loblolly pine (Pinus taeda L.) plantation in tandem with removal of understory biomass has been shown to reduce microbial biomass and dehydrogenase activity by accelerating microbial utilization of a reduced substrate pool (Blazier et al. 2005). Annual or semi-annual traffic with straw harvesting and fertilization equipment has been shown to increase soil bulk density due to compaction (Haywood et al. 1998), which can alter microbial biomass and activity. Soil compaction has been shown to decrease microbial biomass due to low oxygen supplies in compacted soil (Kaiser et al. 1991). However, other studies have shown no changes (Tan et al. 2005; Busse et al. 2006) or increases (Breland and Hansen 1996) in microbial biomass in response to compaction. Powers et al. (2005) found that soil compaction of a wide range of forest soils was dependent on initial bulk density; generally, soils with densities greater than 1.4 Mg m−3 were resistant to compaction. Their study examined effects of traffic at the time of forest harvesting, but repeated traffic associated with annual straw harvesting may differ from forest harvesting in its capacity for soil compaction.
Inorganic fertilizers do not replenish organic matter essential as microbial substrates and may exacerbate soil microbial biomass and activity declines caused by organic matter removal (Blazier et al. 2005). Addition of animal wastes such as broiler poultry litter (a mixture of manure and bedding material from broiler poultry production facilities), which has carbon concentrations of 44% (Adeli et al. 2005), can increase soil microbial biomass and activity in horticultural (Canali et al. 2004) and agricultural (Plaza et al. 2004) soils. Applications of poultry litter have been shown to remedy N and P deficiencies of loblolly pine plantations (Samuelson et al. 1999; Friend et al. 2006) and reduce bulk density (Brye et al. 2004). Due to the robust broiler poultry production industry of the southeastern USA, land application options for poultry litter are sought in some portions of the region due to concerns about the environmental consequences of repeated litter disposal to pastures (Brye et al. 2004). Many broiler poultry production facilities of the region are within the natural range of loblolly pine, which can facilitate use of poultry litter in pine plantations (Friend et al. 2006). However, the influences of broiler litter as a fertilizer source in straw harvesting regimes has not been studied.
Understanding the interactive effects of organic matter removal, fertilization, and fertilizer source on soil microbiological and physical conditions is essential for developing ecologically sustainable management practices for intensive straw harvesting regimes. The objective of this study was to determine the effects of annual straw harvesting, fertilization, and fertilizer source on: (1) soil microbial biomass C (C mic), (2) soil dehydrogenase activity, and (3) key soil physical properties (soil strength, bulk density, porosity, aeration, soil moisture content, organic matter, and available water holding capacity).
Materials and methods
Study site
The study was conducted in a loblolly pine plantation at the LSU AgCenter’s Calhoun Research Station in north central Louisiana (32°30′48″ N, 92°20′53″ W). Soils of the study area are USDA NRCS series Ora and Savannah series, which are fine-loamy, siliceous, thermic Typic Fragiudults (Matthews et al. 1974). The plantation was mechanically planted with 1,119 trees per hectare on retired pasture land in 1990. In May 2000, the plantation was thinned from below to a residual density of 618 trees per hectare.
Treatments
Four treatment regimes, each replicated four times, were conducted for this study:
-
1.
No pine straw harvesting with no fertilization (CONTROL)
-
2.
Annual pine straw harvesting with no fertilization (RAKE)
-
3.
Annual pine straw harvesting with annual application of inorganic fertilizers (RAKE-IN)
-
4.
