Abstract
In this review, we explore the inconsistencies in the data and gaps in our knowledge that exist in what is currently known regarding gill chemosensors which drive the cardiorespiratory reflexes in fish. Although putative serotonergic neuroepithelial cells (NEC) dominate the literature, it is clear that other neurotransmitters are involved (adrenaline, noradrenaline, acetylcholine, purines, and dopamine). And although we assume that these agents act on neurons synapsing with the NECs or in the afferent or efferent limbs of the paths between chemosensors and central integration sites, this process remains elusive and may explain current discrepancies or species differences in the literature. To date it has been impossible to link the distribution of NECs to species sensitivity to different stimuli or fish lifestyles and while the gills have been shown to be the primary sensing site for respiratory gases, the location (gills, oro-branchial cavity or elsewhere) and orientation (external/water or internal/blood sensing) of the NECs are highly variable between species of water and air breathing fish. Much of what has been described so far comes from studies of hypoxic responses in fish, however, changes in CO2, ammonia and lactate have all been shown to elicit cardio-respiratory responses and all have been suggested to arise from stimulation of gill NECs. Our view of the role of NECs is broadening as we begin to understand the polymodal nature of these cells. We begin by presenting the fundamental picture of gill chemosensing that has developed, followed by some key unanswered questions about gill chemosensing in general.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research designed to identify the chemosensors driving cardiorespiratory reflexes in fish and describe the mechanistic basis of chemosensing has expanded dramatically over the last several decades. This work has been the topic of a host of excellent reviews, each with its own special focus (Shelton et al. 1986; Burleson et al. 1992; Glass 1992; Perry and Gilmour 2002; Burleson and Milsom 2003; Vulesevic et al. 2006; Zaccone et al. 2006; Sundin et al. 2007; Gilmour and Perry 2007; Jonz and Nurse 2006; Bailly 2009; Perry et al. 2009; Perry and Abdallah 2012; Milsom 2012; Porteus et al. 2012; Zachar and Jonz 2012; Jonz et al. 2015; Perry et al. 2016; Perry and Tzaneva 2016; Tresguerres et al. 2019; Pan and Perry 2020; Reed and Jonz 2022; Milsom et al. 2022; Perry et al. 2023). We see no need to reiterate the material presented in these reviews but rather wish to explore the inconsistencies in the data and gaps in our knowledge that they reveal. Further, we aim to identify a way forward in addressing unanswered questions. We begin by presenting the fundamental picture of gill chemosensing that has developed, followed by some key unanswered questions about gill chemosensing in general.
Note that in the literature, different authors use different terms to describe gill structure. Some describe the branching structures arising from the gill arches as consisting of gill filaments bearing the lamellae where gas exchange occurs. Others refer to these same structures as primary and secondary lamellae respectively. Throughout this review we will use the former terminology: filaments and lamellae.
The current view
Currently it is the neuroepithelial cells (NECs) found within fish gills that are believed to be the putative gill chemoreceptors responsible for cardiorespiratory reflexes to O2, CO2, ammonia, and lactate. These cells were first described by Dunel-Erb et al. (1982) and were characterized by the presence of dense-cored vesicles containing serotonin (5-HT). These cells were isolated or clustered and were supported by the epithelial basal lamina. They were found on the efferent side of the gill filaments, facing the respiratory water flow and were innervated by a plexus of nerve fibres (Dunel-Erb et al. 1982; Sundin et al. 1986; Bailly et al. 1989; Bailly et al. 1992; Bailly 2009). They were identified as NECs characterized as belonging to the group of amine precursor uptake and decarboxylation (APUD) endocrine cells based on morphology seen in sectioned tissue as described by Pearse (1969) (Fig. 1). The study was performed on multiple species of fish (perch (Perca fluviatilis), trout (Onchorynchus mykiss), pike perch (Stizostedion lucioperca), catfish (Ictalurus melas), black bass (Micropterus dolomieu), eel (Anguilla anguilla), and dogfish (Scyliorhinus canicula) demonstrating the ubiquity of serotonin containing cells in gill filaments. Over the subsequent decades, we have learned much about serotonergic gill NECs in vivo, in vitro, and in primary culture. Next we summarize this information.
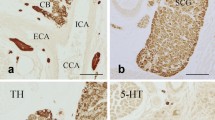
a Longitudinal section of a primary filament of trout showing formaldehyde fluorescence labelling NECs. Note that cells display processes. Secondary arteries (*) and capillaries of lamellae (arrows). b Longitudinal section of a primary filament of trout. Efferent secondary arteries (ea2) are seen in cross section. Several NECs (arrows) are seen resting on the basal lamina (bl) (× 650). c Electron micrograph of basal part of neuroepithelial cell in trout. Some dense cored vesicles are in contact or very close to cell membranes (arrows). Many vesicles are empty. N = nerve within subepithelial area; bl = basal lamina. (× 30,000). d Electron micrograph of a group of NECs in trout. One rests on the basal lamina (bl). Note that both cells are extending processes; the upper cell toward the lower cell, which in turn sends its process in the direction of the basal lamina. Nerve profiles (n) are closely intermingled with smooth muscle fibres (mf). Vascular compartment seen on left belongs to central venous sinus (cvs) (× 10,000) (from Dunel-Erb et al. 1982)
In vivo data
That the O2/CO2 chemoreceptors responsible for cardio-respiratory responses in adult fish are primarily located in the gills was determined by selectively denervating the IXth and Xth cranial nerves innervating the gill arches and demonstrating that the changes in heart rate and breathing to these stimuli were greatly reduced or completely eliminated (see Milsom 2012 for review). While these studies also indicated there were cellular chemoreceptors located elsewhere, in adult fish the contribution of extrabranchial chemoreceptors to cardiorespiratory reflexes was relatively small. The responses to hypoxia in vivo were shown to arise from changes in both internal and external environments; i.e. to changes in the blood and in the water flowing over the gills (see Perry and Gilmour 2007; Milsom 2012 for reviews). While this also appears true for ammonia sensing (Lang et al. 1987; Randall and Ip 2006; Zhang and Wood 2009; Wright and Wood 2012; Porteus et al. 2021), whether this is also true for CO2 remains a topic of debate (Tresguerres et al. 2019; Milsom et al. 2022) (see section on CO2/pH sensing). Sensing of lactate is presumably internal only (Thomsen et al. 2017, 2018; Leonard et al. 2022). An often-overlooked implication of this is that the distribution of chemosensing cells is not uniform: some sense only changes in the blood, some only changes in the water flowing over the gills and some sense both.
While the gills have been shown to be the primary sensing site for respiratory gases, the location (gills, oro-branchial cavity, or elsewhere) and orientation (external/water or internal/blood sensing) of the chemoreceptor cells are highly variable between species of water and air-breathing fish. This is true of the receptors involved in reflex changes in each of the different components of the cardiorespiratory response (breathing frequency, breath amplitude, heart rate, and systemic vascular resistance). Although not universal, the receptors involved in eliciting changes in heart rate and breathing frequency in response to hypoxia and hypercarbia tend to be restricted to the gills while those producing increases in breath amplitude are more widespread, frequently also being found at extrabranchial sites (Milsom 2012). The distribution of the chemoreceptors sensitive to CO2 and ammonia in the gills involved in producing ventilatory responses tends to be more restricted than that of the O2-sensitive chemoreceptors and the specific location of the receptors involved in the various components of the cardiorespiratory response to CO2 can vary from those of the O2-sensitive chemoreceptors (see Milsom 2012 for review).
Studies designed to determine the relative roles of various neurotransmitters and neuromodulators in intact fish by intra-arterial injection reveal that 5-HT, adrenaline, noradrenaline, acetylcholine and purines generally stimulate ventilation, whereas dopamine inhibits ventilation ((O. mykiss) Thomas et al. 1979; Fritsche et al. 1992; (O. mykiss) Burleson and Milsom 1995a, b; (Danio rerio) Shakarchi et al. 2013). Given the focus on 5-HT containing NECs, it is of note that administration of various agonists of 5-HT receptor subtypes 5-HT2 and 5-HT3 led to increases in ventilation frequency and/or amplitude in European eel (A. anguilla) (Janvier et al. 1996), Gulf toadfish (Opsanus beta) (McDonald et al. 2010), and zebrafish larvae (D. rerio) (Shakarchi et al. 2013; Abdallah et al. 2014; Jonz et al. 2015), while antagonists of these receptors block responses to hypoxia. Also, since adenosine and acetylcholine (ACh) are the primary neurotransmitters in the mammalian carotid body, it is notable that ventilatory responses to ACh and to both nicotine and muscarine have been reported in several species (Lenfant and Johansen 1968; Burleson and Milsom 1995b; Shakarchi et al. 2013). However, the muscarinic antagonist, atropine abolished the ventilatory response to hypoxia at very high concentrations in zebrafish (D. rerio)(Rahbar et al. 2016) as well as the hypercarbia-induced ventilatory response in Pacific spiny dogfish (Squalus acanthias) (McKendry et al. 2001), yet atropine had no effect on ventilation in rainbow trout (O. mykiss) (Burleson and Milsom 1995a; 1995b), Adriatic sturgeon (Acipenser naccarii) (McKenzie et al. 1995) or channel catfish (Ictalurus punctatus) (Burleson and Smatresk 1990). The emersion response in the facultative airbreathing mangrove rivulus, K. marmoratus, was accentuated in fish pre-exposed to ACh and attenuated in fish pre-exposed to the nicotinic antagonist, hexamethonium (Regan et al. 2011). As well, exogenous administration of ATP-γ-S, a broad-spectrum purinoceptor agonist, elicited hyperventilation in zebrafish (Coe et al. 2017) and the hyperventilatory response to hypoxia could be inhibited by exogenous application of P2X3 purinoceptor antagonists and adenosine antagonists (Coe et al. 2017; Rahbar et al. 2016; Stecyk and Farrell 2006).
In all of these studies, however, it is not known whether the various agents were acting on neurons synapsing with the NECs or elsewhere in the afferent or efferent limbs of the paths between chemosensors and central integration sites. Certainly, many of them will have been acting at multiple sites and this may explain some of the differences in results between species noted above. Some of the differences may also be due to differences in the concentration of the drugs used or in the ability of the drugs to cross the blood–brain barrier.
In vitro data
In vitro studies on isolated gill arches have been instrumental in describing the distribution of NECs within the gills, the innervation of NECs (afferent and efferent) as well as the possible neurotransmitters and receptors involved in transmission of information to/from the NECs.
Distribution of NECs within the gills
In the pioneering study by Dunel-Erb et al. (1982), NECs were found on the efferent side of the gill filaments facing the respiratory water flow in a variety of species. Subsequent research using a variety of imaging techniques of NECs from sections or whole-mounts of gill preparations have identified 5-HT containing NECs of different sizes in different locations in the gills of a variety of species (Dunel-Erb et al. 1982; Laurent 1984; Bailly et al. 1989, 1992; Zaccone et al. 1992; Goniakowska-Witalińska et al. 1997; Sundin et al. 1998a, b; Jonz and Nurse 2003; Saltys et al. 2006; Vulesevic et al. 2006, Coolidge et al. 2008, Qin et al. 2010, Tzaneva and Perry 2010, Zhang et al. 2011, Porteus et al. 2012, 2013, 2014b; Shakarchi et al. 2013; Zaccone et al. 1997, 2012, 2020; Milsom et al. 2022). To date the only species in which they have not been found are the hagfish (Porteus et al. submitted).
Oxygen chemoreceptors have also been found throughout the orobranchial cavity innervated by cranial nerves V, VII, IX, and X (mostly IX and X). In elasmobranchs, they have also been located to the spiracle and in teleosts that have one, the pseudobranch, both innervated by cranial nerves VII and IX (see Milsom 2012 for review). They have been identified in the carotid labyrinth of catfish (Zaccone et al. 2012) and also in the skin, primarily in larvae but also in adults of some species. Indirect evidence suggests that receptors in the skin may play a significant role in larvae during the first few days post-fertilization when the gills are developing and during which the larvae are dependent on cutaneous gas exchange (Jonz and Nurse 2006; Regan et al. 2011; Coccimiglio and Jonz 2012; Zaccone et al. 2017; Rossi et al. 2020; Cochrane et al., 2021). They may also play a key role in emergence responses in amphibious fish that move onto land when the aquatic environment will not sustain oxidative metabolism (Cochrane et al. 2019).
The remainder of this section focuses on the NECs located exclusively within the gills. Relatively large NECs (7–15 µm in diameter) can be found in the epithelium along the entire length of the filament in zebrafish (Danio rerio), trout (O. mykiss), goldfish (Carasius auratus), killifish (Fundulus heteroclitus), traira (Hoplias malabaricus), trairaõ (Hoplias lacerdae), bowfin (Amia calva), mangrove rivulus (Kryptolebias marmoratus), African bony tongue (Heterotis niloticus), piraracu (Arapaima gigas) sockeye salmon (Oncorhynchus nerka) and medaka (Oryzias latipes) (Coolidge et al. 2008; Jonz and Nurse 2003; Jonz et al. 2004; Saltys et al. 2006; Tzaneva and Perry 2010; Zhang et al. 2011; Porteus et al. 2014a, b; Zaccone et al. 2020, 2022a, b; Leonard et al. 2022; Brink and Milsom, unpublished data). They tend to be more concentrated towards the distal half of the filament and in medaka, the Japanese rice fish (O. latipes), they are clustered exclusively at the filament tip (Porteus et al. 2012) (Fig. 2). Most filamental NECs are located in the deepest part of the filament epithelium very near the efferent filament artery (eFA) facing the respiratory water flow; a strategic position for potentially monitoring changes in arterial or ambient PO2.

(Modified from Porteus et al. 2012)
Distribution of serotonin-containing neuroepithelial cells (NECs) in the gills of various species of fish. Serotonin is stained green for salmon (Oncorhynchus nerka) and goldfish (Carasius auratus) and purple for Japanese rice fish (Oryzias latipes). Note that the NECs are found only along the filaments in salmon but in both the filaments and lamellae in goldfish. In the ricefish they are confined only to the filament tips
Smaller NECs (about half the size of the ones found in the filament) have also been found in the lamellae of all species mentioned above except trout, mangrove rivulus (K. marmoratus) and Atlantic salmon (Coolidge et al. 2008; Jonz and Nurse 2003; Jonz et al. 2004; Saltys et al. 2006; Zhang et al. 2011; Regan et al. 2011; Ghanizadeh-Kazerouni et al. 2024). They tend to be concentrated towards the tips of the lamellae but their distribution can be as plastic as the lamellae themselves. In goldfish (C. auratus) and African bony tongue (H. niloticus), where the interlamellar space fills in, the NECs migrate to the tips of the lamellae when fish are exposed to cold or normoxic water (Tzaneva and Perry 2010; Tzaneva et al. 2011; Zaccone et al. 2022a, b).
Innervated 5-HT-positive cells have also been described in the gill rakers of goldfish and trout, an ideal location for sensing external hypoxia. In goldfish these cells also stained for the synaptic vesicle marker SV2, but not in trout (Coolidge et al. 2008). It was suggested that in trout, these 5-HT-IR cells might be Merkel-like cells associated with taste buds. Indeed, NECs and Merkel-like cells share many similar characteristics (reviewed by Zaccone et al. 1994; Coolidge et al. 2008; Zachar and Jonz 2012).
To date it has been impossible to link the differences in the distribution of NECs to differences in sensitivity to different stimuli or in lifestyles (active versus sluggish; water breathing versus air-breathing, etc.).
Most studies designed to examine the effect of sustained hypoxia on the size and density of NECs have shown in zebrafish, an increase in size of the 5-HT containing cells on the filaments, changes in their shape suggestive of an increase in surface area, and extended cytoplasmic neurone-like processes towards nerve fibres, but no change in density (Jonz et al. 2004; Shakarchi et al. 2013; Pan et al. 2021). However, gill NEC density was increased in one study (Pan et al. 2021) and decreased in another in zebrafish after hyperoxic acclimation (Vulesevic et al. 2006). In the bowfin, a bimodal breather capable of aerial gas exchange, there was also no change in density but an increase in the size of the filamental NECs, primarily due to an increase in length, but only in fish without access to air after exposure to sustained hypoxia (6.0 kPa for 7 days). This was not seen in bowfin exposed to sustained hypoxia with access to air (Porteus et al. 2014b) indicating that the response was to internal/blood changes in PO2. There was also no change in density of NECs on the filaments of African bony tongue (Heterotis niloticu) following exposure to chronic hypoxia (Zaccone et al. 2022a, b). In zebrafish (D. rerio), the filament epithelium also contained a population of 5-HT negative NECs that were only immunopositive for the synaptic vesicle marker SV2 (Jonz and Nurse 2003). After in vivo exposure to chronic hypoxia the number of these 5-HT-negative NECs that occupied the filament epithelium adjacent to the 5-HTcontaining NECs increased. In mangrove rivulus (K. marmoratus), hypoxia acclimation increased NEC size in the gills and skin of adult fish (Regan et al. 2011), and fish reared in normoxic water exhibited an increase in NEC density in the gills and skin following air exposure (Rossi et al. 2020). While there is variation in the changes seen in NECs on exposure to chronic hypoxia, all are consistent with the NECs being sensitive to O2 levels.
Given that acetylcholine (ACh) and ATP are thought to be the primary neurotransmitters in the carotid body in most mammals involved in the acute hypoxic response (Nurse 2005; 2010) and that administration of ACh to fish in vivo produces such a strong response (Burleson and Milsom 1995b), it was surprising that immuno histochemical markers for ACh were not found to label serotonergic NECs in trout (O. mykiss), goldfish (C. auratus) (Porteus et al. 2013), mangrove rivulus (K. marmoratus) (Regan et al. 2011), or zebrafish (D. rerio) (Zachar and Jonz 2017). However, cells that stain positive for either the vesicular ACh transporter (VAChT) or the enzyme choline acetyltransferase (ChAT) (the enzyme involved in the synthesis of ACh), or both, were identified in the gills of several of these species. In zebrafish they were found to be more numerous on the afferent side of the gill filaments but those on the efferent side were situated within 10 μm of the serotonergic NECs (Zachar and Jonz 2017). While the cholinergic cells formed contacts with nerve fibers, they did not stain for SV2 suggesting that the ACh was not contained in vesicles, or as observed in some neurosecretory cells, they do not express SV2 (Pumplin and Getschman 2000). It was suggested that these cells might respond to hypoxia by releasing ACh locally to modulate the serotonergic NECs in a paracrine fashion (also see below in “Conclusions” section) (Zachar and Jonz 2017). More recently VAChT has been found to colocalize with 5HT in the NECs of African bony tongue (H. niloticus) and piraracu (Arapaima gigas) (Zaccone et al. 2020; 2022a, b).