Annual pine straw harvesting with annual application of broiler poultry litter (RAKE-PL)
The CONTROL treatment was conducted to provide an untreated control. The RAKE treatment was carried out to isolate the effects of straw raking on soil microbiological and physical properties. The RAKE-IN and RAKE-PL treatments were conducted to compare the effects of fertilizer source on soil microbial and physical properties in raked soils. Treatments were applied in a randomized complete block design, with soil type as a blocking factor, to 0.1-ha plots. Pine straw harvesting in plots receiving treatments that included straw raking was initiated in fall 2000, and harvesting continued each fall through 2005. Straw harvesting was conducted using a tractor-drawn mechanical rake and baler. Woody and herbaceous vegetation were suppressed prior to initiation of annual straw harvests by applying 4.7 l ha−1 imazapyr (2-[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl]-3-pyridinecarboxylic acid) with a tractor-mounted sprayer in June 2000. In summers before each straw harvest, woody debris was manually removed, and plots designated for straw harvest were rotary-mowed. Both fertilization treatments supplied 193 kg N ha−1 and 102 kg P ha−1. The N application rate was similar to the 224 kg N ha−1 rate recommended by Morris et al. (1992) for southern pine plantations in which straw is annually harvested, with the slight deviation due to calibration limitations of fertilizer-spreading equipment. The use of poultry litter in this study necessitated exceeding the 58 kg P ha−1 rate recommended by Morris et al. (1992) for annually raked pine plantations. Applying the poultry litter with a target N rate of 193 kg N ha−1 was associated with the application of 102 kg P ha−1 as a consequence of the N and P contents of poultry litter, and the application rates of inorganic fertilizers were adjusted to match P applied via poultry litter for consistency among treatments. For the RAKE-IN treatment, N and P were supplied as a mixture of urea and diammonium phosphate applied with a tractor-mounted cyclone seeder. For the RAKE-PL treatment, N and P were supplied as broiler litter purchased from a local broiler poultry production facility that was typically stacked outside of the facility for at least 3 weeks prior to delivery. A tractor-drawn manure spreader was used to broadcast-apply broiler litter to plots. Litter was left on the soil surface after application. Subsamples of litter were collected to determine N and P concentrations of the litter, which averaged 26 g N kg−1 and 13 g P kg−1. Analyses of other nutrients in litter were precluded by budget and laboratory constraints, but Blazier et al. (2008) reported 28 g K kg−1, 28 g Ca kg−1, 6 g Mg kg−1, 2395 mg Fe kg−1, 424 mg Mn kg−1, 263 mg Cu kg−1, 404 mg Zn kg−1, and 45 mg B kg−1 in litter from a similar broiler production facility within 65 km of the facility from which litter was obtained in this study. Application rates of poultry litter averaged 8 Mg ha−1. Nitrogen concentrations of the litter samples were analyzed by the Kjeldahl procedure (Munsinger and McKinney 1982). Phosphorus was determined by nitric acid digestion followed by analysis with inductively coupled plasma spectrometry (Zarcinas et al. 1987) on a Thermo-Jarrell Enviro II (Thermo-Jarrell Ash, Franklin, MA, USA).
Microbial biomass C and dehydrogenase activity
Soil samples to be monitored for C mic and dehydrogenase activity were taken seasonally. Samples were taken in August 2005, November 2005, February 2006, and May 2006. A 2.5-cm diameter punch auger was used to take six samples to a depth of 10 cm from randomly placed subsampling points in each of the 0.1-ha plots. Any organic matter on the soil surface was brushed away immediately prior to sample collection. In each plot, six samples were collected from randomly placed subsampling points; these six subsamples were composited into a single sample for each plot. Samples were refrigerated at 5°C during transportation and storage; samples were stored for a maximum of 4 days prior to C mic and dehydrogenase activity assessment. Mean monthly soil temperatures and total monthly precipitation for the months in which sampling occurred were collected from the climatic reports collected for the Calhoun Research Station as part of the Louisiana Agriclimatic Information System (Louisiana State University Agricultural Center, http://www.lsuagcenter.com/mcms/webtools/viewExternal.aspx?url=http ://www2.lsuagcenter.com/weather/).
The chloroform fumigation–incubation method was used to determine C mic (Jenkinson and Powlson 1976a,b; Luizao et al. 1992). Procedures included a 10-day pre-incubation of soil samples at 25°C followed by fumigation with alcohol-free CHCl3 vapor for 24 h. Samples were incubated at 25°C for 10 days. Respired CO2 was collected with 2 M NaOH, and CO2 was quantified by titration with 0.1 N HCl. The equation developed by Horwath et al. (1996) was used to convert CO2–C to C mic due to the equation’s standardization against direct microscopy and its close correlation with C mic observed by direct microscopy on a broad range of soils. Results are expressed as mg C kg−1 soil on an oven-dry soil basis (105°C, 24 h).
Microbial activity was estimated by determining dehydrogenase activity (Lenhard 1956; Alef 1995). Dehydrogenase, which is only active in viable living cells, serves as an indicator of total microbial metabolic activity (Tabatabai 1994; Camiña et al. 1998). To quantify dehydrogenase, triphenyltetrazolium chloride (TTC) was used as an artificial electron acceptor. Dehydrogenase reduces TTC to red-colored triphenyl formazan (TPF) that can be extracted with methanol and quantified colorimetrically (Thalmann 1968). Results are expressed as μg TPF kg−1 soil on an oven-dry soil basis (105°C, 24 h).
Metabolic activity of microbial communities can be determined by quantifying ratios of intracellular enzymatic activities to soil C mic (Landi et al. 2000; Deng et al. 2006). In this study, microbial metabolic activity was determined for each plot and month as dehydrogenase activity per C mic, as defined by Landi et al. (2000).
Soil physical properties
Six surface (0 to 10 cm) soil cores were randomly taken in all plots in April 2006 using a hammer core sampler with a 4.7-cm diameter and a 10-cm length, and this type of sampling maintained intact the soil surface, which would include any residual broiler litter where applied. Bulk density, porosity, soil moisture content, and air-filled porosity were analyzed for each core sample using procedures of Blake and Hartge (1986) and Danielson and Sutherland (1986). Upon completion of these analyses, values for the subsamples were averaged to yield a plot-level mean for each of the parameters.
In January 2007, six soil core samples were randomly taken in each plot to a 10-cm depth with a 2.5-cm punch auger. Each core was divided into depths of 0 to 5 cm and 5 to 10 cm, and organic matter concentrations were determined in each of these samples using the loss on ignition method (Ben-Dor and Banin 1989). Upon completion of organic matter concentration determination, values for all subsamples per plot were averaged to derive mean organic matter concentrations for each plot and depth. Separate soil core samples were randomly taken in January 2007 using a 2.5-cm punch auger to a 10-cm depth for the determination of available water holding capacity. Six soil samples were randomly subsampled from each plot, and these samples were composited for each plot. A WP4 dewpoint potentiometer (Decagon Devices, Pullman, WA, USA) was used to create soil moisture retention curves from the samples, and available water holding capacity was determined using the soil moisture retention curves (Gee et al. 1992; Brye 2003). In April 2006, soil strength in 0 to 15 and 15 to 30 cm depths was measured with a Scout SCT compaction meter (Spectrum Technologies, Plainfield, IL, USA) at 18 randomly selected sampling points per plot (Bradford 1986). Mean soil strength was derived for each plot and depth.
Statistical analysis
All treatment effects were analyzed for variance (ANOVA) at α = 0.05 using the MIXED procedure of the SAS System (SAS Institute 2006). When an ANOVA indicated significant treatment effects, treatment means were calculated and separated by the DIFF option of the LSMEANS procedure. The DIFF option provided multiple comparisons of treatment means by invoking t tests to determine significant differences between all possible treatment combinations. In order to rectify heterogeneous variances revealed by a null model likelihood ratio test, C mic and dehydrogenase activity values were log-transformed. Microbial biomass C and dehydrogenase activity were analyzed with a repeated measures model with an autoregressive correlation structure with: (1) block, (2) month, (3) treatment, and (4) the interaction between treatment and month as fixed effects. All other variables were measured with a model containing block and treatment as fixed effects. When the null model likelihood ratio test revealed heterogeneous variances in a dataset, the GROUP option of MIXED was utilized to perform ANOVA using different variances for all treatment combinations.
Results and discussion
All treatments that included straw harvesting induced evidence of soil compaction and significantly increased bulk densities (Table 1) to levels 0.6% to 3.3% greater than the 1.75 g cm−3 bulk density defined as a growth-limiting threshold for forests grown on loamy soils (Daddow and Warrington 1983), whereas soil in the CONTROL treatment remained below this threshold. These bulk density increases were also associated with significant declines in porosity in all treatments that included straw harvesting (Table 1). These findings suggest that annual straw harvesting had potential to reduce tree growth through reduced rooting volume and aeration. However, Page-Dumroese et al. (2006) found relatively vigorously growing forests throughout North America on diverse soils exceeding 1.75 g cm−3 in bulk density. In their study of harvesting longleaf pine straw on a sandy loam soil in central Louisiana, Haywood et al. (1998) found that three annual straw harvests increased mean bulk density 7.6% over an unraked control treatment, which was attributed to equipment traffic and increased exposure of mineral soil to rainfall. Our findings of significant 5% to 8% increases in bulk density over the CONTROL treatment after five annual straw harvests compare closely with their results. Based on empirically derived guidelines for soil compaction for US public forestlands, practices such as subsurface tillage to remedy increased bulk densities are not generally recommended unless soil bulk densities are increased by at least 15% relative to untreated areas (Powers et al 1998; Shestak and Busse 2005). Thus, although soil compaction has occurred with annual straw harvesting, the increases in bulk density may not be of significance for maintaining forest growth. Similarities in bulk density and porosity among the RAKE treatment and treatments that included raking and fertilization suggest that the additional trafficking from fertilization equipment each season did not appreciably compact the soil and that straw harvesting was the predominant cause of soil compaction.