Innervation of NECs
As just alluded to, NECs can act as chemosensors involved in cardiorespiratory regulation in multiple ways. They may act directly as receptosecretory paracrine regulators of local cell functions. As well, in many species the filamental NECs that contain 5-HT have a complex innervation pattern (Dunel-Erb et al. 1982; Bailly et al. 1989, 1993; Jonz and Nurse 2003; Saltys et al. 2006; Bailly 2009; Porteus et al. 2012; Reed and Jonz 2022). They are innervated by neurons intrinsic to the gill filaments that are believed to act on vascular shunts and smooth muscle to control gill blood flow. They are also innervated by extrinsic neurons that project to or arise from the central nervous system. These neurons may be efferent in nature (Fig. 1c; showing neural processes containing vesicles, closely positioned near an area of an NEC that is enriched with dense cored vesicles), acting on the NECs to release 5-HT that might act locally in an autocrine or paracrine fashion, or afferent in nature transmitting information to the central nervous system.
The intrinsic neurons of the gills of fish are serotonergic multipolar neurons (Sundin et al. 1986; Bailly et al. 1989; Jonz and Nurse 2003; Bailly 2009; Porteus et al. 2013). In zebrafish, trout and goldfish they have been divided into two groups both running alongside the efferent filament artery (eFA) (Fig. 3a). One group is superficial and closer to the efferent filament epithelium [designated as superficial proximal neurons (SPN)] and the other is deeper behind the eFA [the deep proximal neurons (DPN)] (Jonz and Nurse 2003; Porteus et al. 2013). The superficial proximal neurons extend fibers along the filament epithelium to innervate the NECs and proximally to the junction of the eFA and the efferent branchial artery (eBA). Immunocytochemistry suggests that the NECs may be influenced by these neurons and also reciprocally regulate the activity of the nerve fibres. At the other pole, the nerve endings surround and innervate the base of the eFA. This is the site of a contractile segment or sphincter of the eFA (Nilsson and Sundin 1998; Jonz and Nurse 2003; Bailly 2009). This sphincter is also innervated by cholinergic neurons of the proximal nerve (Bailly et al. 1989; Dunel-Erb et al. 1989, Porteus et al. 2013), as well as by extrinsic sympathetic and parasympathetic fibers (Bailly and Dunel-Erb 1986; Dunel-Erb and Bailly 1986). In the zebrafish, the proximal neurons of the filament are the major source of innervation at the eFA base. Given that this has now been described in at least ten species of fish, this suggests that in all teleosts a mechanism of local neural control involving proximal neurons allows for adjustments in vascular tone through a vasoconstrictor effect on the eFA base induced by release of 5-HT from local serotonergic fibers, acting postsynaptically on the eFA sphincter and regulating blood flow in the gill (Sundin et al. 1986; Bailly et al. 1989; Sundin 1995).
Innervation pattern of zebrafish gill NECs in the filament. a Intrinsic innervation showing nerve endings of superficial proximal neurons and deep proximal neurons terminating at the base of the efferent filament artery (site of sphincter) and extension of superficial proximal neurons nerve fibres toward NECs and deep proximal neuron fibers toward chain neurons (with varicose processes), respectively. The proximal neurons do not innervate the lamellae and are both serotonergic and cholinergic. The chain neurons in some species double label for both serotonin and ACh. b Extrinsic innervation illustrating formation of a nerve bundle composed of nerve fibers emanating from the branchial nerve of the gill arch that gives rise to a nerve plexus surrounding the efferent filament artery. Fibers of the nerve plexus (with arrows) innervate the sphincter at the base of the efferent filament artery and also the filament NECs and extend out to the respiratory lamellae where they also innervate the lamellar NECs in some species. The extrinsic innervation contains cholinergic, serotonergic and sympathetic and parasympathetic neurons. Serotonergic nerve fibres are less common beneath the more numerous distal NECs towards the tips of the gill filament. The NECs in the distal half of the filament synapse with sympathetic catecholaminergic neurons (Illustration by Jacelyn Shu, modified from Jonz and Nurse 2003)
An additional group of serotonergic neurons, the chain neurons (ChNs), were found in zebrafish, on the efferent aspect of the filament as part of the DPN (Fig. 3a). Bipolar, serotonergic neurons running parallel to the eFA had been described previously (Sundin et al. 1998a, b; Sundin and Nilsson 2002). The varicosities observed along the chain fibers suggest that these neurons may form synaptic connections with other structures (Jonz and Nurse 2003). In trout and goldfish, chain neurons double-labeled with VAChT and 5-HT antibodies (Porteus et al. 2013).
Most of the sympathetic nerves enter the gills via the metatrematic rami of branchial nerves IX and X. These give rise to the extrinsic innervation of the NECs which arise from a plexus of nerve fibers originating from a nerve bundle located between the central venous sinus (CVS) and the eFA (Bailly 2009) (Fig. 3b). These nerve fibers travel around the eFA and project to filament and lamellar epithelia where they contact NECs. The nerve plexus provides innervation to both serotonergic and nonserotonergic NECs of the filament epithelium (Jonz and Nurse 2003; Bailly 2009). This appears to be the only innervation of the serotonergic NECs of the lamellae, as they do not appear to receive innervation from the SPN (Jonz and Nurse 2003). Their processes may release 5-HT and/or other neurotransmitters acting on filament ionocytes, similar to neuronal or paraneuronal 5-HT in other epithelia. In the bowfin (Amia calva) the lamellar NECs do not appear to be innervated nor to stain for SV2 (Porteus et al. 2014b). On the one hand, negative labeling for SV2 is not sufficient to conclude that synaptic vesicles are not present, since some sensory/secretory cells do not express SV2 (Pumplin and Getschman 2000), while on the other hand, these cells may play a role in sequestering and metabolizing excess circulating 5-HT (Sebatiani et al. 2022). VAChT has also been found in the extrinsic nerve bundle supplying the gill filament in trout and goldfish, consistent with reports of cholinergic nerve fibers in perch (P. fluviatilis) coursing along the efferent filament artery (including the sphincter region) and the efferent lamellar arterioles (Porteus et al. 2013). In perch, denervation of the pre and meta-trematic nerve decreased acetylcholinesterase (ACHE) staining indicating that these cholinergic fibres were extrinsic to the gill (Bailly and Dunel-Erb 1986).
Possible neurotransmitters and receptors involved in transmission of information to/from the NECs
Tyrosine hydroxylase, the rate limiting enzyme in the synthesis of catecholamines, was not found in the 1st gill arches of trout, goldfish or in the Indian catfish (Heteropneustes fossilis) (Zaccone et al. 2003; Porteus et al. 2013). This was surprising given that sympathetic innervation of the gill vasculature supplying the efferent filament artery sphincter and the nutritive vasculature has been well documented in studies of most teleost species studied to date (Sundin and Nilsson 1998). This most likely reflects the scarcity of adrenergic nerves (Sundin and Nilsson 1998) that likely function together with circulating catecholamines in response to hypoxia (Reid 1999). However, tyrosine hydroxylase has been found in nerves of the gill filaments in zebrafish (Reed et al. 2023) and in the pseudobranch of trout (Porteus et al. 2013).
Serotonergic nerve fibres are less common beneath the more numerous distal NECs towards the tips of the gill filament. The NECs in the distal half of the filament synapse with sympathetic catecholaminergic neurons of the extrinsic innervation (Bailly 2009). This suggests that the activity of the distal NECs may be modulated by the sympathetic nervous system. However, numerous synaptic-like contacts between the NECs and the nerve endings display ultrastructural features of afferent synapses suggesting that the NECs may modulate the activity of the sympathetic nerves (Bailly 2009; Zaccone et al. 2017).
The cells with serotonin-containing vesicles in the lamellae of most species are not innervated (Saltys et al. 2006; Coolidge et al. 2008). This is consistent with detailed reviews of gill morphology and branchial innervation that do not describe any innervation extending deep into the lamellae (Laurent and Dunel 1980; Wilson and Laurent 2002; Sundin and Nilsson 2002), although Jonz and Nurse (2003) have reported innervated lamellae in the zebrafish. To the extent that NECs have been identified in the pseudobranch of fish, they too are not innervated (Jonz and Nurse 2003).
There have been very few studies directly examining the discharge profiles of O2 chemoreceptors in the gills of fish. Single fibre recordings in tuna (Thunnus albacares) demonstrated that some chemoreceptors only sensed changes in external (water) O2, some only sensed changes in internal (blood) O2 and some sensed both—suggesting they were situated in different locations within the gill epithelia (Milsom and Brill 1986).
The effects of various neurochemicals on afferent discharge in the glossopharyngeal nerve (cranial nerve IX) were examined in an isolated and perfused first gill arch preparation from rainbow trout (Burleson and Milsom 1995a). Afferent neural activity increased in response to NaCN and hypoxic perfusate indicating that responses were at least in part from O2 sensitive chemoreceptors. Sympathetic agonists (epinephrine, norepinephrine and isoproterenol) had little or no effect on neural activity while 5-HT and dopamine produced only a brief, modest burst followed by a mild inhibition of neural discharge. However, external application of 5-HT to the gill surfaces of spiny dogfish (S. acanthias) increased discharge of afferent nerve fibers in the gill filaments (Poole and Satchell 1979). A modest stimulation of breathing frequency has also been seen in response to application of a 5-HT agonist (2-m-5-HT), and a reduction in response to the 5-HT antagonist (MDL 72222) in zebrafish larvae (Jonz et al. 2015). Acetylcholine and nicotine were potent neurochemical stimulants while muscarine had only a slight effect (Burleson and Milsom 1995a). Atropine completely blocked the effects of acetylcholine on receptor discharge but only slightly inhibited and delayed responses to hypoxia and NaCN (Burleson and Milsom 1995a). It was suggested that cholinergic mechanisms were more likely to be involved in eliciting cardiorespiratory reflexes from O2 sensitive chemoreceptors in the gills than either adrenergic or serotonergic mechanisms but that the transduction process involved in O2-chemoreception was complex and not dependent on any single one of the neurochemicals tested (Burleson and Milsom 1995a). As NECs characteristically contain 5-HT, it was surprising that 5-HT elicited only a modest transient burst of chemoreceptor activity. Notably, the neural responses to exogenous application of neurochemicals in this study (Burleson and Milsom 1995a) used doses that were the same as those used in intraarterial injections in in vivo studies (Burleson and Milsom 1995b) that produced significant cardiorespiratory responses. This suggests that most of those responses were due to stimulation at unknown sites outside the gills. The bottom line, however, is that the roles of any of these chemicals in the receptor control of cardiorespiratory reflexes in fish is still to be determined.
Cells in primary culture
Electrophysiological studies of NECs in multicellular heterogeneous culture provide direct evidence that NECs act as chemosensors for O2, CO2, ammonia and lactate (Jonz et al. 2004; Burleson et al. 2006; Qin et al. 2010; Zachar et al. 2017; Zachar and Jonz 2012; Zhang et al. 2011; Abdallah et al. 2014; Leonard et al. 2022). These cells were identified in culture by compartmentalized uptake of the vital dye Neutral Red. Whole-cell, voltage and current-clamp recordings revealed that in response to hypoxia (PO2 = 25–140 mmHg) these NECs depolarized due to inhibition of a background K+ conductance, similar to that seen in carotid body glomus cells where this change in receptor potential leads to calcium influx and release of neurotransmitters (Jonz et al. 2004). Similar results were subsequently found in gill NECs of channel catfish (I. punctatus) (Burleson et al. 2006). Later demonstration that isolated NECs from adult goldfish (C. auratus) respond to hypoxia by Ca2+-dependent vesicular recycling support this scenario for neurotransmitter release from NECs (Zachar et al. 2017). This suggests that this series of events is fundamental to O2 sensing and appears to have arisen early in vertebrate evolution, and persists in mammalian O2 chemoreceptors (Jonz 2018). The extent to which this scenario also applies to sensing of CO2, ammonia and lactate will be discussed in later sections of this review. It must be noted, however, that a caveat to this story is that to date, all studies on single cells have been on the larger 5-HT containing NECs and that there is no direct evidence that 5-HT is directly released by these NECs during exposure to any of the various stimuli.
Nicotinic receptors have been reported in the gills of the Asian catfish (H. fossilis). The nicotinic receptor α7 subunit is expressed in the NECs and mucous cells in the respiratory air sac and the gills (Lauriano et al. 2021). The AChR γ-like subunit has also been detected in low levels in the gills of the X-ray tetra (Pristella maxillaris) (Ma et al. 2021). In zebrafish, the nicotinic receptor α2b subunit gene, chrna2b, was highly expressed in both NECs and neurons while the α6 subunit gene (chrna6) and β3a subunit gene (chrnb3a) were primarily expressed in NECs. The β4 subunit gene (chrnb4) was primarily expressed in neurons (Pan et al. 2022). It was suggested that the location of these subunits supports a model in which VAChT-positive cells release ACh during hypoxic stimulation, leading to excitatory post-synaptic or paracrine effects on ACh receptors of neurons or NECs (Pan et al. 2022). The presence of P2X3 receptors on NECs on the tips of zebrafish lamellae (Jonz and Nurse 2003; Rahbar et al. 2016) also suggests that neurotransmitter release by the NECs may be modulated by ATP. It has also been suggested that 5-HT released by the NECs may act on the NECs themselves in an autocrine or paracrine fashion. The gene encoding an inhibitory 5-HT1A receptor is more prevalent in NECs than in any other cell type in the gill (Pan et al. 2022). TH, the key enzyme involved in catecholamine synthesis as well as nNOS have also been recorded in the NECs of various species (Zaccone et al. 2008, 2020, 2022).
Specific chemosensing mechanisms
While much of what has been described so far comes from studies of hypoxic responses in fish, changes in O2, CO2, ammonia and lactate have all been shown to elicit cardio-respiratory responses and all have been suggested to arise from stimulation of gill NECs. Our view of the role of NECs is broadening as we begin to understand the polymodal nature of these cells. Here we explore how gill NEC sensing extends beyond O2 and CO2/pH to the third respiratory gas, ammonia, and a by-product of anaerobic metabolism, lactate. In the sections that follow we present the data in support of this as well as the gaps and inconsistencies in our knowledge.
Oxygen sensing
Summary
Fish gills are multifunctional organs that coordinate respiratory and environmental gas sensing with respiratory gas exchange, ionic and acid–base regulation, and excretion of nitrogenous compounds (Evans et al. 2005; Pan et al. 2022). Fish sense O2 levels across dynamic temporal and spatial levels. Acute hypoxia sensing (scale of seconds to minutes) couples rapid changes in environmental or tissue O2 levels to membrane depolarization and neurochemical release by specialized chemosensing cells, followed by activation of respiratory neural pathways (Loenarz et al. 2011; Hockman et al. 2017; Baik and Jain 2020; Perry et al. 2023). Longer duration hypoxic events (hours to weeks) additionally evoke the PHD-HIF-pVHL pathway, which couples O2 levels to gene transcriptional regulation (McElroy and Chandel 2017; Pelster and Egg 2018; Mandic et al. 2021b).
Mechanisms
The physiological mechanisms used by mammals for sensing O2 are relatively well understood and supported by robust and detailed evidence. In contrast, many aspects of the mechanistic basis of O2 chemosensing in teleost gills remain mysterious. What data there are, however, suggest that many aspects of the acute and chronic signaling pathways underlying O2 chemosensing are evolutionarily conserved (Jonz 2018). The following is a short review of some of the general features of the signaling pathways of the two major types of mammalian O2 sensory cells: the glomus cells in the carotid body that sense the O2 levels in arterial blood (internal hypoxia); and the pulmonary neuroepithelial cells (PNECs) and aggregated nodal clusters of PNECs, the pulmonary neuroepithelial bodies (PNEBS) that respond only to airway hypoxia (external hypoxia).
Carotid body type I cells
Type I (glomus) cells within carotid bodies elicit the acute mammalian hyperventilatory responses to hypoxia (Gonzalez et al. 1994; Weir et al. 2005; Buckler 2015; Caravagna and Seaborn 2016; McElroy and Chandel 2017; Baik and Jain 2020). Glomus cells are highly perfused with blood and densely innervated. During acute hypoxia, glomus cells detect and couple blood levels of O2 (also CO2/acid) to a net depolarizing receptor potential and subsequent downstream signaling events including neurochemical release (Fig. 4). Critical plasma membrane bound ion channels are involved in this process; specifically, the non-voltage-dependent 2 pore Potassium channels (K2P) TASK 1, TASK 3 and TASK1/TASK3 heterodimers that are associated with maintaining cellular resting potential (Bittner et al. 2010; Buckler 2015). These channels are not blocked by voltage dependent K+ channel blockers TEA and 4 AP; are blocked by quinidine; and are inhibited by hypoxia, which stabilizes a closed channel state. Together with this closure, ion pumps and exchangers contribute to depolarization, with ensuing opening of voltage gated calcium channels. The resulting calcium influx triggers transmitter release from glomus cells including ACh, serotonin, and ATP (Buckler 2015; Olschewski et al. 2017). Sensory activity in the carotid body is also strongly correlated with mitochondrial electron transport chain (ETC) activity (Chang 2017; Holmes et al. 2018; Ortega-Sáenz and López-Barneo 2020). Carotid body TASK channels in excised patches (voltage clamped) show no direct O2 sensitivity (Buckler 2015). In one model, hypoxia inhibits the ETC, resulting in a drop in intracellular MgATP which then modulates TASK channel activity. The MgATP decline is linked to the extreme, atypical hypoxia sensitivity of carotid body mitochondria (Varas et al. 2007; Keith et al. 2013; Buckler 2015). In a recent transcriptome analysis of the intracellular modulation of glomus cells, hypoxia evoked depolarization revealed two critical molecules (Gao et al. 2017). These were mitochondrial Cox4i2 (cytochrome c oxidase subunit IV isoform 2), which is expressed among a restricted set of known hypoxia responsive cell types; and the mitochondrial protein HIGD1, which is required to confer the extreme sensitivity and specificity in O2 sensing characteristic of glomus cells (Nurse 2017; Gao et al. 2017; Timón-Gómez et al. 2022). Clearly O2 sensing in chemoreceptor cells relies on multiple, interactive biophysical and metabolic properties as opposed to one or a small number of O2 sensing molecules (Lopez-Barneo et al. 2016a; b; Gao et al. 2017).