Although all treatments that included raking induced similar bulk density and porosity changes, other investigated soil properties differed among treatments (Tables 1 and 2). In the uppermost 5 cm of soil, the RAKE and RAKE-IN treatments both had soil strengths 46% greater than the CONTROL treatment. In the southern USA, soil strength rather than bulk density was found to be the critical impedance factor governing root penetration through soil profiles (Taylor and Burnett 1964). Soil strengths above 2 MPa have been defined as highly compacted because of demonstrated root growth restrictions (Taylor and Gardner 1963; Tiarks and Haywood 1996), and soil strengths of the RAKE and RAKE-IN treatments exceeded 2 MPa. Available water holding capacity was also reduced by the RAKE and RAKE-IN treatments relative to the CONTROL treatment. These findings suggest that the RAKE and RAKE-IN treatments made soil less amenable for root growth in the uppermost 5 cm of soil, which is the predominant zone in which tree roots, particularly fine roots, grow (Gilman 1987).
Because straw harvesting involves removal of pine straw and woody debris as well as suppression of understory vegetation, pine roots would be the predominant source of soil organic matter (in the form of root biomass and exudates) in straw harvesting regimes. Restriction of pine root growth due to compaction could thus reduce belowground supplies of organic matter and exacerbate the impacts of removal of organic matter from the soil surface. Due to the dependence of soil microbial populations on labile sources of soil organic matter, reduced organic matter supplies can reduce soil microbial biomass and activity (Gallardo and Schlesinger 1994; Donegan et al. 2001; Högberg et al. 2001). However, soil organic matter concentrations, C mic, and dehydrogenase activity of the RAKE treatment were similar to those of the CONTROL treatment (Table 2, Fig. 1). The absence of changes in C mic in response to the RAKE treatment contrasts with findings of Tan et al. (2008) in their study of forest floor removal in a boreal forest soil. However, in their study forest floor removal similarly had no effect on dehydrogenase activity. Busse et al. (2006) found that soil microbial biomass and activity (defined as CO2 respiration) were relatively unaffected by complete forest floor removal at several forested sites in North America. In our study, soil microbial biomass and dehydrogenase activity was sustained by the RAKE treatment despite the forest floor removals and apparent compaction resulting from the treatment.
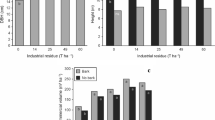
Microbial biomass C (a) and dehydrogenase activity (b) in a loblolly pine plantation in north central Louisiana averaged over four seasonal assessments in response to an absence of straw harvesting and fertilization (CONTROL), annual straw harvesting for 5 years (RAKE), annual straw harvesting and fertilization with an inorganic blend of N and P for 5 years (RAKE-IN), and annual straw harvesting and fertilization with broiler poultry litter for 5 years (RAKE-PL). Vertical bars in each column are standard deviations. For each variable, columns headed by different letters differ significantly at P < 0.05
The RAKE-IN treatment had a more pronounced effect on soil physical and microbiological properties. Relative to the CONTROL treatment, only the RAKE-IN treatment had greater soil strengths at both soil depths, reduced moisture content, and reduced soil organic matter concentrations (Tables 1 and 2). Repeated fertilization with inorganic nitrogen has been shown to reduce soil organic matter concentrations by increasing decomposition rates (Khan et al. 2007). Increased soil strength in response to the RAKE-IN treatment may have been due to the reductions in soil organic matter concentrations caused by this treatment. Soil strength tends to increase with decreasing soil organic matter concentrations because soil organic matter serves as organic aggregate binding and bonding material (Munkholm et al. 2002). The relatively lower moisture content and available water holding capacity of the RAKE-IN treatment is consistent with its lower soil organic matter content because organic matter fosters soil moisture retention (Plaza et al. 2004; Powers et al. 2005). Relatively high microbial metabolic activity found in August 2005 in response to the RAKE-IN treatment provides evidence that inorganic fertilization produced short-term increases in microbial organic matter decomposition (Fig. 2). The relatively low C mic and dehydrogenase activity observed across all measurement periods in response to the RAKE-IN treatment in this study (Fig. 1) is likely due to the propensity of this treatment to reduce soil organic matter.
Dehydrogenase activity/microbial biomass C ratio in a loblolly pine plantation in north central Louisiana in four seasonal assessments in response to an absence of straw harvesting and fertilization (CONTROL), annual straw harvesting for 5 years (RAKE), annual straw harvesting and fertilization with an inorganic blend of N and P for 5 years (RAKE-IN), and annual straw harvesting and fertilization with broiler poultry litter for 5 years (RAKE-PL). Vertical bars in each column are standard deviations. For each variable, columns headed by different letters differ significantly at P < 0.05
Poultry litter did not alter soil physical and biological properties in a manner similar to inorganic fertilizers. The RAKE-PL treatment was characterized by soil moisture content, strength, organic matter concentrations, and available water holding capacity similar to the CONTROL treatment (Tables 1 and 2). However, as with the RAKE-IN treatment, the relatively high microbial metabolic activity found in August 2005 provides some evidence that poultry litter induced short-term stimulations in microbial decomposition of organic matter (Fig. 2). The RAKE-PL treatment may have adequately replenished organic matter lost through straw harvesting and microbial decomposition because broiler poultry litter is relatively carbon-rich (Adeli et al. 2005). Surface applications of poultry litter to agricultural soils have been shown to raise soil organic matter and C contents to a 15-cm depth (Kingery et al. 1994). The RAKE-PL treatment was associated with soil strength, organic matter, moisture, C mic, and dehydrogenase activity similar to the CONTROL treatment (Table 2, Fig. 1). These results suggest that use of poultry litter as a fertilizer source in an annual straw harvest regime was superior to inorganic fertilizers in sustaining soil quality. However, the RAKE-PL treatment was characterized by lower air-filled porosity than the CONTROL treatment (Table 1), so there may have been a compaction potential associated with this treatment (Tekeste et al. 2007).
No treatments altered seasonal trends in C mic and dehydrogenase activity. The C mic values of this study are similar to the range of 100 to 160 mg C kg−1 reported by Lee and Jose (2003) for a 7-year-old fertilized loblolly pine plantation in Florida. The C mic values of this study are somewhat lower than the seasonal range of 150 to 800 mg C kg−1 observed in 3- and 4-year-old loblolly pine plantations receiving fertilization and vegetation suppression treatments in southeastern Oklahoma (Blazier et al. 2005). The dehydrogenase activity values of this study are somewhat lower than the seasonal range of 10 to 100 μg TPF g−1 reported in the Blazier et al. (2005) study. Due to the consistencies in methodology between our study and the Blazier et al. (2005) study, these differences are likely due to differences in soil type and plantation age. The relatively high C mic observed in November 2005 is similar to the findings of Tan et al. (2005), in which seasonal C mic in boreal forests in North America were highest in fall. Probably, these high C mic values in fall depend on root mortality that occurs in this period under loblolly pine (King et al. 2002). Dehydrogenase activity and microbial metabolic activity were likely driven by soil moisture and temperature conditions, with the relatively drier, warmer months exhibiting less activities than wetter, more temperate months (Figs. 3 and 4). Average soil temperatures for August 2005, November 2005, February 2006, and May 2006 were 28°C, 17°C, 10°C, and 24°C, respectively. Total precipitation for the months of August 2005, November 2005, February 2006, and May 2006 was 5.4, 3.6, 18.6, and 5.8 cm, respectively.
Conclusions
Annual straw harvesting had the potential to compact soil, and annually applying inorganic fertilizers exacerbated reductions in rooting volume and moisture availability by promoting more pronounced reductions in soil organic matter. These changes in soil physical properties were associated with reductions in C mic and dehydrogenase activity. Straw harvesting conducted without fertilization and with annual fertilization with broiler poultry litter was not associated with significant changes in soil organic matter, C mic, and dehydrogenase activity. These findings suggest that soil quality in this plantation in an annual straw harvesting regime could be best sustained by either reducing frequency of inorganic fertilization or using poultry litter as an annual fertilizer source. While this study provides evidence that poultry litter is an ecologically superior annual fertilizer source for this intensive straw harvesting system, it was conducted at a single site. Before broad assertions about the impacts of annual straw harvesting, fertilization, and fertilizer source on soil microbiological and physical properties can be made, it is imperative that a wider range of sites be tested in a similar manner. It is also important to ascertain the longevity of the changes in soil microbiological and physical properties observed in this study and whether these changes affect forest productivity.