Proposed oxygen sensing mechanism in NECs. A decrease in PO2 releases the inhibition of cystathionine-β-synthase (CBS) and cystathionine-δ-lyase (CSE) and thus increases H2S production (1). It is likely that H2S acts on K+ channels to close them and therefore cause an decrease in resting membrane potential (2). This leads to the activation of rapidly inactivating voltage-activated K+ (KV) channels (3) followed by the opening of voltage dependent Ca2+ channels (CaV) and/or release of intracellular calcium stores (4). The increase in internal Ca2+ concentration leads to the fusion of vesicles and release of neurotransmitters (5). Created with BioRender.com
PNECs/PNEBs
Pulmonary neuroendocrine cells may be solitary (PNECs), or clustered PNECs (pulmonary neuroendothelial bodies, NEBs). They are O2 sensitive, intrapulmonary secretory cells found within mammalian airways. PNECs are interspersed through lung alveoli; and NEBs are located at airway bifurcations and heavily innervated by vagal nodose neurons. These cells secrete both serotonin and calcitonin gene related peptide (Noguchi et al. 2020; Shivaraju 2021), and are derived from endoderm as are fish gill NECs (Hockman et al. 2017). The complete function of PNECs/NEBs is unknown, but they likely act as mammalian hypoxia airway sensors and reduce airway resistance and modulate pulmonary arterial constriction in response to hypoxia (Youngson et al. 1993; Cutz and Jackson 1999; Cutz et al. 2004; Ratcliffe et al. 2016; McElroy and Chandel 2017; Baik and Jain 2020; Noguchi et al. 2020). PNECs/NEBs are thought to sense hypoxia via a plasma membrane O2 sensitive Kv channel (KO2) complexed using the O2 sensor NADPH-oxidase (NOX2)(Fu et al. 2000). H2O2 is a messenger that gates (KO2); however other K+ channels (including TASK channels) and NOX variations may contribute to the complete depolarizing response to hypoxia. Currently, however, the characteristics and contributions of their mitochondria are not as well understood as those of glomus cells. Hypoxia triggered attenuation of the K+ current(s) results in depolarization, with subsequent opening of voltage dependent calcium channels and calcium dependent exocytosis of serotonin and CGRP (Youngson et al. 1993; Fu et al. 2000; Cutz et al. 2013).
Gill NECs
Gill NECs have features in common with both PNECs and glomus cells relevant to their function as O2 sensing cells. Transcriptional analysis of zebrafish gill filament single cell mRNA profiles revealed similarities in mitochondria related molecules (e.g. ubiquinone subunit ndufa4l2a and cytochrome c oxidase) between glomus cells and NECs (Gao et al. 2017; Pan et al. 2022). Therefore, gill NECs may show specific, extreme sensitivity to environmental O2, similar to glomus cells.
In fish, carbon monoxide (CO), nitric oxide (NO), and hydrogen sulphide (H2S) are known as gasotransmitter signalling molecules which have critical roles in the physiological regulation of the cardiorespiratory centre (see Perry and Tzaneva 2016 for a review). There is growing evidence that these endogenously produced gases are involved with O2 sensing mechanisms. Briefly, CO is produced by heme oxygenase (HO) proteins which breakdown heme into Fe2+ and CO (Tenhunen et al. 1969). When levels of CO rise, there is an inhibition of ventilatory control, which appears to be temperature dependent and involved with inhibiting L-type voltage gated Ca2+ channels in NECs. NO is synthesized from L-arginine by nitric oxide synthase (NOS) in a reaction that requires NADPH and O2. The involvement of NO in O2 sensing relies heavily on mammalian literature, where NO acts to inhibit CB output (Kline et al. 1998), however, in fish, NO acts as a neurotransmitter in the ventilatory response, but its role in oxygen sensing is less understood in lower vertebrates (Perry and Tzaneva 2016).
In zebrafish, H2S has been shown to be involved in the oxygen sensing mechanism (Porteus et al. 2014a). Oxygen inhibits the H2S biosynthetic enzymes cystathionine-β-synthase (CBS) and cystathionine-δ-lyase (CSE). Therefore, as oxygen in the NEC is reduced, H2S concentration increases. Knockdown of CBS and CSE and exposure to inhibitors has been shown to decrease the hypoxic ventilatory response of zebrafish larvae and adults, respectively (Porteus et al. 2014a). Addition of H2S using sodium sulfide increased breathing frequency in larvae and increased Ca2+ in isolated NECs from adult zebrafish (Porteus et al. 2014a). CSE, but not CBS was localized to larval NECs, indicating that H2S is involved in oxygen sensing in NECs (Fig. 4).
Direct information about the O2 sensory physiology of fish gill NECs has been derived from a small number of critical patch clamp (voltage and current clamp) studies on single cells from heterogeneous primary cultures. Putative gill NECs from zebrafish expressed K+ currents (IKO2 or IKB) that were blocked by quinidine but were insensitive to voltage dependent K+ current blockers TEA or 4-AP (Jonz et al. 2004). These channels were similar to the hypoxia responsive "background" K+ current in glomus cells, and inhibition of the K+ current by hypoxia was dependent upon O2 tension. In channel catfish, putative NECs responded to hypoxia with either inhibition or potentiation of a voltage dependent K+ current. The cells with hypoxia-inhibited K+ channels seemed morphologically more like glomus cells and the NECs from zebrafish. There was no specific K+ channel pharmacology reported in this study, but cyanide caused an irreversible decrease in K+ conductance in both types of cells (Burleson et al. 2006). Lastly, putative NECs from hypoxia-tolerant goldfish (C. auratus) were minimally responsive to hypoxia, but depolarized in response to anoxia and cyanide, as assessed with current clamp experiments (Zachar and Jonz 2017).
The O2-dependent regulation of gene transcription via the PHD-HIF-pVHL pathway
The canonical PHD-HIF-pVHL hypoxia inducible factor (HIF) pathway is a key regulator of O2 homeostasis and governs changes in gene transcription associated with hypoxic events, and the subject of many excellent reviews (Kaelin and Ratcliffe 2008; Mills et al. 2018; Pelster and Egg 2018; Mandic et al. 2021b). The following is a summary of key data relevant to HIF signaling in teleost O2 chemosensing.
HIF is a heterodimeric protein composed of HIF-α subunits and HIF-β subunits. Only the HIF-α subunit is affected by O2 levels. O2 availability determines the activity and stability of HIF-α, via prolyl hydroxylase factor (PHD) which hydroxylates residues within HIF-α. Factor inhibiting HIF (FIH) proteins contribute to the HIF-α response to hypoxia. With adequate O2 levels, PHDs hydroxylate HIF-α, which is then recognized by von Hippel-Lindau tumor-suppressor protein (pVHL) leading to HIF-α degradation. Decreases in O2 result in decreases in O2 dependent hydroxylation of HIF-α, and a subsequent failure of VHL targeting for degradation. Consequently, HIF-α accumulates in the nucleus and complexes with HIF β. Subsequently the HIF heterodimer binds to hypoxia response elements of specific genes followed by gene transcription. The time-dependent HIF-1α to HIF-2α transition (two common HIF isoforms), sometimes called the "HIF switch", arises from different durations of hypoxia exposure, resulting in different patterns of gene regulation in response to hypoxia (Lobada 2012). HIF-1α tends to regulate acute responses while HIF-2α appears to be associated with prolonged or chronic hypoxic events (Holmquist-Mengelbier et al. 2019; Bartoszewski 2019; Jaskiewicz et al. 2022). Thus, through the PHD-HIF-pVHL pathway, hypoxia stabilizes HIF alpha and promotes transcription of genes supporting an organism's response to hypoxia (Fig. 4).
Fish, relative to mammals, are especially vulnerable to changing O2 levels in aquatic environments or those caused by anthropogenic impacts (Mandic and Regan 2018). Additionally, in fish, oxygen availability and HIF signaling are linked with photoperiod and molecular clocks (Egg et al. 2013; Pelster and Egg 2018). The HIF pathway is a key component of O2 chemosensing which orchestrates whole animal, tissue, and cellular events dependent on O2 metabolism over time spans longer than an hour. Hypoxia increases levels of HIF-1α isoforms in nearly 30 species of fish (Pelster and Egg 2018; Mandic et al. 2021a; Pan et al. 2022), including hypoxia tolerant species (Carassius carassius; Rissanen et al. 2006; Sollid et al. 2006) and hypoxia sensitive species (O. mykiss, Soitamo et al. 2001).
HIF signaling plays a role in the teleost hyperventilatory response to hypoxia. Generally, in evolution, gene duplication results in increased genetic diversity, serving as substrate for evolutionary adaptation (Rytkönen and Storz 2011; Rytkönen et al. 2011). Genome-wide gene duplication events in teleost evolution gave rise to duplicates of three paralogs of HIF-1α: hif-1α, hif-2α and hif-3α (Rytkönen et al. 2013). The HIF alpha duplicated paralogs were subsequently lost in most teleost lineages, but some sub-families of the hypoxia tolerant cyprinids (such as the zebrafish Danio rerio) retained them. Positive selection on the duplicated paralogs may have conferred greater adaptability of cyprinids to hypoxic environments (Mandic et al. 2021a, b). For example, HIF-1α may contribute to hypoxia tolerance as a result of hypoxia pre-exposure, as shown in zebrafish (Chen et al. 2013; Mandic et al. 2020, 2021a).
Additional gene duplications relevant to hypoxia in teleosts are currently being revealed. One example found in zebrafish are two orthologs for the hypoxia inducible gene IGFBP-1 (Insulin-like growth factor binding protein). IGFBP-1 modulates IGF and subsequently hypoxia-induced embryonic growth and developmental time course (Kamei et al. 2008). Heme oxygenase (HO) is likely involved in modulation of calcium sensitive BK K+ channels associated with acute O2 sensing in mammals (Williams et al. 2004). Two HO-2 genes (HO-2a and -2b) are found in zebrafish (Tzaneva and Perry 2014; Perry and Tzaneva 2016) as well as the hypoxia sensitive blunt-snouted bream Megalobrama amblycephala (Zhang et al. 2017), and therefore may be highly relevant to acute O2 sensing (see above). These and other results of gene duplication in teleost evolution may diversify the perspective on processes involved in hypoxia sensing.
The involvement and mechanisms of HIF signaling in acute and chronic hyperventilatory responses to hypoxia are not well known and are of critical interest (reviewed by Mandic et al. 2019). Hypoxia tolerance is reduced in adult zebrafish with the loss of HIF-1α (Joyce and Perry 2020; Mandic et al. 2020). The hyperventilatory response (HVR) occurs in specific "time domains" (Powell et al. 1998; Porteus et al. 2011), and the HVR can be influenced by HIF signaling. However, immediate to acute (seconds to about 60 min) events in the HVR are not likely to be influenced in adult fish by HIF-1α signaling (Mandic et al. 2019; Mandic et al. 2021a). In fish the acute phase of the HVR can be prolonged through a time span of minutes to hours, and this phase is modulated by HIF 1α (Perry and Tanzeva 2016; Mandic et al. 2019) via nNOS (nitric oxide synthase) (Porteus et al. 2015). Further, nNOS is localized to larval skin and gill NECs, as shown by immunohistochemical labeling (Porteus et al. 2015). This last finding is especially noteworthy demonstrating a direct correlation between HIF signaling, the teleost HVR, and O2 chemosensing by gill NECs.
Other contributions of the HIF pathway to acute O2 chemosensing in fish are unfortunately sparse. However, a powerful recent study used a single cell transcriptomic analysis of cellular responses to chronic (2 weeks) hypoxia versus normoxia. Sixteen different cellular transcriptomic profiles of gill filament cells of the zebrafish (D. rerio) were constructed (Pan et al. 2022). Single cell type transcriptomic profiles revealed that gill NECs were specifically enriched for two critical components of O2 molecular sensing. As mentioned above, features of glomus cell O2 sensitive mitochondria were highly enriched in the NEC specific transcript profile. In addition, HIF pathway associated genes were also strongly and specifically enhanced in NECs, including G-protein signaling proteins (Rgs4 and Rgs5). Both Rgs 4 and Rgs5 are targets of HIF in glomus cells. Further Rgs5a is a target of HIF-2α in O2 sensitive adrenal medullary cells (Gao et al. 2017). All these findings support zebrafish gill NECs as endowed with enhanced O2 sensory abilities in acute and chronic time periods.
Inconsistencies
Many inconsistencies associated with gill O2 chemosensing are reviewed in other sections, so what follows is a more focused evaluation of some issues confounding our understanding of mechanisms of O2 sensing from cellular and in situ Studies.
Ambiguity within cellular electrophysiology studies
The whole cell patch clamp technique was used for recording the O2 response properties of K+ currents in putative NECs in primary cell culture (Jonz et al. 2004; Burleson et al. 2006; Zachar and Jonz 2017). It is known that K+ ion channels in glomus cells are not directly modulated by O2, since isolated membrane patches containing these channels are not directly O2 sensitive (Buckler et al. 2000; Buckler 2015). Further, K+ channels in PNECs/NEBs require modulation by intracellular molecules to couple them to O2 levels (Fu et al. 2000). As remarked on (Jonz et al. 2015) the whole cell patch clamp configuration opens a window through the plasma membrane into the cytosol, which can lead to dialysis of cytosolic constituents including calcium and second messengers. This can distort the behavior of K+ currents that are modulated by cytosolic factors in response to acute hypoxia or anoxia. Additionally, plasma membrane K+ channel properties were also used as defining features to identify candidate hypoxia sensory cells within heterogenous gill filament primary cultures. Therefore mis-identification of NECs or non-identification of other cells responsive to acute hypoxia may have resulted. Voltage clamp with perforated patches, which pass monovalent cations through pore forming molecules in the electrode solution (Linley 2013) might be more useful for characterizing the O2 responsive currents of cells by retaining intracellular organelles, modulators, and calcium. Modifying voltage clamp protocols therefore becomes particularly important due to the known modulation of K+ channels by products of cellular respiration in glomus cells and PNECs/NEBs (Buckler et al. 2000; Buckler 2015).
Cell identity stemming from heterogeneous gill filament cultures
Single cell studies of the O2 chemosensory properties of gill NECs are difficult to interpret due to challenges in identifying NECs among multi-cellular populations. Gill NECs, dissociated and placed in acute or primary cultures, are typically identified by labeling with the vital dye Neural Red (NR). NR partitions into acidic compartment of cells which could potentially label many different cell types including neurons and ionocytes, as well as NECs. Also, larger cells within the mixtures of dissociated cells might be chosen for recording, which is another biasing parameter for candidate NEC selection. With the finding of VAChT+ cells in zebrafish gill filaments (Zachar et al. 2017), distinguishing between seronergic versus cholinergic cells becomes problematic.
As a case in point, a study in zebrafish used the fluorescent styryl dye FM 1–43 to label cells for levels of vesicular activity in the plasma membrane, to measure secretory activity in response to the hypoxia mimetic NaCN (Jonz et al. 2015). The FM 1–43 labelled cells in situ which looked like NECs in terms of their location, size, and morphology, but they were not counter-stained with a confirmatory immunohistochemical probe for serotonin. This study itself showed interesting results: putative neutral red + NECs in vitro labelled more brightly in the aerobic metabolism blocker NaCN than control cells. This effect was blocked by the calcium channel blocker cadmium, which decreases vesicular activity. However, the cell populations used in these experiments likely contained cholinergic cells as well as serotonergic NECs (Zachar et al. 2017). Interpreting the data could have been clarified by differentiation between the putative gill NECs and other cells present in culture.
Serotonin transporters (SERTs) are expressed in NECs of zebrafish gill filaments, as revealed by transcriptome analysis (Pan et al. 2022). Also, SERTs are known to be present in the gill tissue of channel catfish, although the presence of SERT mRNA or plasma membrane bound protein has not yet been pinpointed to gill NECs (Amadour and McDonald 2018). Therefore, one potential method of labeling living serotonergic NECs more specifically is with APP + , a fluorescent substrate for monoamine transporters. APP + enters cells through serotonin transporters (SERTs), eventually localizing to mitochondria (Karpowicz et al. 2013; Li et al 2022). One caveat, however, is that APP + can enter cells via DATs (dopamine transporters) or NETs (norepinephrine transporters) but with lower efficacy (Karpowicz et al. 2013). Consequently, any dissociated DAT + neurons might label with APP + in mixed cell cultures (Reed et al. 2023). APP + could be used to label NECs on filaments, pre-or post-dissociation, perhaps in conjunction with NR, cell size, or FM dyes (Jonz et al. 2015) when placed into culture.
Gaps and future directions
Relative to mammalian O2 sensors, knowledge of acute and chronic O2 sensing at the cellular level is still indeterminate in fish gills. Revised experimental strategies, such as those outlined above, might augment the findings of some original experiments. Serotonergic NECs fit most criteria for gill O2 chemosensors, but resolution of the associated O2 sensory network(s) involved (as well as other candidate chemosensors) remains terra incognita.
Morphometric changes in response to chronic hypoxia in candidate O2 sensory cells have been a reliable but indirect means of identifying the cells involved in O2 sensing (Jonz et al. 2004; Burleson et al. 2006; Regan et al. 2011; Shakarchi et al. 2013; Porteus et al. 2014b, Rossi et al. 2020; Pan et al. 2021). However, these measurements are often influenced by fixation and sectioning, wherein the topography of the sensory circuit becomes lost. A feasible test of transmitter release from identified cells could consist of the use of mapping gill filaments using correlated light and electron microscopy (CLEM) (Begemann and Galic 2016; Friedrichsen et al. 2022; De Boer et al. 2015). Use of ultra rapid freezing under high pressure could capture very rapid chemotransmission events such as degranulation or vesicular fusion in identified NECs, neurons, or other secretory cells in response to acute or chronic hypoxia (Watanabe 2016; Baatsen et al. 2021). High resolution, accurate and precise morphometric qualities could be then mapped in identified cells. This approach could be applied across species and represent a “next-best-thing-to-in vivo” proxy for identifying O2/CO2 sensory cells underpinning the HVR.
CO2/pH sensing
Summary
The initial evidence for the location of CO2 sensing sites in fish gills came from denervation experiments that showed that the ventilatory responses to CO2 were abolished by the denervation of branchial arches, with the 1st gill arch being more important than the rest in some fish species (Burleson and Smatresk 2000; Sundin et al. 2000; Perry and Reid 2002; McKendry and Perry 2001; Florindo et al. 2004). Fish also responded to elevated PCO2 with a tachycardia, rather than the bradycardia seen in response to hypoxia (Miller et al. 2014). Experiments designed to test external (water) versus internal (blood) CO2 sensing indicate that these chemoreceptors primarily respond to changes in water PCO2 and specifically to changes in CO2 rather than pH (reviewed by Milsom et al. 2022). Whether receptors exist in the gills that can respond specifically to changes in the CO2 of arterial blood remains unclear. Indirect evidence suggests there may be. Following exhaustive exercise in normoxic water, ventilation remains elevated although arterial PO2 returns to normal. Arterial PCO2, however, also remains elevated and pH decreased. Reducing the post-exercise acidosis with carbonic anhydrase injections reduces elevated ventilation (Wood and Munger 1994).
More recently direct electrophysiology and Ca2+ imaging experiments have shown that some NECs depolarize in response to changes in CO2 as well as in O2 implicating NECs as the CO2 chemoreceptors (Qin et al. 2010; Abdallah et al. 2014). In adult zebrafish, only a subset of NECs responded to both CO2 and O2 (Qin et al. 2010), indicating that there might be different subpopulations of NECs responsible for chemoreception of these different respiratory gases. That the receptors that also respond to changes in PCO2 produce different changes in heart rate than those responding to hypoxia suggest that those responding to changes in PCO2 have different central projections than those that respond only to changes in PO2.
Mechanisms
The exact sensing mechanism for an increase in CO2 in the water is unknown but the general sensing pathways follow the oxygen sensing pathway, with an increase in PCO2, producing inhibition of potassium channels leading to retention of K+ which in turn leads to membrane depolarization, activating voltage gated Ca2+ channels. This results in an increase in internal calcium concentration, followed by the release of neurotransmitters (Fig. 5).
Proposed CO2 sensing mechanism in NECs. CO2 enters the NECs and reacts with water to produce bicarbonate and protons through the action of carbonic anhydrase, CA (1). Either CO2 or the associated decrease in cellular pH cause the closing of K+ channels (2). TASK-2 channels are a type of background K+ channels that are present in zebrafish. The closing of K+ channels in turn causes a decrease in resting membrane potential. This leads to the opening of voltage-dependent Ca2+ channels (CaV) and/or release of Ca2+ from intracellular calcium stores (3). The increase in internal Ca2+ concentration leads to the fusion of vesicles and release of neurotransmitters (4) Created with BioRender.com
Although the exact sensing mechanisms are unknown, cytosolic carbonic anhydrase (CA) is likely involved as it catalyzes the hydration reaction of CO2 to bicarbonate and protons, contributing to the acidification of the cell. Specifically, cytosolic CA17a has been found in the NECs of both zebrafish adults (Qin et al. 2010) and larvae (Miller et al. 2014; Kunert et al. 2022) and it is the isoform thought to be involved in CO2 sensing. The inhibition of CA by acetazolamide (Miller et al. 2014; Qin et al. 2010), by morpholino knockdown of CA2-like/ca17a (Miller et al. 2014) or by CRISPR/Cas9 knockout of ca17a (Kunert et al. 2022) generally reverses the cardioventilatory responses to hypercapnia, supporting an important role of CA in sensing CO2 (but see discrepancies below). There is a residual response of NECs to hypercapnia after CA inhibition which presumably reflects intracellular acidification occurring at the uncatalysed rate of CO2 hydration. This suggests that the presence of CA in CO2-sensing cells allows a more rapid and vigorous response (Qin et al. 2010).
Background K+ channels have been involved in CO2 sensing in adult zebrafish (Qin et al. 2010). TWIK-related tandem pore domain acid-sensitive K+ (TASK-2) channels are a type of background K+ channel recently identified in both adult gill (Peña-Münzenmayer et al. 2014) and larval epidermal NECs of zebrafish (Koudrina et al. 2020). Zebrafish TASK-2 channels expressed in a mammalian cell line (HEK-293) have been shown to respond by inhibition to either a decrease in intracellular pH, or an increase in PCO2 independent of changes in pH (Peña-Münzenmayer et al. 2014), making them ideal for sensing CO2 in fish gills. In zebrafish larvae, TASK-2 channels have been found in most epidermal NECs (Koudrina et al. 2020), but these have not been specifically localized to gill NECs in adult fish (only in gill tissues) or other fish species. The closing of K+ channels, and the change in voltage that follows, triggers the influx of Ca2+ from internal or external stores. Ca2+ has been shown to primarily come from internal, rather than external stores in zebrafish (Abdallah et al. 2015a), and it’s unclear if this is the case for other fish species as well. This in turn is thought to lead to the fusion of synaptic vesicles with the cell membrane and the release of neurotransmitters. Exactly which neurotransmitters are involved in CO2 sensing remains unknown (Perry et al. 2023).
Inconsistencies
Key genes not identified in single cell RNAseq
Despite the experimental, immunohistochemical and pharmacological evidence suggesting that TASK-2 channels are involved in CO2 sensing, a recent single cell RNA-seq study on NECs did not detect the genes for these channels in adult NECs (Pan et al. 2022). Of note, TASK-2 channel genes have been found in whole gill extracts from adult zebrafish (Peña-Münzenmayer et al. 2014), but the TASK-2 protein has only been localized in the skin NECs of zebrafish larvae. Skin NECS are a population of cells that might be different than the branchial NECs identified in adults, providing a basis for this discrepancy. This is also similar to the situation for cytosolic CA, where the gene ca17a was not detected in the single cell RNAseq study (Pan et al. 2022), but cytosolic CA has been identified using immunohistochemistry in adult NECs (Abdalla et al. 2014). It remains unclear why these genes were not found in adult NECs using RNAseq (Pan et al. 2022), but might reflect an insufficient depth of coverage during sequencing in that study.
Stimulus in whole animal versus single cell experiments
Several in vivo studies, have shown that ventilation changes in response to increases in external PCO2 but not pH in most species (see above). Consistent with this, electrophysiological measurements on isolated NECs indicated that NECs respond to external PCO2 and not to changes in external pH. However, an internal decrease in pH was also necessary for depolarization of the NECs (Qin et al. 2010). In contrast, Ca2+ imaging studies show that Ca2+ concentration of the NECs does not increase in response to increases in external PCO2 alone, only when there is also an associated drop in external pH, and that they do not respond to a decrease in internal pH alone (Abdallah et al. 2014). It is hard to reconcile this discrepancy between whole animal and cellular level responses and between electrophysiological and calcium release data.
Changes in NEC size in different species in response to hypercapnia
Some studies have shown that a decrease in oxygen level increases the size or density of NECs just as in mammalian glomus cells, likely due to the increase in neurotransmitter cycling and storage (Jonz et al. 2004; Regan et al. 2011). However, evidence of the effects of change in external PCO2 on NEC size is scarce and discrepant. Acclimation of zebrafish to hypercapnia for 28d did not cause any change in density or size of NECs, but a 7-day exposure to 5% CO2 increased cell density in mangrove rivulus (K. marmoratus; Robertson et al. 2015) and three spine stickleback (Soor et al. unpublished data). These observations are hard to interpret due to the use of different species, different experimental stimuli, and different outcomes.
Role of carbonic anhydrase in CO2 sensing
The role of cytosolic CA in the sensing process is unclear as internal acidification alone did not cause an increase in internal Ca2+ in zebrafish (Abdallah et al. 2014). Additionally, application of acetazolamide did not abolish the increase in internal Ca2+ in response to 5% CO2 (Abdallah et al. 2014), however this is a very high PCO2 for this species. As noted above, however, this may reflect intracellular acidification occurring at the uncatalyzed rate of CO2 hydration suggesting that the presence of CA in CO2-sensing cells allows a more rapid and vigorous response (Qin et al. 2010).
Tachycardia versus bradycardia
An interesting conundrum is how some NECs can respond to both CO2 and O2, but give rise to different responses in heart rate (tachycardia and bradycardia, respectively) when the neurotransmitter that is released is thought to be serotonin under both circumstances (although serotonin has not been shown to be released in response to either of these gases). This would indicate that perhaps different neurotransmitters are involved in the response to these two different stimuli or that the NECs that response to CO2 and O2 project to different areas of the brain than those that respond to O2 only and thus lead to different whole animal responses.
Gaps and future directions
Although much progress has been made regarding CO2 sensing in NECs in the past decade, there are still large gaps in our knowledge. While several oxygen sensing mechanisms have been proposed, we still don’t know what the exact sensing mechanism is for CO2, and whether the sensing involves molecular CO2 or a change in pH (internal, external or trans-membrane difference) or both. Furthermore, although it’s assumed that 5-HT is the primary neurotransmitter released by NECs in response to this stimulus, this has not been confirmed and other neurotransmitters have not been considered. Moreover, nothing is known about the higher brain centers involved in the integration of the sensory information that gives rise to the cardiorespiratory response to CO2 in fish. Lastly, there is no direct evidence linking the sensing of CO2 by NECs to changes in ventilation or heart rate directly, in adults or in larvae.
Ammonia
Summary
The respiratory gas aside from O2 and CO2 that has gained attention as a respiratory gas in fish is ammonia (Zhang and Wood 2009; Zhang et al. 2011; Zhang et al. 2013; De Boeck and Wood 2015; Zhang et al. 2015). Ammonia, which is toxic to fish in high concentrations exists in two forms: either as a dissolved gas (NH3) or the ammonium cation (NH4+). Generally, NH4+ is the predominant chemical species at physiological pH due to the high pK (~ 9.1 at 28 °C) of the equilibrium reaction. For simplicity, throughout this section ammonia refers to total NH3 and NH4+, unless otherwise stated. In ammoniotelic teleosts, ammonia represents ~ 70% of the nitrogenous waste produced from the catabolism of proteins. As post-prandial blood levels rise, ammonia is continuously excreted from the gills. Similar results are seen post strenuous exercise. On the other hand, high external ammonia, from either densely populated aquaculture facilities or areas with heavy eutrophication, also causes blood ammonia levels to rise. In either case, fish must actively attempt to match rate of ammonia excretion with rate of nitrogenous waste production to maintain ammonia levels within an optimal range. Both increasing levels of internal and external ammonia lead to increases in ventilation. While some ammonia may pass by simple diffusion through the cell membranes of the gills, most ammonia movement through the branchial epithelium is facilitated by channels (Rh glycoproteins) (Nakada et al. 2007; Nawata et al. 2007). It has been suggested that ammonia acts on internal receptors only and that external HEA only stimulates ventilation after the ammonia diffuses into the gills (Zhang et al. 2015; De Boeck and Wood 2015; Eom et al. 2019) This makes sense as under natural conditions, HEA is not as common as elevated internal ammonia from feeding or exhaustive exercise and, therefore, internal detection would be more physiologically relevant. It has now been shown that serotonergic NECs on all gill arches in juvenile rainbow trout respond to ammonia and appear to be the peripheral ammonia chemosensing cells. The adaptive advantages of increasing ventilation following feeding and/or exercise to enhance O2 uptake and excrete ammonia are evident but the role of increases in ventilation in response to high external ammonia (HEA) is less evident (see inconsistencies below).
To date, ammonia has been shown to trigger hyperventilation in rainbow trout (Zhang and Wood 2009; Zhang et al. 2011; Zhang et al. 2013; Zhang et al. 2015), zebrafish (Perry and Tzaneva 2016; Porteus et al. 2021), dogfish shark (Squalus acanthias suckleyi; De Boeck and Wood 2015), and Pacific hagfish (Eptatretus stoutii; Eom et al. 2019). In most of these species, the hyperventilatory response has been shown to be due to ammonia itself and not the changes in blood acid–base status which are often associated with experimental ammonia treatments (Zhang and Wood 2009). It has been suggested based on hagfish studies that ventilatory responses to all three gases in vertebrates arose in the myxine lineage (Perry et al. 2009; Eom et al. 2019). In this regard, it is interesting that elasmobranchs such as the dogfish shark respond to ammonia with hyperventilation even though these fish do not excrete metabolic nitrogenous wastes but instead retain nitrogen as urea as an osmoregulatory strategy in seawater (Hazon et al. 2003). Given that these groups of fish have very different strategies to cope with nitrogenous wastes and that mammals also possess these peripheral and/or central ammonia chemoreceptors (Wichser and Kazemi 1974), it is suggestive that this innate response to ammonia arose early in vertebrate evolution and has been conserved.
Mechanisms
As mentioned above, hypoxia and hypercapnia both cause inhibition of background K+ currents in NECs (Jonz et al. 2004; Burleson et al. 2006; Qin et al. 2010) leading to depolarization and an influx of Ca2+ via voltage-gated Ca2+ channels. This leads to neurotransmitter and/or neuromodulator release and afferent nerve activation. It was originally hypothesized by Randall and Ip (Randall and Ip 2006) that ammonia initiated this same cascade. Subsequently, Zhang et al. (2011) observed two different types of [Ca2+]i responses of NECs in culture to high NH4+ perfusion: a slow response and a fast-plus-slow response. The fast response was similar in shape and magnitude to the response to high K+ supporting the hypothesis that ammonia leads to depolarization and the opening of voltage-gated Ca2+ channels. The slow response might result from intracellular acidosis associated with ammonia washout. Interestingly, while NH4+ is known to pass through K+ channels, the permeability of these channels to NH4+ is only 10–30% that of K+ (Randall and Ip 2006). The similar rapid responses to both K+ and ammonia suggested a more rapid mechanism of ammonia entry into the NECs (Zhang et al. 2011). It was suggested that Rh glycoproteins, known for their role in transporting ammonia (Nawata et al. 2010; reviewed in Wright and Wood 2009), facilitated ammonia entry into chemoreceptive cells (Zhang et al. 2015) contributing to the fast response. However, recent data suggest that, in both adult and larval zebrafish, the response of NECs to HEA does not require Rh proteins; therefore, leaving the basis of the rapid response of NECs to ammonia unresolved (see inconsistencies below).
Inconsistencies
Ventilatory frequency versus amplitude
In adult zebrafish and rainbow trout, acute exposure to ammonia caused increases in ventilation amplitude but not frequency (Zhang et al. 2011; Porteus et al. 2021). This is consistent with the ventilatory response to hypercapnia, but not hypoxia. In rainbow trout, when ammonia was injected intravascularly, however, small increases in breathing frequency in addition to large elevations of amplitude were observed (Zhang and Wood 2009; Eom et al. 2020). In spiny dogfish (De Boeck and Wood 2015) both ventilatory amplitude and frequency increase during acute ammonia exposure, although the increase in amplitude was much more drastic in the dogfish than rainbow trout (De Boeck and Wood 2015; Zhang et al. 2009). In contrast to adult zebrafish, larval zebrafish [4 days post fertilization (dpf)] show an increase in ventilation frequency in response to HEA, however, due to their size, amplitude was not measured (Porteus et al. 2021). The different effects on the frequency and amplitude of ventilation between hypoxia and CO2/ammonia are suggestive of different receptors projecting to diverse integrating sites in the CNS rather than of a single shared population of chemosensing cells. In other words, if the chemoreceptors are NECs, those that sense ammonia and CO2 have a different afferent innervation than those that sense changes in O2. Or perhaps the thresholds or specific sensors for amplitude and breathing frequency are different. There may also be subsets of NECs with different neurotransmitters and neuromodulators which are released upon stimulation acting on different afferent nerves.
Convection versus diffusion limitation
Originally it was hypothesized that branchial ammonia excretion was dependent only on diffusion suggesting that only ammonia gradients (PNH3) and/or NH4+ electrochemical gradients, dictated movement from the blood to the external environment or vice versa (Randall and Ip 2006). Increasing ventilation when plasma ammonia levels are elevated would reduce ammonia levels in the gill boundary water as it diffuses from the gills and increase the net diffusion gradient. This hypothesis, however, was made prior to the knowledge of the presence of Rhesus (Rh) glycoproteins in the gills and their associated metabolon which facilitates ammonia transport (Hung et al. 2007; Nawata et al. 2007). Our current understanding is that both convection and diffusion influence ammonia excretion. Hyperventilation is successful at increasing ammonia excretion across the gills once diffusive limitation has been eliminated by activation of the Rh metabolon system (Eom et al. 2020). More specifically, during rest when the plasma ammonia concentration is low, diffusion limits excretion rates. This was shown in resting trout by attempting to manipulate ventilation using either hypoxia or hyperoxia to stimulate ammonia excretion, which was unsuccessful. After exercise or during digestion of a meal, however, greater convection of water due to increased ventilation enables more ammonia to be washed away facilitated by the Rh metabolon which prevents diffusion trapping of ammonia (Eom et al. 2020). It should be noted that in trout, heart rate is not influenced by ammonia loading or hyperoxia and only declines following hypoxia (Eom et al. 2020). In the case of HEA, ammonia diffuses into plasma and ultimately causes toxicity. While increasing ventilation would be predicted to enhance this uptake, ultimately, plasma concentrations will equilibrate by reducing ammonia accumulation at the boundary layer, it once again reduces the buildup of metabolically produced ammonia.
Role of rhesus glycoproteins
It was recently shown, using immunohistochemistry, that Rh proteins (Rhcgb, Rhbg or Rhag) in zebrafish are not co-localized with 5-HT in gill filament NECs (Porteus et al. 2021). Additionally, rhcgb knockout fish have similar responses as wildtype fish when acutely stimulated with HEA. Similarly, in larval zebrafish, Rhcgb, Rhbg and Rhag were not detectable using IHC, however, in rhcgb knockout fish, the ventilatory response to HEA was attenuated. Overall, the data suggest that, in both adult and larval zebrafish, the response of NECs to HEA does not require Rh proteins leaving the basis of the rapid response of NECs to ammonia unresolved.