References
Adeli A, Sistani KR, Bal, a MF, Rowe DE (2005) Phosphorus dynamics in broiler litter-amended soils. Commun Soil Sci Plant Anal 36:1099–1115 doi:10.1081/CSS-200056876
Alef K (1995) Dehydrogenase activity. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic, San Diego, CA, pp 228–231
Ben-Dor E, Banin A (1989) Determination of organic matter content in arid-zone soils using a simple “loss on ignition” method. Commun Soil Sci Plant Anal 20:1675–1695
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis, part 1: physical and mineralogical methods,. 2nd edn. SSSA, Madison, WI, pp 363–375
Blazier MA, Hennessey TC, Deng SP (2005) Effects of fertilization and vegetation control on microbial biomass carbon and dehydrogenase activity in a juvenile loblolly pine plantation. For Sci 51:449–459
Blazier MA, Gaston LA, Clason TR, Farrish KW, Oswald BP, Evans HA (2008) Nutrient dynamics and tree growth of silvopastoral systems: impact of poultry litter. J Environ Qual 37:1546–1558
Bradford JM (1986) Penetrability. In: Klute A (ed) Methods of soil analysis, Part 1: physical and mineralogical methods,. 2nd edn. SSSA, Madison, WI, pp 463–478
Breland TA, Hansen S (1996) Nitrogen mineralization and microbial biomass as affected by soil compaction. Soil Biol Biochem 28:655–663 doi:10.1016/0038-0717(95)00154-9
Brye KR (2003) Long-term effects of cultivation on particle size and water-retention characteristics determined using wetting curves. Soil Sci 168:459–468 doi:10.1097/00010694-200307000-00001
Brye KR, Slaton NA, Norman RJ, Savin MC (2004) Short-term effects of poultry litter form and rate on soil bulk density and water content. Commun Soil Sci Plant Anal 35:2311–2325 doi:10.1081/CSS-200030655
Busse MD, Cochran PH, Barrett JW (1996) Changes in ponderosa pine site productivity following removal of understory vegetation. Soil Sci Soc Am J 60:1614–1621
Busse MD, Ratcliff AW, Shestak CJ, Powers RF (2001) Glyphosate toxicity and the effects of long-term vegetation control on soil microbial communities. Soil Biol Biochem 33:1777–1789 doi:10.1016/S0038-0717(01)00103-1
Busse MD, Beattie SE, Powers RF, Sanchez FG, Tiarks AE (2006) Microbial community responses in forest mineral soil to compaction, organic matter removal, and vegetation control. Can J Res 36:577–588 doi:10.1139/x05-294
Camiña F, Trasar-Cepeda C, Gil-Sotres F, Leirós C (1998) Measurement of dehydrogenase activity in acid soils rich in organic matter. Soil Biol Biochem 30:1005–1011 doi:10.1016/S0038-0717(98)00010-8
Canali S, Tinchera A, Intrigliolo F, Pompili L, Nisini L, Mocali S et al (2004) Effect of long term addition of composts and poultry manure on soil quality of citrus orchards in Southern Italy. Biol Fertil Soils 40:206–210 doi:10.1007/s00374-004-0759-x
Daddow RL, Warrington GE (1983) Growth-limiting soil bulk densities as influenced by soil texture. USDA Forest Service, Watershed Sys Dev Group Rep WSDG-TN-00005
Danielson RE, Sutherland PL (1986) Porosity. In: Klute A (ed) Methods of soil analysis, part 1: physical and mineralogical methods,. 2nd edn. SSSA, Madison, WI, pp 443–461
Deng SP, Parham JA, Hattey JA, Babu D (2006) Animal manure and anhydrous ammonia amendment alter microbial carbon use efficiency, microbial biomass, and activities of dehydrogenase and amidohydrolases in semiarid agroecosystems. Appl Soil Ecol 33:258–268 doi:10.1016/j.apsoil.2005.10.004
Diaz-Raviña M, Acea MJ, Carballas T (1993) Microbial biomass and its contribution to nutrient concentrations in forest soils. Soil Biol Biochem 25:25–31 doi:10.1016/0038-0717(93)90237-6
Dickens ED (1999) Effect of inorganic and organic fertilization on longleaf pine tree growth and pine straw production. In: Haywood JD (ed) Proceedings of the 10th Biennial Southern Silvicultural Research Conference, Shreveport, LA, Feb. 16–18, 1999. Gen. Tech. Rep. SRS-30, Asheville, NC. USDA Forest Service, Southern Research Station. pp 464–468
Donegan KK, Watrud LS, Seidler RJ, Maggard SP, Shiroyama T, Porteous LA et al (2001) Soil and litter organisms in Pacific Northwest forests under different management practices. Appl Soil Ecol 18:159–175 doi:10.1016/S0929-1393(01)00155-X
Duryea ML, Edwards JC (1989) Pine-straw management in Florida’s forest. FL Coop Ext Serv. Inst Food Agric Sci Cir 831, University of Florida, Gainsville, FL, 5 p
Fauci F, Dick RP (1994) Microbial biomass as an indicator of soil quality: effects of long-term management and recent soil amendments. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. SSSA, Madison, WI, pp 229–234