Gill arches involved in ammonia sensing
In adult rainbow trout, the acute hyperventilatory response to ammonia was completely abolished with the removal of gill arches I and II, suggesting that ammonia sensors are only found within these two arches. However, all four gill arches immunostained for 5-HT-NECs and NECs cultured from all four arches responded to high ammonia with a raise in [Ca2+]i as shown by fura-2 ratiometric calcium imaging (Zhang et al. 2011). Additionally, following exposure to chronic (28-d) ammonia, the size of the NECs on all four gill arches decreased (Zhang et al. 2011). Explaining the difference between in vivo and in vitro results remains problematic.
Gaps and future directions
Chemosensory cascade
Currently little is known regarding the chemosensory cascade triggered by increased ammonia. Intracellular ratiometric calcium imaging is a powerful tool to directly demonstrate the activation of NECs from the three respiratory gases and, with the use of agonists and antagonists, to establish upstream mechanisms. All three stimuli cause a rapid increase in intracellular calcium ([Ca2+]i) in rainbow trout (Zhang et al. 2011) and zebrafish (Porteus et al. 2021) suggesting they employ similar mechanisms. NECs isolated from gill arches I through IV of rainbow trout showed a similar response to both high K+ and ammonia (Zhang et al. 2011). However, when an ammonia response was compared with that to CO2, in zebrafish, the magnitude of the [Ca2+]i increase was substantially lower with ammonia; ~ 30% compared to 800% with hypercapnia (Porteus et al. 2021) suggesting sensitivity differences between the two stimuli, however, more studies are required to substantiate this possibility. Furthermore, in the studies of Zhang et al. (2011), ammonia caused two types of [Ca2+]i responses, a slow and a fast-plus-slow response. The fast response was similar to the response to exogenous K+. The slow response in both types was delayed and occurred after the removal of ammonia and was thought to be linked to the change in pHi due to its long recovery time. Indeed, intracellular acidification has been proposed as one of the main mechanisms of CO2 sensing in fish (Qin et al. 2010) and has been demonstrated in the mammalian system (Lahiri and Forster 2003). In future investigations, it would be beneficial to monitor the change of pHi in NECs responding to ammonia. Sorting out similarities and differences in the response of NECs to O2, CO2 and ammonia remains an important goal.
External versus internal ammonia sensing
In adult rainbow trout, the acute hyperventilatory response to ammonia was delayed by ~ 30–40 min when the first gill arch (gill arch I) was removed and completely abolished with the removal of gill arches I and II. Based on these vivo results, Zhang and colleagues (2011) initially hypothesized that gill arch I receptors could be responsible for sensing external waterborne ammonia which rapidly diffused across the epithelial and mucus barrier and explaining the quick response time to external ammonia when gill arch I is functional. Whereas, because of the time delay when gill arch I was removed, arch II receptors might sense only internal plasma ammonia, consistent with the time it would take for internal plasma ammonia levels to rise following external exposure. This would suggest that rhesus glycoproteins were only present in the epithelia of the first gill arch. Alternatively, in teleosts, gill arch I is innervated by a branch of the glossopharyngeal nerve (IX), whereas gill arches I-IV are innervated by the vagus nerve (X; Milsom and Burleson 2007). This difference in innervation may also explain the different roles of arch I and II NECs in ammonia sensing. Yet another alternative theory would be the involvement of the pseudobranch in ammonia sensing. The pseudobranch contains 5-HT positive NECs in zebrafish (Jonz and Nurse 2003) and would represent an internal ammonia sensor as it is not exposed to the external environment. These are hypotheses that are yet to be tested.
Integration of chemosensory signals
To the best of our knowledge, there has only been one study assessing the integration of the ventilatory response to ammonia along with those of O2 and CO2. In that study the hyperventilatory response to HEA was abolished following exposure to hyperoxia (Porteus et al. 2021). This brings us back to our earlier comment about the adaptive advantages of increasing ventilation on exposure to HEA and indicates that the need to increase O2 supply for metabolism outweighs the need to excrete ammonia. To further complicate these interactions, chronic exposure to HEA ablates the ventilatory response to ammonia (Zhang et al. 2011), suggesting that sensitivity can be altered by chronic and maybe intermittent exposures. Regardless, more information is required on the integration of the chemosensing signals to determine if they are additive, synergistic, or antagonistic in nature.
Alternative to gill ammonia sensing
Although it is clear that NECs are involved with the hyperventilatory response to ammonia, there is strong evidence that the brain is also involved and may even be the primary driver of the ventilatory response to elevated internal levels of ammonia (Zhang et al. 2013). Indeed, central chemoreceptors of the brain in mammals are a second site of ventilatory sensitivity to respiratory gases and previous research has shown that ammonia concentrations of the brain tissue were more strongly correlated to increases in ventilation than plasma or cerebrospinal fluid concentrations (Wichser and Kazemi 1974). Along these same lines in fish, ammonia readily passes through the blood–brain barrier in fish (Wright et al. 2007) and mRNA expressions of the Rhbg and Rhcg1 glycoproteins have been found in the brain of trout (Nawata et al. 2007; Zhang et al. 2013). In trout, ventilation was more strongly correlated with brain ammonia than plasma ammonia (Zhang et al. 2013). Injecting the surface of the hindbrain of trout caused immediate hyperventilation, providing direct evidence of central chemoreception in teleost (Eom and Wood 2021a, b). Overall, there is building evidence that ammonia in the brain could function in driving ventilation in fish.
Lactate
Summary
Much less is known about the effects of lactate on gill chemosensing cells and we are still heavily reliant on the mammalian literature to help guide us on understanding the role of this metabolite in regulating breathing. Lactate is a by-product of anaerobic metabolism and is uniquely equipped to signal inadequate oxygen availability in cells. More specifically, during normoxic conditions, lactate levels are kept low due to the reversible reaction of lactate dehydrogenase to form pyruvate, which is readily accepted for use in the tricarboxylic acid cycle (TCA). However, in hypoxia, the TCA cycle slows down causing a buildup of pyruvate shifting the pyruvate-lactate equilibrium towards lactate accumulation. In this way, lactate is an ideal signaling molecule to activate the cardiorespiratory system to improve oxygen supply to the tissues (Thomsen et al. 2019). Lactate is also commonly used as an energy source in several tissues, including the heart, and is a key substrate for gluconeogenesis in the liver (Brooks 2020). Hypoxia and exercise both induce an increase in blood lactate levels and circulating lactate in both fish and mammals induces a dose-dependent increase in ventilation which is independent of pH (Thomsen et al. 2019, 2017; Torres-Torrelo et al. 2021).
Lactate is gaining more attention as a signaling molecule in the respiratory control of vertebrates (Chang et al. 2015; Thomsen et al. 2019, 2017; Torres-Torrelo et al. 2021; Leonard et al. 2022). In mammals, lactate is sensed by O2 and CO2/H+ peripheral chemoreceptors (i.e. glomus cells) located in the carotid body (Torres-Torrelo et al. 2021) which are responsible for maintaining overall homeostasis of the cardiorespiratory system (Nurse 2005). In rainbow trout and the air-breathing catfish Pangasius, increases in plasma lactate have been shown to increase ventilation and activate the hypoxic ventilatory response (Thomsen et al. 2017, 2019). Removal of the afferent input either by denervation or removal of the first gill arch attenuated lactate sensing, supporting a role for gill NECs in this process (Thomsen et al. 2017, 2019). More recently, it was confirmed in killifish, using single cell calcium imaging techniques, that the cells that sense lactate were also sensitive to high K+ and, in some cases, to both hypercapnia and high K+ (Leonard et al. 2022). It was shown that only L-lactate, not D-lactate, induces the dose-dependent increase in ventilation and bradycardia at physiologically relevant concentrations and constant pH (Thomsen et al. 2019) (Fig. 6).
Proposed ammonia sensing mechanism in NECs. It is unclear how ammonia enters NECs or is sensed (1). Ammonia is thought to cause the closing of K+ channels (2). The closing of K+ channels in turn causes a decrease in resting membrane potential. This leads to the opening of voltage-dependent Ca2+ channels (CaV) and influx of Ca2+ (3). The increase in internal Ca2+ concentration leads to the fusion of vesicles and the release of neurotransmitters (4) Created with BioRender.com
Mechanisms
The underlying cellular and molecular mechanisms involved with lactate chemosensing are not fully understood. Based on a mammalian study by Chang and colleagues, it was suggested that the molecular receptor involved in lactate sensing was Olfr78 which is readily found in the carotid body glomus cells (Chang et al. 2015). This was later disproven based on evidence that lactate sensing was preserved in Olf78r-deficient glomus cells (Torres-Torrelo et al. 2021, 2018). Similarly, the olfactory receptor OR51E2-like gene in trout, which is expressed in all tissues, was proposed as a putative lactate receptor in the gills (Thomsen et al. 2019) along with the HCAR1 receptor which is highly expressed in the gill tissues. However, more recent evidence suggests that lactate is co-transported with protons (H+) into the glomus cells via the monocarboxylic acid transporters (MCTs) where it is converted to pyruvate by lactate dehydrogenase (l-LDH because d-LDH is not present in vertebrate cells). The conversion of lactate to pyruvate increases the cytosolic NADH/NAD+ ratio, causes membrane depolarization, and a rise in intracellular [Ca2+] (Torres-Torrelo et al. 2021). In fish, there is growing support for a similar role of the MCTs in the lactate sensing pathway (Fig. 7). Firstly, recent single-cell transcriptomic analysis of hypoxia-exposed zebrafish NECs showed upregulation of slc16a3 gene expression coding for the monocarboxylate transporter MCT4 (Pan et al. 2022). Secondly, recent studies in killifish provide evidence that fish NECs sense physiological levels of lactate (5–10 mM) via MCT transporters using a non-specific MCT1/2/4 blocker, cyano-4-hydroxycinnamate (Leonard et al. 2022). The molecular identity of the exact MCT transporter involved needs further characterization.
Proposed lactate sensing mechanism in NECs. Lactate is co-transported with protons (H+) into the glomus cells via the monocarboxylic acid transporters (MCTs) (1). Once in the cells it is proposed that lactate is converted to pyruvate by lactate dehydrogenase (LDH), converting NAD+ to NADH. The change in NAD+ to NADH ratio likely causes K+ channels to close (2). This in turn causes a decrease in resting membrane potential and gives rise to the opening of voltage dependent Ca2+ channels (CaV) and the influx of Ca2+ (3). The increase in internal Ca2+ concentration leads to the fusion of vesicles and release of neurotransmitters (4). Created with BioRender.com
Inconsistencies
Pyruvate versus Lactate
Pyruvate, at concentration surpassing in vivo conditions, caused a rise in intracellular Ca2+ in NECs when applied in the absence of lactate. These levels of pyruvate have been shown to stimulate catecholamine secretion from carotid body glomus cells. A similar effect was observed in the mammalian system, and it was hypothesized that high extracellular levels of pyruvate would increase pyruvate transport into the cell shifting the pyruvate-lactate equilibrium via LDH towards increased lactate and the incipient oxidation of NADH to NAD+ + H+ causing cell acidification leading to membrane depolarization (Torres-Torrelo et al. 2021). However, Thomsen and colleagues found no effect of physiologically relevant levels of pyruvate on gill ventilation, possibly suggesting that in vivo, lactate conversion into pyruvate is not the main driving force of the HVR by lactate (Thomsen et al. 2019).
Gaps and future directions
Alternatives to gill lactate sensing
Time course experiments comparing the time delay of the ventilatory response to lactate injections into either the dorsal aorta versus ventral aorta (Thomsen et al. 2019) provide indirect evidence that the gills are the main site of plasma lactate sensing. Little information is available describing which gill arches are involved in lactate chemosensing. Based on studies of O2-chemosensing in rainbow trout, it appears that receptors on the first gill arch influence the heart rate, whereas receptors on the other gill arches and pseudobranch influence ventilation with receptors on the first gill arch having the greatest effect (Daxboeck and Holeton 1978; Perry and Reid 2002; Zhang et al. 2011). Thomsen and colleagues also demonstrated that removal of the first gill arch caused a reduction in the ventilatory responses to both NaCN and lactate (Thomsen et al. 2019) indicating that receptors on the first gill arch also play a predominant role in lactate sensing. The exact sites and roles of the NECs involved in lactate chemosensing remain to be determined.
Signal transduction cascade
Although there is limited information, lactate transduction pathways appear to mimic those of hypoxia and hypercapnia to a certain degree; at least with respect to membrane depolarization leading to the opening of voltage-gated Ca2+ channels and a rise in [Ca2+]i. This was demonstrated in killifish where nifedipine (0.5 µM), a blocker of voltage-gated L-type Ca2+ channels, reversibly blocked this lactate induced rise of [Ca2+]i (Leonard et al. 2022). Lactate sensing appears to start with the transport of lactate into NECs via a MCT, leading to membrane depolarization, Ca2+ entry through voltage-gated L-type Ca2+ channels (Leonard et al. 2022), and potentially neurotransmitter (5-HT) release (Thomsen et al. 2019). However, we have yet to determine whether the membrane depolarization is caused by intracellular acidification and generation of intracellular signals such as NADH and ROS, which as been shown in mammals (Torres-Torrelo et al. 2021).
Neurotransmitter involvement
In rainbow trout, it appears that only 5-HT and not acetylcholine mediates downstream lactate signaling leading to the HVR. Specifically, the 5-HT3 receptor antagonists decreased the ventilatory response to both sodium cyanide (NaCN) and lactate (Thomsen et al. 2019). Atropine only abolished bradycardia but had no effect on the ventilatory response. This has not been shown in more than one fish species. Furthermore, co-localization studies using immunohistochemistry are required to further confirm the placement of serotonergic receptors on the afferent nerves placed in close contact with MCT-positive NECs.
Hypoxia versus lactate
There is sound evidence that lactate is not necessary for the general HVR response, however, it is not yet clear whether the response to lactate is a component of the HVR or serves to modulate the intensity of the HVR as plasma lactate levels change. Clearly, future studies would be valuable to identify the cellular role of lactate.
Questions arising
What exactly are fish gill NECs?
NECs have been defined as either neuroendocrine cells or neuroepithelial cells, both of which are accurate descriptions as these cells both contain bioactive substances (are neuroendocrine) and are derived from epithelia. An important question though is which epithelia are they derived from?
Many parallels have been drawn between fish NECs and mammalian glomus cells of the carotid body. Glomus cells and NECs have similar cell ultrastructure; they are mitochondria rich and contain numerous dense core vesicles containing neuroactive substances (Fig. 1). They have similar innervation patterns: both mammalian glomus cells and the NECs in the first gill arch of fish are innervated by the glossopharyngeal nerve. The NECs in the first gill arch are also innervated by the vagus nerve as are the NECs in all other gill arches, as well as the glomus cells in amphibians, reptiles and birds (Jonz and Nurse 2009; Milsom and Burleson 2007; Jonz and Zaccone 2009). Both NECs and glomus cells respond to hypoxia by depolarizing due to the closing of a background K+ channel (Buckler et al. 2000; Jonz et al. 2004). Furthermore, the carotid arteries on which the carotid bodies sit are derived from the arteries of the same embryonic origin as those perfusing the first pair of gill arches in fish. Given the parallels, there also has been a tendency to draw homologies between the NECs and the chromaffin cells of the carotid body.
In the landmark paper of Dunel-Erb and colleagues, however, it was clearly stated that “These cells (NECs) are considered neuroepithelial cells, similar to those found within the wall of lung airways in mammals and nonmammalian vertebrates” (Dunel-Erb et al. 1982). This refers to the neuroepithelial cells and neuroepithelial bodies (NEBs) found within the lungs and airways of all air-breathing vertebrates, from the air-breathing fishes to mammals (hence, jointly referred to as PNECs to designate their pulmonary location) (Zaccone et al. 2009). The distinction between chromaffin cells and PNECs and NEBs arises from the embryological origin of the two groups of cells. Carotid body chromaffin cells are derived from neural ectoderm of the neural crest during development while the neuroepithelial cells of airways are derived from undifferentiated but committed neuroepithelial progenitors from embryonic endoderm (Yeger et al. 2009). The antibody for Human Natural Killer-1 (HNK1) carbohydrate labels neural crest cells in many, but not all vertebrates, and has been used to trace cell migration (Bronner-Fraser 1986). While it was shown that filamental NECs in goldfish (C. auratus), trout (O. mykiss) and bowfin (A. calva) did not label with the HNK-1 antibody (Porteus et al. 2013, 2014b) or zn-12 (an antibody which recognizes the same epitope) in other studies (e.g. Jonz et al. 2003) suggesting fish NECs were not derived from the neural crest, these studies were not definitive. The carbohydrate epitope recognized by the HNK1 antibody (Voshol et al. 1996) is borne by multiple glycoproteins and glycolipids, and gene or antigen expression in itself cannot indicate lineage (Hockman et al. 2017). The more recent study of Hockman and colleagues using genetic lineage-tracing, neural crest-deficient mutants in zebrafish, and physical fate-mapping in frog and lamprey, however, strongly supports the contention that gill NECs are not neural crest-derived, but endoderm-derived, like PNECs. They appear to develop from the lining of the mouth and gills (Hockman et al. 2017).
Before examining parallels between PNECs and the NECs found in the gills of fish, we need to examine how similar the various NECs described in the gills of fish are and the criteria that have been used to define them as NECs. From the preceding discussion we have described (1) large 5HT-positive NECs in the proximal filament that receive both intrinsic and extrinsic innervation, (2) large 5HT-positive NECs in the distal filament that receive primarily extrinsic innervation, (3) small NECs in the filament that stain for SV2 but are 5HT-negative and innervated by the extrinsic nerves, (4) small 5HT-positive NECs in the lamellae that are innervated, and (5) small 5HT-positive NECs in the lamellae that are not innervated and that do not stain for SV2. Note that it has been suggested that the small 5-HT-negative NECs along the filament could be immature NECs still undergoing differentiation (Jonz and Nurse 2003). It has also been suggested that the small 5HT-positive NECs in the lamellae that are not innervated and that do not stain for SV2, play a role in scavenging excess 5HT from the circulation and degrade it.
With this in mind we note that the solitary PNECs seen in air-breathing fish and amphibia have the following attributes. They are of the closed type (i.e. not exposed to the environment but deeper in the epithelium), are not innervated, do contain dense cored vesicle with 5-HT as the primary amine, can contain a variety of other bioactive substances and are suggested to play a paracrine role associated with local mucous activity or smooth muscle contraction (Goniakowska-Witalińska et al. 2009). The strongest parallel we see here is with the gill lamellar NECs (number 4 in the list above).
The PNECs that cluster together to form PNEBs, however, are innervated by branches of the vagus nerve and respond to environmental (airway) O2 and CO2 (Lauweryns et al. 1978; Youngson et al. 1993; Livermore et al. 2015). They contain dense cored vesicles that store bioactive substances; amines (serotonin) and a variety of peptides. Hypoxia leads to acute inhibition of K+ channels leading to depolarization, Ca2+ influx via voltage gated Ca2+ channels and neurotransmitter exocytosis (Youngsen et al. 1993; Fu et al. 2002). They receive both afferent and efferent innervation. Morphologically they appear very similar to the glomus cells of the carotid body (Cutz et al. 2009). As a result, there are also many parallels between gill NECs and the PNEBs found in mammalian airways.
Unfortunately, innervation patterns also do not allow us to determine whether gill NECs are homologous to carotid body glomus cells or airway PNEBs. There are O2 sensitive chemoreceptors on all gill arches. However, while all gill arches receive vagal innervation, only the first gill arch is innervated by the hypoglossal nerve. While the carotid bodies in mammals are innervated only by the hypoglossal nerve, in amphibians, and non-chelonian reptiles, they are innervated by both the hypoglossal and vagus nerves and in chelonian reptiles and birds they are innervated only by the vagus nerve (Milsom and Burleson 2007).
Thus, at present drawing homologies between carotid body chromaffin cells, NECs on the first gill arch, NECs on other gill arches, and pulmonary NECs remains equivocal and controversial. While it is possible that the NECs on the first gill arch are homologous to carotid body chromaffin cells while those on the other gill arches are homologous to the PNECs, the vasculature perfusing the third and fifth gill arches become the aorta and pulmonary arteries, each of which also contain O2 chemoreceptors (the aortic bodies and pulmonary O2 receptors respectively). Resolving this issue will require fate mapping of the origin of the different types of NECs on all gill arches in a variety of species.
To add to the confusion, presently there is no conclusive evidence that PNECs, PNEBs or fish gill NECs are associated with the control of respiration.
How essential is serotonin?
It is perhaps paradoxical that so much research has focused on the one neurotransmitter, serotonin, that has only modest effects in evoking neural discharge in fish (Burleson and Milsom 1995b) as well as the one least involved in the response to hypoxia in mammals (Nurse 2005). There is no doubt that 5-HT is ubiquitous being found in almost all NECs, and consequently has frequently been used, almost exclusively, in many studies to identify NECs. Serotonergic NECs have been found on the gill arch, gill rakers, gill filaments and lamellae, the pseudobranch and labyrinth in species that have them, and in the skin. Evidence of its role in O2 chemosensing in larvae and adult fish is compelling, but all indirect (see Perry et al. 2023 for review).
Several observations raise questions about the extent to which hypoxia leads to the release of 5-HT from NECs. While there is evidence that trout NECs appear “degranulated” after hypoxia exposure (Dunel-Erb et al. 1982), numerous studies record an increase in the size and surface area of NECs exposed to chronic hypoxia (Jonz et al. 2004; Vulesevic et al. 2006; Regan et al. 2011; Shakarchi et al. 2013; Porteus et al. 2014b; Rossi et al. 2020; Zaccone et al. 2022a, b). Several studies using activity dependent dyes [SR101, also known as Texas red (TXR)] used to label active synapses, and the vesicular activity indicator dye FM 1–43) have been used to evaluate the activity of NECs during hypoxia. SR101 incorporation via synaptic vesicle recycling in gill tissue was assessed from fish that were exposed to acute (30 min) normoxia and hypoxia. Surprisingly, in trout and goldfish, serotonin-immunoreactive NECs never took up SR101. Furthermore, neither bipolar neurons nor the extrinsic nerve bundle took up the activity dependent dye suggesting that these cells were not highly active in hypoxia. However mitochondrial rich cells (MRCs) did take up the dye, in normoxic but not hypoxic conditions, an observation showing that the labeling protocol was effective (Porteus et al. 2013). Jonz et al. (2015) found that only a few NECs of the filament and respiratory lamellae were FM1-43-positive. Interestingly, in dissociated Neutral Red positive, putative NECs that were acutely exposed to sodium cyanide (NaCN) FM1-43 fluorescence was significantly increased, but FM1-43 fluorescence did not increase significantly in hypoxia (PO2 = 11 mm Hg) (Zachar and Jonz, unpublished observations reported in Jonz et al. 2015). Finally, Pan et al. (2021) using transgenic lines of zebrafish larvae, found that larvae lacking the tryptophan hydroxylase 1a gene, the rate limiting enzyme for 5-HT synthesis, displayed a higher ventilation rate when exposed to hypoxia compared to wild-types, and that although tryptophan hydroxylase 1b mutants exhibited a lower ventilation rate, they still exhibited a significant rise in ventilation in hypoxia. This led the authors to conclude that 5-HT in locations other than NECs may play a dominant role in regulating the hypoxic ventilatory response.
The net conclusion from all these studies is that despite all the focus on serotonin to date, it does not appear to be an essential component of the O2 sensing pathway.
What is the role of other neurotransmitters?
To be part of a chemosensing system producing cardiorespiratory reflexes, NECs must release neurotransmitters onto receptors found on extrinsic neurons innervating them. Receptors found on the NECs themselves could contribute to chemoreceptor reflexes by modulating NEC function or provide efferent stimulation regulating a paracrine function. The latter are reviewed in the last paragraph of “The current view” section (isolated cells). It is the former that are reviewed here.
Besides 5-HT, NECs have now been shown to contain a host of neurotransmitters and neuropeptides, (or stain for the antibodies of the enzymes involved in neurotransmitter synthesis or transmembrane transport) in different combinations. These include ACh, catecholamines, neuropeptides (enkephalin, metenkephalins, neuron specific enolase, calbindin D28 K), and the gaseous neurotransmitters, NO, hydrogen sulfide (H2S), and carbon monoxide (Bailly 2009; Dunel-Erb et al. 1982; Mauceri et al. 1999; Milsom and Burleson 2007; Jonz et al. 2016; Perry et al. 2009; Porteus et al. 2012, 2014a, 2015; Regan et al. 2011; Tzaneva et al. 2016; Zaccone et al. 2003, 2006, 2017, 2018; Zachar et al. 2017). The extent to which these substances colocalize with one another in the NECs of different species is complex. There are two excellent reviews that explore these data in detail, that of Pan and Perry (2020) and that of Reed and Jonz (2022) (Table 1). The conclusion that each comes to, however, is that the roles of any of these chemicals in the receptor control of cardiorespiratory reflexes in fish is still to be determined. Here is a very brief summary.
Catecholamines might seem likely candidates given their roles as neuromodulators in the carotid bodies of mammals (Nurse 2005). Tyrosine hydroxylase (TH), which catalyzes the rate limiting step in this synthesis of catecholamines, has routinely been used for identifying carotid body glomus cells. Immunohistochemical labeling for TH did not reveal any structures in the first gill arch of either trout or goldfish, although TH-immunoreactive fibers were found sparsely distributed in the filament in the pseudobranch of trout (Porteus et al. 2013). TH has also been shown to colocalize with nNOS in the NECs and nerve fibres of some species (Zaccone et al. 2008) and has recently been localized to nerve fibres of the gill filaments and respiratory lamellae of zebrafish (Reed et al. 2023). Hockman and colleagues (2017) using immunostaining for TH found TH positive cells, at least some of which appeared to be innervated, in the walls of the anterior cardinal veins in the gill arches of ammocoete sea lamprey. They also found neural crest-derived catecholaminergic cells associated with zebrafish pharyngeal arch blood vessels. These cells were few and occurred in loose aggregates. Hockman et al. speculated that the carotid body may have evolved via the aggregation of such cells.
To add to the confusion, while there are studies indicating that catecholamines may inhibit ventilatory responses to hypoxia, there is no evidence that they stimulate ventilation. The recent study of Reed et al. (2023) identified dopamine active transporter (dat) and vesicular monoamine transporter (vmat2) expression in neurons of the gill filaments innervating NECs and suggest that D2 receptors on presynaptic NECs provide a feedback mechanism that attenuates the chemoreceptor response to hypoxia. Similarly, it was previously shown that the β-adrenoreceptor antagonist propranolol inhibited O2 receptor discharge from perfused O. mykiss first gill arch preparations (Burleson and Milsom 1990), while β-adrenergic stimulation of the same preparation using adrenaline, noradrenaline or isoproterenol had almost no effect on neural activity from chemoreceptor afferent neurons (Burleson and Milsom 1995a).
Thus, at present a role of catecholamines in gill receptor chemosensing is equivocal.
The gasotransmitters are also likely candidates and have been reported to be present in the NECs of several species based on the presence of key enzymes necessary for their production (Mauceri et al. 1999; Tzaneva and Perry 2014; Porteus et al. 2014a, b; Porteus et al. 2015). NO has been shown to stimulate ventilation in zebrafish larvae but inhibit it in adults (Porteus et al. 2015). Carbon monoxide (CO) has been shown to inhibit ventilation in zebrafish and goldfish (Tzaneva and Perry 2014, 2016); and H2S has also been shown to stimulate ventilation in zebrafish (Porteus et al. 2014a). Whether these results are responses to release of the transmitters from the NECs, however, remains to be determined.
A role of the purinergic nervous system in O2 sensing is also a possibility. Purinergic blockade with aminophylline, an A1 and A2 receptor antagonist blocked the ventilatory response to hypoxia in the common carp (Cyprinus carpio) (Stecyk and Farrell 2006) and the epaulette shark (Hemiscyllium ocellatum) (Stensløkken et al. 2004) and the A2a receptor antagonist, SCH58261 blocked the ventilatory response to hypoxia in zebrafish (Coe et al. 2017). Similarly, the purinergic receptor antagonist, PPADS, which targets purinergic P2X2 and P2X3 receptors, inhibited the hyperventilatory response to hypoxia in zebrafish (Coe et al. 2017). Immunohistochemical staining of P2X3 receptors showed colocalization with 5-HT-positve NECs in the tips of zebrafish lamellae (Jonz and Nurse 2003) but also with serotonergic neurons (Rahbar et al. 2016) suggesting the possibility of reciprocal excitation.
ACh is another likely candidate. As noted earlier, ventilatory responses to ACh and to both nicotine and muscarine have been reported in several species (Lenfant and Johansen 1968; Burleson and Milsom 1995b; Shakarchi et al. 2013). Furthermore, the emersion response in the facultative airbreathing mangrove rivulus, K. marmoratus, was accentuated in fish pre-exposed to ACh and attenuated in fish pre-exposed to the nicotinic antagonist, hexamethonium (Regan et al. 2011; Wright et al. 2012). In zebrafish, its effects were dependent on developmental stage. Neither exogenous application of ACh nor of the nicotinic ACh receptor antagonist, hexamethonium affected ventilation frequency in early-stage zebrafish larvae (7–10 d.p.f) but both did in late stage larvae (14–21 d.p.f) (Shakarchi et al. 2013).
ACh and 5-HT almost never colocalize in NECs. However, NEC-like cells that stain positive for either the vesicular ACh transporter (VAChT) or the enzyme choline acetyltransferase (ChAT) (the enzyme involved in the synthesis of ACh), or both, have been described in zebrafish where they were found on both the afferent and efferent aspects of each gill filament (Zachar et al. 2017). They were more numerous on the afferent side of the gill filaments but those on the efferent side were situated within 10 μm of the serotonergic NECs and appeared to be innervated. The nicotinic receptor α2b subunit gene (chrna2b) and the β4 subunit gene (chrnb4) are highly expressed in neurons (Pan et al. 2022). However, the ACh containing NECs did not stain for SV2 and so it was suggested that these cells might release ACh locally, perhaps via non-vesicular mechanisms present in non-neuronal cholinergic cells (for example Chavez et al. 2011; Nassenstein 2015). Subsequently ACh might then modulate the nearby serotonergic NECs by paracrine signaling (Zachar et al. 2017).
Furthermore, administration of the muscarinic antagonist, atropine abolished the ventilatory response to hypoxia at very high concentrations in zebrafish (D. rerio) (Rahbar et al. 2016) as well as the hypercarbia-induced ventilatory response in Pacific spiny dogfish (S. acanthias) (McKendry et al. 2001), but had no effect on ventilation in rainbow trout (O. mykiss) (Burleson and Milsom 1995a, b), Adriatic sturgeon (Acipenser naccarii) (McKenzie et al. 1995) or channel catfish (I. punctatus) (Burleson and Smatresk 1990). And, while atropine completely blocked the effects of acetylcholine on receptor discharge in afferent neurons in trout, it only delayed the responses to hypoxia and NaCN (Burleson and Milsom 1995a).
In summary, currently there is no direct evidence of any neurotransmitters being released during hypoxia and the data just presented make it fair to say (to paraphrase Perry and Pan) “the primary neurochemicals involved in respiratory chemoreception in fish are not clearly identified yet” (Perry and Pan 2020).
What putative roles for gill NECs have strong support?
It now seems probable that gill NECs are homologous to the PNECs found in the lungs and airways of all air breathing vertebrates. These cells can occur as isolated cells (PNECs) or in clusters (neuroepithelial bodies or PNEB). In airbreathing fish solitary PNECs have been identified in the lungs or gas exchange organs and are typically found on the basal membrane. They contain 5-HT and several neuropeptides (monamine peptides and purine neurotransmitters) and may or may not be innervated. In the lungfish Protopterus aethiopicus they are innervated and found on the pneumatic duct (Adriaensin et al. 1990; Zaconne et al. 1989b). In the birchir (both Polypterus ornatipinnis and P. delbezi) they are found in the airbladder in small dispersed islets of ciliated epithelia with goblet cells and are not innervated (Zaconne et al. 1989a). In the bowfin (A. calva), they are also not innervated (Goniakowska-Witalińska 1997). In the teleosts, and the more derived actinopterygians, they have not been found in the facultative air breathing catfish Pangasius hypopthalmus but have been found in posterior chamber of the intestine, which is used for gas exchange in bronze Corydoras (Corydoras aeneus) (Podkowa and Goniakowska-Witalińska 2002). This is a group of airway chemosensors for which functional roles have not yet been established but as in amphibians, they have been associated with vaso- and bronchoconstriction (Goniakowska-Witalińska et al. 2009). Lastly, NECs may have a role in neuro-immuno-modulation, as evidenced by their expression of the antimicrobial piscidin 1, enkephalins, and the neuropeptide modulator GABA B R1, in the Asian catfish Heteropneustes fossilis (Zaccone et al. 2022a, b).
A role for gill NECs in control of vascular tone would then seem reasonable and is also well supported by indirect evidence. As alluded to throughout this review, gill NECs could act in either an autocrine/paracrine or neuroendocrine fashion in multiple ways (Fig. 1d). All gill NECs may respond to hypoxia by releasing 5-HT onto neighboring vascular muscle cells. Those that are innervated have all been shown to possess receptors for various neurotransmitters and may not only respond directly to hypoxia by releasing 5-HT but may be induced to release 5-HT by their innervation. Jonz and Nurse (2003) raised the possibility that extrinsic or intrinsic nerve fibers innervating filament NECs in the zebrafish could be efferent and allow for a centrally mediated neurosecretory role of NECs. The relationship between gill NECs along the proximal efferent filamental artery (eFA) and the contractile segment or sphincter at the junction of the eFA and the efferent branchial artery (eBA) was described in “The current view” section and is strongly implicated in the control of gill blood flow (Nilsson and Sundin 1998; Jonz and Nurse 2003; Bailly 2009). In vivo examination of rainbow trout gill vasculature exposed to hypoxia demonstrated constriction of the eFA (Sundin and Nilsson 1997) and injections of 5-HT caused vasoconstriction of the efferent filament artery and stopped blood flow to the distal part of the filament, just as hypoxia does (Sundin et al. 1995; Sundin et al. 1998a, b).
This is the most likely situation for gill NECs in the lamellae, particularly in species in which the lamellar gill NECs are not innervated. Under resting conditions, fish face an osmo-respiratory compromise. Fish need sufficient gas exchange surface to meet metabolic demands but to reduce surface area to prevent the loss of ions across the gill (Randall et al. 1973; Nilsson 1986; Sardella and Brauner 2007; Wood and Eom 2021). They do this by perfusing only two-thirds or less of the lamellae under resting conditions (Booth 1978; Farrell et al. 1979) and in many species by increasing the interlamellar cell mass (ILCM) (Nilsson 2007). In hypoxia the ILCM is reduced, and the respiratory surface area is increased by increasing perfusion as well as by pillar cell contraction (Smith and Johnson 1977; Stensløkken et al. 2006). Interestingly, lamellar NECs were not present in the trout, and in vivo studies of the microcirculation of the rainbow trout gill did not show direct vasodilation in the lamellae via pillar cell contraction (Sundin and Nilsson 1997).
Parenthetically it is also possible that this is the primary role of the NECs that have been identified in the skin of early-stage larvae as well as the adults of some species acting to divert blood flow away from hypoxic areas and increase cutaneous gas exchange efficiency. Thus, while serotonergic NECs have been proposed to be the primary O2 chemosensors in fish gills, eliciting the full range of cardiorespiratory reflexes, changes in breathing frequency, breath amplitude, heart rate and vascular resistance, there is only strong evidence for the last of these.
Conclusions
Serotonergic NECs have been proposed to be the primary peripheral chemosensing cells for O2, CO2/pH, ammonia and lactate in fish gills, eliciting the full range of cardiorespiratory reflexes. These include changes in breathing frequency, breath amplitude, heart rate and vascular resistance. However, there is only strong evidence for a role of serotonergic NECs in controlling vascular tone, either by exciting intrinsic nerves acting on vascular shunts or by paracrine effects directly on vascular smooth muscle. It now appears that fish gill NECs are not homologous with mammalian carotid chromaffin cells and we suggest that in future they be referred to as gill NECs to stress their homology with the PNECs found in vertebrate lungs. Both gill NECs and PNECs are derived from undifferentiated but committed neuroepithelial progenitor cells from embryonic endoderm. It is a paradox that it is the presence of serotonin that has been used to identify gill NECs yet serotonin does not appear to be an essential component of the chemosensing pathways. In fact, currently there is no direct evidence of any neurotransmitter being released from activated gill NECs. Finally to add to the confusion, there appear to be 1) large 5HT-positive NECs in the proximal filament that receive both intrinsic and extrinsic innervation, 2) large 5HT-positive NECs in the distal filament that receive primarily extrinsic innervation, 3) small NECs in the filament that stain for the vesicular marker, SV2 but are 5HT-negative and innervated by the extrinsic nerves, 4) small 5HT-positive NECs in the lamellae that are innervated, and 5) small 5HT-positive NECs in the lamellae that are not innervated and that do not stain for SV2. Some of these NECs appear to sense only changes in the blood, some only changes in the water flowing over the gills and some sense both. Furthermore, some sense only O2 and some both O2 and CO2. It is assumed that those sensing CO2 also sense ammonia. Lastly, it appears that while there are NECs on all gill arches that are depolarized by the presence of lactate, only a subset on the first two gill arches produce changes in ventilation. Given the knowledge gaps and inconsistencies in the data pointed out in this review. our net conclusion is that we are still a long way from understanding fish gill chemosensing.