Friend AL, Roberts SD, Schoenholtz SH, Mobley JA, Gerard PD (2006) Poultry litter application to loblolly pine forests: growth and nutrient containment. J Environ Qual 35:837–848 doi:10.2134/jeq2005.0244
Gallardo A, Schlesinger WH (1994) Factors limiting microbial biomass in the mineral soil and forest floor of a warm-temperate forest. Soil Biol Biochem 26:1409–1415 doi:10.1016/0038-0717(94)90225-9
Gee GW, Campbell MD, Campbell GS, Campbell JH (1992) Rapid measurement of low soil water potentials using a water activity meter. Soil Sci Soc Am J 56:1068–1070
Gilman EF (1987) Where are tree roots? Env Hort Dept, Fl Coop Ext Serv, Inst Food Ag Sci, Univ Fl, Ext Bull ENH137
Harris JA (2003) Measurements of the soil microbial community for estimating the success of restoration. Eur J Soil Sci 54:801–808 doi:10.1046/j.1351-0754.2003.0559.x
Haywood JD, Tiarks AE, Elliott-Smith ML, Pearson HR (1995) Management of longleaf pine stands for pine straw harvesting and the subsequent influence on forest productivity. In: Edwards MB (ed) Proceedings of the 8th Biennial Southern Silvicultural Research Conference, Auburn, AL, Nov. 1–3, 1994. Gen. Tech. Rep. SRS-1, Asheville, NC. USDA Forest Service, Southern Research Station, pp 218–288
Haywood JD, Tiarks AE, Elliott-Smith ML, Pearson HA (1998) Response of direct seeded Pinus palustris and herbaceous vegetation to fertilization, burning, and pine straw harvesting. Biomass Bioenergy 14:157–167 doi:10.1016/S0961-9534(97)10029-0
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN et al (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792 doi:10.1038/35081058
Horwath WR, Paul EA, Harris D, Norton J, Jagger L, Horton KA (1996) Defining a realistic control for the chloroform fumigation–incubation method using microscopic counting and 14C-substrates. Can J Soil Sci 76:459–467
Jenkinson DS, Powlson DS (1976a) The effects of biocidal treatments on metabolism in soil-I. Fumigation with chloroform. Soil Biol Biochem 8:167–177 doi:10.1016/0038-0717(76)90001-8
Jenkinson DS, Powlson DS (1976b) The effects of biocidal treatments on metabolism in soil-V: A method for measuring soil biomass. Soil Biol Biochem 8:209–213 doi:10.1016/0038-0717(76)90005-5
Kaiser EA, Walenzik G, Heinemeyer O (1991) The influence of soil compaction in decomposition of plant residues and on microbial biomass. In: Wilson WS (ed) Advances in soil organic matter research: the impact on agriculture and the environment. Royal Society of Chemistry, Cambridge, pp 207–216
Khan SA, Mulvaney RL, Ellsworth TR, Boast CW (2007) The myth of nitrogen fertilization for soil carbon sequestration. J Environ Qual 36:1821–1832 doi:10.2134/jeq2007.0099
King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty PM (2002) Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol 154:389–398 doi:10.1046/j.1469-8137.2002.00393.x
Kingery WL, Wood CW, Delaney DP, Williams JC, Mullins GL (1994) Impact of long-term land application of broiler litter on environmentally related soil properties. J Environ Qual 23:139–147
Landi L, Renella G, Moreno JL, Falchini L, Nannipieri P (2000) Influence of cadmium on the metabolic quotient, l-:d-glutamic acid respiration ratio and enzyme activity:microbial biomass under laboratory conditions. Biol Fertil Soils 32:8–16 doi:10.1007/s003740000205
Lee K, Jose S (2003) Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a fertilization gradient. For Ecol Manage 185:263–273
Lenhard G (1956) The dehydrogenase activity in soil as a measure of the activity of soil microorganisms. Z. Pflanzenernah Dung Bodenkd 73:1–11 doi:10.1002/jpln.19560730102
Lopez-Zamora I, Duryea ML, McCormac Wild C, Comerford NB, Neary DG (2001) Effect of pine needle removal and fertilization on tree growth and soil P availability in a Pinus elliottii Engelm. Var. elliottti stand. For Ecol Manage 148:125–134
Luizao RC, Bonde TA, Rosswall T (1992) Seasonal variation of soil microbial biomass: the effects of clearfelling a tropical rainforest and establishment of pasture in the central Amazon. Soil Biol Biochem 24:805–813 doi:10.1016/0038-0717(92)90256-W
Matthews SD, Reynolds EF, Colvin GP, Weems TA, Ray CA, Seaholm JE et al (1974) Soil Survey of Ouachita Parish, Louisiana. Soil Conservation Service, US Department of Agriculture. US Government Printing Office, Washington, DC
Mills R, Robertson DR (1991) Production and marketing of Louisiana pine straw. Louisiana Cooperative Extension Service, Louisiana State University Agricultural Center. Pub 2430:9
Morris LA, Jokela EJ, O, Conner JB Jr (1992) Silvicultural guidelines for pinestraw management in the southeastern United States. Ga For Comm For Res Pap 88:11