References
Abdallah S, Jonz MG, Perry S (2014) Extracellular H+ induces Ca2+ signals in respiratory chemoreceptors of zebrafish. Pflugers Arch-Eur J Physiol 467:399–413. https://doi.org/10.1007/s00424-014-1514-2
Adriaensen D, Scheuermann DW, Timmermans J-P, De Groodt-Lasseel MHA (1990) Neuroepithelial endocrine cells in the lung of the lungfish Protopterus aethiopicus. Cells Tissues Organs 139:70–77. https://doi.org/10.1159/000146981
Baatsen P, Gabarre S, Vints K et al (2021) Preservation of fluorescence signal and imaging optimization for integrated light and electron microscopy. Front Cell Dev Biol 9:737621. https://doi.org/10.3389/fcell.2021.737621
Baik AH, Jain IH (2020) Turning the oxygen dial: balancing the highs and lows. Trends Cell Biol 30:516–536. https://doi.org/10.1016/j.tcb.2020.04.005
Bailly Y (2009) Serotonergic neuroepithelial cells in fish gills. In: Zaccone G, Cutz E, Adriaensen D, Nurse CA, Mauceri A (eds) Airway chemoreceptors in the vertebrates. Science Publishers, Enfield, pp 235–268
Bailly Y, Dunel-Erb S, Geffard M, Laurent P (1989) The vascular and epithelial serotonergic innervation of the actinopterygian gill filament with special reference to the trout, Salmo gairdneri. Cell Tissue Res. https://doi.org/10.1007/BF00239455
Bailly Y, Dunel-Erb S, Laurent P (1992) The neuroepithelial cells of the fish gill filament: indolamine-immunocytochemistry and innervation. Anat Rec 233:143–161. https://doi.org/10.1002/ar.1092330118
Bartoszewski R, Moszyńska A, Serocki M et al (2019) Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J 33:7929–7941. https://doi.org/10.1096/fj.201802650RR
Begemann I, Galic M (2016) Correlative light electron microscopy: connecting synaptic structure and function. Front Synaptic Neurosci 8:28. https://doi.org/10.3389/fnsyn.2016.00028
Bittner S, Budde T, Wiendl H, Meuth SG (2010) From the background to the spotlight: TASK channels in pathological conditions. Brain Pathol 20:999–1009. https://doi.org/10.1111/j.1750-3639.2010.00407.x
Bronner-Fraser M (1986) Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol 115:44–55. https://doi.org/10.1016/0012-1606(86)90226-5
Brooks GA (2020) The tortuous path of lactate shuttle discovery: from cinders and boards to the lab and ICU. J Sport Health Sci 9:446–460. https://doi.org/10.1016/j.jshs.2020.02.006
Buckler KJ (2015) TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch Eur J Physiol 467:1013–1025. https://doi.org/10.1007/s00424-015-1689-1
Buckler KJ, Williams BA, Honore E (2000) An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol 525:135–142. https://doi.org/10.1111/j.1469-7793.2000.00135.x
Burleson ML, Milsom WK (1995a) Cardio-ventilatory control in rainbow trout: I. Pharmacology of branchial, oxygen-sensitive chemoreceptors. Respir Physiol 100:231–238. https://doi.org/10.1016/0034-5687(95)91595-X
Burleson ML, Milsom WK (1995b) Cardio-ventilatory control in rainbow trout: II. Reflex effects of exogenous neurochemicals. Respir Physiol 101:289–299. https://doi.org/10.1016/0034-5687(95)00029-D
Burleson ML, Smatresk NJ (1990) Evidence for two oxygen-sensitive chemoreceptor loci in channel catfish, Ictalurus punctatus. Physiol Zool 63:208–221. https://doi.org/10.1086/physzool.63.1.30158162
Burleson ML, Smatresk NJ (2000) Branchial chemoreceptors mediate ventilatory responses to hypercapnic acidosis in channel catfish. Comp Biochem Physiol A Mol Integr Physiol 125:403–414. https://doi.org/10.1016/S1095-6433(00)00167-7
Burleson ML, Milsom WK (2003) Comparative aspects of O2 chemoreception: Anatomy, physiology, and environmental adaptations. In: Lahiri S, Semenza GL, Prabhakar NR (eds) In: oxygen sensing: responses and adaptation to hypoxia. Marcel Dekker, New York, pp 685–707. https://doi.org/10.1201/b14819-44
Burleson ML, Mercer SE, Wilk-Blaszczak MA (2006) Isolation and characterization of putative O2 chemoreceptor cells from the gills of channel catfish (Ictalurus punctatus). Brain Res 1092:100–107. https://doi.org/10.1016/j.brainres.2006.03.085
Caravagna C, Seaborn T (2016) Oxygen Sensing in Early Life. Lung 194:715–722. https://doi.org/10.1007/s00408-016-9908-x
Chang AJ (2017) Acute oxygen sensing by the carotid body: from mitochondria to plasma membrane. J Appl Physiol 123:1335–1343. https://doi.org/10.1152/japplphysiol.00398.2017
Chang AJ, Ortega FE, Riegler J et al (2015) Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 527:240–244. https://doi.org/10.1038/nature15721
Chávez J, Vargas MH, Cruz-Valderrama JE, Montaño LM (2011) Non-quantal release of acetylcholine in guinea-pig airways: role of choline transporter: non-quantal release of acetylcholine in guinea-pig airways. Exp Physiol 96:460–467. https://doi.org/10.1113/expphysiol.2010.056440
Chen K, Cole RB, Rees BB (2013) Hypoxia-induced changes in the zebrafish (Danio rerio) skeletal muscle proteome. J Proteomics 78:477–485. https://doi.org/10.1016/j.jprot.2012.10.017
Coccimiglio ML, Jonz MG (2012) Serotonergic neuroepithelial cells of the skin in developing zebrafish: morphology, innervation and oxygen-sensitive properties. J Exp Biol. https://doi.org/10.1242/jeb.074575
Cochrane PV, Rossi GS, Tunnah L et al (2019) Hydrogen sulphide toxicity and the importance of amphibious behaviour in a mangrove fish inhabiting sulphide-rich habitats. J Comp Physiol B 189:223–235. https://doi.org/10.1007/s00360-019-01204-0
Cochrane PV, Jonz MG, Wright PA (2021) The development of the O2-sensing system in an amphibious fish: consequences of variation in environmental O2 levels. J Comp Physiol B 191:681–699. https://doi.org/10.1007/s00360-021-01379-5
Coe AJ, Picard AJ, Jonz MG (2017) Purinergic and adenosine receptors contribute to hypoxic hyperventilation in zebrafish (Danio rerio). Comp Biochem Physiol A Mol Integr Physiol 214:50–57. https://doi.org/10.1016/j.cbpa.2017.09.013
Coolidge EH, Ciuhandu CS, Milsom WK (2008) A comparative analysis of putative oxygen-sensing cells in the fish gill. J Exp Biol 211:1231–1242. https://doi.org/10.1242/jeb.015248
Cutz E, Jackson A (1999) Neuroepithelial bodies as airway oxygen sensors. Respir Physiol 115:201–214. https://doi.org/10.1016/S0034-5687(99)00018-3
Cutz E, Fu WX, Yeger H, Pan J, Nurse CA (2009) Oxygen sensing in mammalian pulmonary epithelial bodies. In: Zaccone G, Cutz E, Adriaensen D, Nurse CA, Mauceri A (eds) Airway chemoreceptors in the vertebrates. Science Publishers, Enfield, pp 269–290
Cutz E, Fu XW, Yeger H (2004) Methods to study neuroepithelial bodies as airway oxygen sensors. In: Methods in enzymology. Elsevier, London, pp 26–40
Cutz E, Pan J, Yeger H et al (2013) Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Semin Cell Dev Biol 24:40–50. https://doi.org/10.1016/j.semcdb.2012.09.003
Daxboeck C, Holeton GF (1978) Oxygen receptors in the rainbow trout, Salmo gairdneri. Can J Zool 56:1254–1259. https://doi.org/10.1139/z78-180
De Boeck G, Wood CM (2015) Does ammonia trigger hyperventilation in the elasmobranch, Squalus acanthias suckleyi? Respir Physiol Neurobiol 206:25–35. https://doi.org/10.1016/j.resp.2014.11.009
De Boer P, Hoogenboom JP, Giepmans BNG (2015) Correlated light and electron microscopy: ultrastructure lights up!. Nat Methods 12:503–513. https://doi.org/10.1038/nmeth.3400
Dunel-Erb S, Bailly Y (1986) The sphincter of the efferent filament artery in teleost gills: II. Sympathetic innervation. J Morphol 187:239–246. https://doi.org/10.1002/jmor.1051870209
Dunel-Erb S, Bailly Y, Laurent P (1982) Neuroepithelial cells in fish gill primary lamellae. J Appl Physiol 53:1342–1353. https://doi.org/10.1152/jappl.1982.53.6.1342
Dunel-Erb S, Bailly Y, Laurent P (1989) Neurons controlling the gill vasculature in five species of teleosts. Cell Tissue Res 255:567–573. https://doi.org/10.1007/BF00218792
Egg M, Köblitz L, Hirayama J et al (2013) Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol Int 30:510–529. https://doi.org/10.3109/07420528.2012.754447
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364:656–665. https://doi.org/10.1056/NEJMra0910283
Eom J, Wood CM (2021a) Understanding ventilation and oxygen uptake of Pacific hagfish (Eptatretus stoutii), with particular emphasis on responses to ammonia and interactions with other respiratory gases. J Comp Physiol B 191:255–271. https://doi.org/10.1007/s00360-020-01329-7
Eom J, Wood CM (2021b) Brain and gills as internal and external ammonia sensing organs for ventilatory control in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol A Mol Integr Physiol 254:110896. https://doi.org/10.1016/j.cbpa.2021.110896
Eom J, Giacomin M, Clifford AM et al (2019) Ventilatory sensitivity to ammonia in the Pacific hagfish (Eptatretus stoutii), a representative of the oldest extant connection to the ancestral vertebrates. J Exp Biol. https://doi.org/10.1242/jeb.199794
Eom J, Fehsenfeld S, Wood CM (2020) Is ammonia excretion affected by gill ventilation in the rainbow trout Oncorhynchus mykiss? Respir Physiol Neurobiol 275:103385. https://doi.org/10.1016/j.resp.2020.103385
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177. https://doi.org/10.1152/physrev.00050.2003
Florindo LH, Reid SG, Kalinin AL et al (2004) Cardiorespiratory reflexes and aquatic surface respiration in the neotropical fish tambaqui (Colossoma macropomum): acute responses to hypercarbia. J Comp Physiol [b] 174:319–328. https://doi.org/10.1007/s00360-004-0417-5
Friedrichsen K, Ramakrishna P, Hsiang J-C et al (2022) Reconstructing neural circuits using multiresolution correlated light and electron microscopy. Front Neural Circ 16:753496. https://doi.org/10.3389/fncir.2022.753496
Fritsche R, Thomas S, Perry SF (1992) Effects of serotonin on circulation and respiration in the rainbow trout Oncorhynchus mykiss. J Exp Biol 173:59–73. https://doi.org/10.1242/jeb.173.1.59
Fu XW, Wang D, Nurse CA et al (2000) NADPH oxidase is an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild-type and oxidase-deficient mice. Proc Natl Acad Sci USA 97:4374–4379. https://doi.org/10.1073/pnas.97.8.4374
Fu XW, Nurse CA, Wong V, Cutz E (2002) Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J Physiol 539:503–510. https://doi.org/10.1113/jphysiol.2001.013071
Gao L, Bonilla-Henao V, García-Flores P et al (2017) Gene expression analyses reveal metabolic specifications in acute O2-sensing chemoreceptor cells. J Physiol 595:6091–6120. https://doi.org/10.1113/JP274684
Ghanizadeh-Kazerouni E, Wilson JM, Jones SRM, Brauner CJ (2024) Characteristics of a gill resection—regeneration model in freshwater laboratory-reared Atlantic salmon (Salmo salar). Aquaculture 579:740210. https://doi.org/10.1016/j.aquaculture.2023.740210
Glass ML (1992) Ventilatory responses to hypoxia in ectothermic vertebrates. In: Wood SC, Weber RE, Hargens AR, Millard RW (eds) Physiological adaptations in vertebrates, respiration, circulation, and metabolism. Marcel Dekker, New York, pp 97–118
Gilmour KM, Perry SF (2007) Fish Physiology v25 sensory systems neuroscience. In: Hara TJ, Zielinski B (eds) Branchial chemoreceptor regulation of cardiorespiratory function. Elsevier, USA, pp 97–151. https://doi.org/10.1016/s1546-5098(06)25003-9
Goniakowska-Witalińska L (1997) Neuroepithelial bodies and solitary neuroendocrine cells in the lungs of amphibia. Microsc Res Tech 37:13–30. https://doi.org/10.1002/(SICI)1097-0029(19970401)37:1%3C13::AID-JEMT3%3E3.0.CO;2-X
Goniakowska-Witalińska L, Pecio A, Podkowa D (2009) Neuroendocrine cells in the lungs of amphibians and air-breathing fishes. In: Zaccone G, Cutz E, Adriaensen D, Nurse CA, Mauceri A (eds) Airway chemoreceptors in the vertebrates—structure evolution and function. Science Publishers, Enfield, pp 99–123
Hazon N, Wells A, Pillans RD et al (2003) Urea based osmoregulation and endocrine control in elasmobranch fish with special reference to euryhalinity. Comp Biochem Physiol B Biochem Mol Biol 136:685–700. https://doi.org/10.1016/S1096-4959(03)00280-X
Hockman D, Burns AJ, Schlosser G et al (2017) Evolution of the hypoxia-sensitive cells involved in amniote respiratory reflexes eLife 6:e21231. https://doi.org/10.7554/eLife.21231
Holmes AP, Ray CJ, Coney AM, Kumar P (2018) Is carotid body physiological O2 sensitivity determined by a unique mitochondrial phenotype? Front Physiol 9:562. https://doi.org/10.3389/fphys.2018.00562
Holmquist-Mengelbier L, Fredlund E, Löfstedt T et al (2019) Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell 10:413–423. https://doi.org/10.1016/j.ccr.2006.08.026
Hung CYC, Tsui KNT, Wilson JM et al (2007) Rhesus glycoprotein gene expression in the mangrove killifish Kryptolebias marmoratus exposed to elevated environmental ammonia levels and air. J Exp Biol 210:2419–2429. https://doi.org/10.1242/jeb.002568
Janvier J-J, Peyraud-Wazenegger M, Soulier P (1996) Mediation of serotonin-induced hyperventilation via 5-HT3-receptor in European eel Anguilla anguilla. J Comp Physiol B 165:640–646. https://doi.org/10.1007/BF00301132
Jaśkiewicz M, Moszyńska A, Króliczewski J et al (2022) The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell Mol Biol Lett 27:109. https://doi.org/10.1186/s11658-022-00408-7
Jonz MG, Fearon IM, Nurse CA (2004) Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J Physiol 560:737–752. https://doi.org/10.1113/jphysiol.2004.069294
Jonz MG (2018) Insights into the evolution of polymodal chemoreceptors. Acta Histochem 120:623–629. https://doi.org/10.1016/j.acthis.2018.08.008
Jonz MG, Nurse CA (2003) Neuroepithelial cells and associated innervation of the zebrafish gill: a confocal immunofluorescence study. J Comp Neurol 461:1–17. https://doi.org/10.1002/cne.10680
Jonz MG, Nurse CA (2006) Ontogenesis of oxygen chemoreception in aquatic vertebrates. Respir Physiol Neurobiol 154:139–152. https://doi.org/10.1016/j.resp.2006.01.004
Jonz MG, Zaccone G (2009) Nervous control of the gills. Acta Histochem 111:207–216. https://doi.org/10.1016/j.acthis.2008.11.003
Jonz MG, Zachar PC, Da Fonte DF, Mierzwa AS (2015) Peripheral chemoreceptors in fish: a brief history and a look ahead. Comp Biochem Physiol A Mol Integr Physiol 186:27–38. https://doi.org/10.1016/j.cbpa.2014.09.002
Jonz MG, Buck LT, Perry SF et al (2016) Sensing and surviving hypoxia in vertebrates. Ann N Y Acad Sci 1365:43–58. https://doi.org/10.1111/nyas.12780
Joyce W, Perry SF (2020) Hypoxia inducible factor-1 α knockout does not impair acute thermal tolerance or heat hardening in zebrafish. Biol Lett 16:20200292. https://doi.org/10.1098/rsbl.2020.0292
Kaelin WG, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402. https://doi.org/10.1016/j.molcel.2008.04.009
Kamei H, Lu L, Jiao S et al (2008) Duplication and diversification of the hypoxia-inducible IGFBP-1 gene in Zebrafish. PLoS ONE 3:e3091. https://doi.org/10.1371/journal.pone.0003091
Karpowicz RJ, Dunn M, Sulzer D, Sames D (2013) APP+, a fluorescent analogue of the neurotoxin MPP+, is a marker of catecholamine neurons in brain tissue, but not a fluorescent false neurotransmitter. ACS Chem Neurosci 4:858–869. https://doi.org/10.1021/cn400038u
Keith J, Buckler KJ, Turner PJ (2013) Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol 591:3549–3563. https://doi.org/10.1113/jphysiol.2013.257741
Kline DD, Yang T, Huang PL, Prabhakar NR (1998) Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J Physiol 511:273–287. https://doi.org/10.1111/j.1469-7793.1998.273bi.x
Koudrina N, Perry SF, Gilmour KM (2020) The role of TASK-2 channels in CO2 sensing in zebrafish (Danio rerio). Am J Physiol Regul Integr Compar Physiol 319:R329–R342. https://doi.org/10.1152/ajpregu.00132.2020