Munkholm LJ, Schjønning P, Debosz K, Jensen HE, Christensen BT (2002) Aggregate strength and mechanical behavior of a sandy loam under long-term fertilization treatments. Eur J Soil Sci 53:129–137 doi:10.1046/j.1365-2389.2002.00424.x
Munsinger RA, McKinney R (1982) Modern Kjeldahl systems. Am Lab 14:76–79
Page-Dumroese DS, Jurgensen MF, Tiarks AE, Ponder F Jr, Sanchez FG, Fleming RL et al (2006) Soil physical property changes at the North American long-term soil productivity study sites: 1 and 5 years after compaction. Can J Res 36:551–564 doi:10.1139/x05-273
Plaza C, Hernández D, García-Gil JC, Polo A (2004) Microbial activity in pig slurry-amended soils under semiarid conditions. Soil Biol Biochem 36:1577–1585 doi:10.1016/j.soilbio.2004.07.017
Powers RF, Tiarks AE, Boyle JR (1998) Assessing soil quality: Practicable standards for sustainable forest productivity in the United States of America. In: Adams MB (ed) The contribution of soil science to the development of and implementation of criteria and indicators of sustainable forest management. SSSA Special Publ No 53. SSSA, Madison WI, pp 53–80
Powers RF, Scott DA, Sanchez FG, Voldseth RA, Page-Dumroese D, Elioff JD, Stone DM (2005) The North American long-term soil productivity experiment: findings from the first decade of research. For Ecol Manage 220:31–50
Powlson DS, Brookes PC (1987) Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol Biochem 19:159–164 doi:10.1016/0038-0717(87)90076-9
Pritchett WL, Fisher RF (1987) Forest soil biology. In: Properties and management of forest soils, 2nd edn. Wiley, New York, NY, pp 77–94
Roise JP, Chung J, Lancia R (1991) Red-cockaded woodpecker habitat management and longleaf pine straw production: an economic analysis. S J Appl For 15:88–92
Samuelson LJ, Wilhoit J, Stokes T, Johnson J (1999) Influence of poultry litter fertilization on an 18-year-old loblolly pine stand. Commun Soil Sci Plant Anal 30:509–518
SAS Institute (2006) Base SAS 9.1.3 procedures guide. SAS Institute, Cary, NC
Shestak CJ, Busse MD (2005) Compaction alters physical but not biological indices of soil health. Soil Sci Soc Am J 69:236–246
Tabatabai MA (1994) Soil enzymes. In: Bigham JM (ed) Methods of soil analysis part 2: microbiological and biochemical properties. SSSA, Madison, WI, pp 775–833
Tan X, Chang SX, Kabzems R (2005) Effects of soil compaction and forest floor removal on soil microbial properties and N transformations in a boreal forest long-term soil productivity study. For Ecol Manage 217:158–170
Tan X, Chang SX, Kabzems R (2008) Soil compaction and forest floor removal reduced microbial biomass and enzyme activities in a boreal aspen forest soil. Biol Fertil Soils 44:471–479 doi:10.1007/s00374-007-0229-3
Taylor HM, Burnett E (1964) Influence of soil strength on root-growth habits of plants. Soil Sci 98:174–180
Taylor HM, Gardner HR (1963) Penetration of cotton seedling taproots as influenced by bulk density, moisture content, and strength of soil. Soil Sci 96:153–156
Tekeste M, Hatzhghi DH, Stroonsnijder L (2007) Soil strength assessment using threshold probability approach on soils from three agro-ecological zones in Eritrea. Biosystems Eng 98:470–478
Thalmann A (1968) Zur methodic der bestimmung der dehydrogenaseaktivität im boden mittels triphenyltetrazolumchlorid (TTC). Landwirtsch Forsch 21:249–258
Tiarks AE, Haywood JD (1996) Site preparation and fertilization effects on growth of slash pine for two rotations. Soil Sci Soc Am J 60:1654–1663
Wagner GH, Wolf DC (1999) Carbon transformations and soil organic matter formation. In: Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA (eds) Principles and applications of soil microbiology. Prentice Hall, Upper Saddle River, NJ, pp 218–258
Weaver T (1998) Managing poultry manure nutrients. Agric Res 46:12–13
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-nutrient analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146
Author information
Authors and Affiliations
Corresponding author
Additional information
This publication has been approved by the Director of the Louisiana Agricultural Experiment Station as manuscript 2008-256-1648.
Rights and permissions
About this article
Cite this article
Blazier, M.A., Patterson, W.B. & Hotard, S.L. Straw harvesting, fertilization, and fertilizer type alter soil microbiological and physical properties in a loblolly pine plantation in the mid-south USA. Biol Fertil Soils 45, 145–153 (2008). https://doi.org/10.1007/s00374-008-0316-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-008-0316-0