Kunert E, Joyce W, Pan YK et al (2022) Role of cytosolic carbonic anhydrase Ca17a in cardiorespiratory responses to CO2 in developing zebrafish (Danio rerio). Am J Physiol Regul Integr Compar Physiol 323:R532–R546. https://doi.org/10.1152/ajpregu.00050.2022
Lahiri S, Forster RE (2003) CO2/H+ sensing: peripheral and central chemoreception. Int J Biochem Cell Biol 35:1413–1435. https://doi.org/10.1016/S1357-2725(03)00050-5
Lang T, Peters G, Hoffmann R, Meyer E (1987) Experimental investigations on the toxicity of ammonia: effects on ventilation frequency, growth, epidermal mucous cells, and gill structure of rainbow trout Salmo gairdneri. Dis Aquat Org 3:159–165. https://doi.org/10.3354/dao003159
Lauriano ER, Capillo G, Icardo JM et al (2021) Neuroepithelial cells (NECs) and mucous cells express a variety of neurotransmitters and neurotransmitter receptors in the gill and respiratory air-sac of the catfish Heteropneustes fossilis (Siluriformes, Heteropneustidae): a possible role in local immune defence. Zoology 148:125958. https://doi.org/10.1016/j.zool.2021.125958
Lauweryns JM, Cokelaere M, Lerut T, Theunynck P (1978) Cross-circulation studies on the influence of hypoxia and hypoxaemia on neuro-epithelial bodies in young rabbits. Cell Tissue Res. https://doi.org/10.1007/BF00225336
Lenfant C, Johansen K (1968) Respiration in the African Lungfish Protopterus Aethiopicus. J Exp Biol 49:437–452. https://doi.org/10.1242/jeb.49.2.437
Leonard EM, Weaver FE, Nurse CA (2022) Lactate sensing by neuroepithelial cells isolated from the gills of killifish (Fundulus heteroclitus). J Exp Biol 225:jeb245088. https://doi.org/10.1242/jeb.245088
Loenarz C, Coleman ML, Boleininger A et al (2011) The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Reports 12:63–70. https://doi.org/10.1038/embor.2010.170
Li M, Chen Q, Zhang Y-W (2022) Determining ligand and Ion-Induced conformational vhanges in serotonin transporter with its fluorescent substrates. IJMS 23:10919. https://doi.org/10.3390/ijms231810919
Linley JE (2013) Perforated whole-cell patch-clamp recording. In: Gamper N (ed) Ion channels. Humana Press, Totowa, pp 149–157
Livermore S, Zhou Y, Pan J et al (2015) Pulmonary neuroepithelial bodies are polymodal airway sensors: evidence for CO2/H+ sensing. Am J Physiol Lung Cell Mol Physiol 308:L807–L815. https://doi.org/10.1152/ajplung.00208.2014
Loboda A, Jozkowicz A, Dulak J (2012) HIF-1 versus HIF-2—Is one more important than the other? Vascul Pharmacol 56:245–251. https://doi.org/10.1016/j.vph.2012.02.006
López-Barneo J, González-Rodríguez P, Gao L et al (2016a) Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am J Physiol Cell Physiol 310:C629–C642. https://doi.org/10.1152/ajpcell.00265.2015
López-Barneo J, Ortega-Sáenz P, González-Rodríguez P et al (2016b) Oxygen-sensing by arterial chemoreceptors: mechanisms and medical translation. Mol Aspects Med 47–48:90–108. https://doi.org/10.1016/j.mam.2015.12.002
Ma K, Chen Y, Zhou L et al (2021) Cloning and characterization of nicotinic acetylcholine receptor γ-like gene in adult transparent Pristella maxillaris. Gene 769:145193. https://doi.org/10.1016/j.gene.2020.145193
Mandic M, Regan MD (2018) Can variation among hypoxic environments explain why different fish species use different hypoxic survival strategies? J Exp Biol 221:jeb161349. https://doi.org/10.1242/jeb.161349
Mandic M, Tzaneva V, Careau V, Perry SF (2019) Hif-1α paralogs play a role in the hypoxic ventilatory response of larval and adult zebrafish (Danio rerio). J Exp Biol. https://doi.org/10.1242/jeb.195198
Mandic M, Best C, Perry SF (2020) Loss of hypoxia-inducible factor 1α affects hypoxia tolerance in larval and adult zebrafish (Danio rerio). Proc R Soc B 287:20200798. https://doi.org/10.1098/rspb.2020.0798
Mandic M, Bailey A, Perry SF (2021a) Hypoxia inducible factor 1-α is minimally involved in determining the time domains of the hypoxic ventilatory response in adult zebrafish (Danio rerio). Respir Physiol Neurobiol 294:103774. https://doi.org/10.1016/j.resp.2021.103774
Mandic M, Joyce W, Perry SF (2021b) The evolutionary and physiological significance of the Hif pathway in teleost fishes. J Exp Biol 224:jeb231936. https://doi.org/10.1242/jeb.231936
Mauceri A, Fasulo S, Ainis L et al (1999) Neuronal nitric oxide synthase (nNOS) expression in the epithelial neuroendocrine cell system and nerve fibers in the gill of the catfish, Heteropneustes fossilis. Acta Histochem 101:437–448. https://doi.org/10.1016/S0065-1281(99)80044-0
McDonald MD, Gilmour KM, Walsh PJ, Perry SF (2010) Cardiovascular and respiratory reflexes of the gulf toadfish (Opsanus beta) during acute hypoxia. Respir Physiol Neurobiol 170:59–66. https://doi.org/10.1016/j.resp.2009.12.012
McElroy GS, Chandel NS (2017) Mitochondria control acute and chronic responses to hypoxia. Exp Cell Res 356:217–222. https://doi.org/10.1016/j.yexcr.2017.03.034
Mckendry JE, Milsom WK, Perry SF (2001) Branchial CO2 receptors and cardiorespiratory adjustments during hypercarbia in Pacific spiny dogfish (Squalus acanthias). J Exp Biol 204:1519–1527. https://doi.org/10.1242/jeb.204.8.1519
McKendry JE, Perry SF (2001) Cardiovascular effects of hypercarbia in rainbow trout (Oncorhynchus mykiss): a role for externally oriented chemoreceptors. J Exp Biol 204:115–125. https://doi.org/10.1242/jeb.204.1.115
McKenzie DJ, Taylor EW, Bronzi P, Bolis CL (1995) Aspects of cardioventilatory control in the adriatic sturgeon (Acipenser naccarii). Respir Physiol 100:45–53. https://doi.org/10.1016/0034-5687(94)00121-F
Miller SH, Zarate S, Smith EH et al (2014) Effect of elevated pCO2 on metabolic responses of porcelain crab (Petrolisthes cinctipes) larvae exposed to subsequent salinity stress. PLoS ONE 9:e109167. https://doi.org/10.1371/journal.pone.0109167
Mills DB, Francis WR, Vargas S et al (2018) The last common ancestor of animals lacked the HIF pathway and respired in low-oxygen environments. Elife 7:e31176. https://doi.org/10.7554/eLife.31176
Milsom WK (2012) New insights into gill chemoreception: receptor distribution and roles in water and air breathing fish. Respir Physiol Neurobiol 184:326–339. https://doi.org/10.1016/j.resp.2012.07.013
Milsom WK, Brill RW (1986) Oxygen sensitive afferent information arising from the first gill arch of yellowfin tuna. Respir Physiol 66:193–203. https://doi.org/10.1016/0034-5687(86)90072-1
Milsom WK, Burleson ML (2007) Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir Physiol Neurobiol 157:4–11. https://doi.org/10.1016/j.resp.2007.02.007
Milsom WK, Gilmour KM, Perry S et al (2022) Control of Breathing in Ectothermic Vertebrates. In: Prakash YS (ed) Comprehensive physiology, 1st edn. Wiley, London, pp 3869–3988
Nakada T, Westhoff CM, Kato A, Hirose S (2007) Ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB j 21:1067–1074. https://doi.org/10.1096/fj.06-6834com
Nassenstein C, Wiegand S, Lips KS et al (2015) Cholinergic activation of the murine trachealis muscle via non-vesicular acetylcholine release involving low-affinity choline transporters. Int Immunopharmacol 29:173–180. https://doi.org/10.1016/j.intimp.2015.08.007
Nawata CM, Hung CCY, Tsui TKN et al (2007) Ammonia excretion in rainbow trout (Oncorhynchus mykiss): evidence for Rh glycoprotein and H+-ATPase involvement. Physiol Genomics 31:463–474. https://doi.org/10.1152/physiolgenomics.00061.2007
Nawata CM, Hirose S, Nakada T et al (2010) Rh glycoprotein expression is modulated in pufferfish (Takifugu rubripes) during high environmental ammonia exposure. J Exp Biol 213:3150–3160. https://doi.org/10.1242/jeb.044719
Nilsson S (1986) Control of gill blood flow. In: Nilsson S, Holmgren S (eds) Fish physiology: recent advances. Springer, Dordrecht, pp 86–101. https://doi.org/10.1007/978-94-011-6558-7_5
Nilsson GE (2007) Gill remodeling in fish–a new fashion or an ancient secret? J Exp Biol 210:2403–2409. https://doi.org/10.1242/jeb.000281
Noguchi M, Furukawa KT, Morimoto M (2020) Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease. Disease Models Mech 13:dmm046920. https://doi.org/10.1242/dmm.046920
Nurse CA (2005) Neurotransmission and neuromodulation in the chemosensory carotid body. Autonom Neurosci Basic Clin 120:1–9
Nurse CA (2010) Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol 95:657–667. https://doi.org/10.1113/expphysiol.2009.049312
Nurse CA (2017) A sensible approach to making sense of oxygen sensing. J Physiol 595:6087–6088. https://doi.org/10.1113/JP274880
Olschewski A, Veale EL, Nagy BM et al (2017) TASK-1 (KCNK3) channels in the lung: from cell biology to clinical implications. Eur Respir J 50:1700754. https://doi.org/10.1183/13993003.00754-2017
Ortega-Sáenz P, Moreno-Domínguez A, Gao L, López-Barneo J (2020) Molecular mechanisms of acute oxygen sensing by arterial chemoreceptor cells, role of Hif2α. Front Physiol 11:614893. https://doi.org/10.3389/fphys.2020.614893
Pan YK, Perry SF (2020) Neuroendocrine control of breathing in fish. Mol Cell Endocrinol 509:110800. https://doi.org/10.1016/j.mce.2020.110800
Pan W, Scott AL, Nurse CA, Jonz MG (2021) Identification of oxygen-sensitive neuroepithelial cells through an endogenous reporter gene in larval and adult transgenic zebrafish. Cell Tissue Res 384:35–47. https://doi.org/10.1007/s00441-020-03307-5
Pan W, Godoy RS, Cook DP et al (2022) Single-cell transcriptomic analysis of neuroepithelial cells and other cell types of the gills of zebrafish (Danio rerio) exposed to hypoxia. Sci Rep 12:10144. https://doi.org/10.1038/s41598-022-13693-1
Peña-Münzenmayer G, Niemeyer MI, Sepúlveda FV et al (2014) Zebrafish and mouse TASK-2 K+ channels are inhibited by increased CO2 and intracellular acidification. Pflugers Arch-Eur J Physiol 466:1317–1327. https://doi.org/10.1007/s00424-013-1365-2
Pearse A (1969) The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the apud series and the embryologic, physiologic, and pathologic implications of the concept. J Histochem Cytochem 17:303–313. https://doi.org/10.1177/17.5.303
Pelster B, Egg M (2018) Hypoxia-inducible transcription factors in fish: expression, function and interconnection with the circadian clock. J Exp Biol 221:jeb163709. https://doi.org/10.1242/jeb.163709
Perry SF, Abdallah S (2012) Mechanisms and consequences of carbon dioxide sensing in fish. Respir Physiol Neurobiol 184:309–315. https://doi.org/10.1016/j.resp.2012.06.013
Perry SF, Gilmour KM (2002) Sensing and transfer of respiratory gases at the fish gill. J Exp Zool 293:249–263. https://doi.org/10.1002/jez.10129
Perry SF, Reid SG (2002) Cardiorespiratory adjustments during hypercarbia in rainbow trout Oncorhynchus mykiss are initiated by external CO2 receptors on the first gill arch. J Exp Biol 205:3357–3365. https://doi.org/10.1242/jeb.205.21.3357
Perry SF, Tzaneva V (2016) The sensing of respiratory gases in fish: mechanisms and signalling pathways. Respir Physiol Neurobiol 224:71–79. https://doi.org/10.1016/j.resp.2015.06.007
Perry S, Esbaugh A, Braun M, Gilmour K (2009) Gas transport and gill function in water-breathing fish. In: Glass ML, Wood M (eds) Cardio-respiratory control in vertebrates: comparative and evolutionary aspects. Springer, Heidelberg, pp 5–42
Perry S, Kumai Y, Porteus CS et al (2016) An emerging role for gasotransmitters in the control of breathing and ionic regulation in fish. J Comp Physiol B 186:145–159. https://doi.org/10.1007/s00360-015-0949-x
Perry SF, Pan YK, Gilmour KM (2023) Insights into the control and consequences of breathing adjustments in fishes-from larvae to adults. Front Physiol 14:1065573. https://doi.org/10.3389/fphys.2023.1065573
Podkowa D, Goniakowska-Witalinska L (2002) Adaptations to the air breathing in the posterior intestine of the catfish (Corydoras aeneus, Callichthyidae). A histological and ultrastructural study. Folia Biol (krakow) 50(1–2):69–82
Poole CA, Satchell GH (1979) Nociceptors in the gills of the dogfish Squalus acanthias. J Comp Physiol 130:1–7. https://doi.org/10.1007/BF02582968
Porteus C, Hedrick MS, Hicks JW et al (2011) Time domains of the hypoxic ventilatory response in ectothermic vertebrates. J Comp Physiol B 181:311–333. https://doi.org/10.1007/s00360-011-0554-6
Porteus CS, Brink DL, Milsom WK (2012) Neurotransmitter profiles in fish gills: putative gill oxygen chemoreceptors. Respir Physiol Neurobiol 184:316–325. https://doi.org/10.1016/j.resp.2012.06.019
Porteus CS, Brink DL, Coolidge EH et al (2013) Distribution of acetylcholine and catecholamines in fish gills and their potential roles in the hypoxic ventilatory response. Acta Histochem 115:158–169. https://doi.org/10.1016/j.acthis.2012.06.004
Porteus CS, Abdallah SJ, Pollack J et al (2014a) The role of hydrogen sulfide in the control of breathing in hypoxic zebrafish (Danio rerio). J Physiol 592:3075–3088. https://doi.org/10.1113/jphysiol.2014.271098
Porteus CS, Wright PA, Milsom WK (2014b) Characterization of putative oxygen chemoreceptors in bowfin (Amia calva). J Exp Biol 217:1269–1277. https://doi.org/10.1242/jeb.098467
Porteus CS, Pollack J, Tzaneva V et al (2015) A role for nitric oxide in the control of breathing in zebrafish (Danio rerio). J Exp Biol 218(3746–3753): https://doi.org/10.1242/jeb.127795
Porteus C, Kumai Y, Abdallah SJ et al (2021) Respiratory responses to external ammonia in zebrafish (Danio rerio). Comp Biochem Physiol A Mol Integr Physiol 251:110822. https://doi.org/10.1016/j.cbpa.2020.110822
Powell FL, Milsom WK, Mitchell GS (1998) Time domains of the hypoxic ventilatory response. Respir Physiol 112:123–134
Pumplin DW, Getschman E (2000) Synaptic proteins in rat taste bud cells: appearance in the Golgi apparatus and relationship to α-gustducin and the Lewisb and A antigens. J Comp Neurol 427:171–184. https://doi.org/10.1002/1096-9861(20001113)427:2%3c171::AID-CNE1%3e3.0.CO;2-W
Qin Z, Lewis JE, Perry SF (2010) Zebrafish (Danio rerio) gill neuroepithelial cells are sensitive chemoreceptors for environmental CO2. J Physiol 588:861–872. https://doi.org/10.1113/jphysiol.2009.184739
Rahbar S, Pan W, Jonz MG (2016) Purinergic and cholinergic drugs mediate hyperventilation in Zebrafish: evidence from a novel chemical screen. PLoS ONE 11:e0154261. https://doi.org/10.1371/journal.pone.0154261
Randall D, Cameron J (1973) Respiratory control of arterial pH as temperature changes in rainbow trout Salmo gairdneri. Am J Physiol Legacy Cont 225:997–1002. https://doi.org/10.1152/ajplegacy.1973.225.4.997
Randall DJ, Ip YK (2006) Ammonia as a respiratory gas in water and air-breathing fishes. Respir Physiol Neurobiol 154:216–225. https://doi.org/10.1016/j.resp.2006.04.003
Ratcliffe P, Pan J, Bishop T, et al (2016) Hyperplasia and hypertrophy of pulmonary neuroepithelial bodies, presumed airway hypoxia sensors, in hypoxia-inducible factor prolyl hydroxylase-deficient mice. HP. https://doi.org/10.2147/HP.S103957
Reed M, Jonz MG (2022) Neurochemical signalling associated with gill oxygen sensing and ventilation: a receptor focused mini-review. Front Physiol 13:940020. https://doi.org/10.3389/fphys.2022.940020
Reed M, Pan W, Musa L et al (2023) A role for dopamine in control of the hypoxic ventilatory response via D2 receptors in the zebrafish gill. J Compar Neurol. https://doi.org/10.1002/cne.25548
Regan KS, Jonz MG, Wright PA (2011) Neuroepithelial cells and the hypoxia emersion response in the amphibious fish Kryptolebias marmoratus. J Exp Biol 214:2560–2568. https://doi.org/10.1242/jeb.056333
Rissanen E, Tranberg HK, Sollid J et al (2006) Temperature regulates hypoxia-inducible factor-1 (HIF-1) in a poikilothermic vertebrate, crucian carp (Carassius carassius). J Exp Biol 209:994–1003. https://doi.org/10.1242/jeb.02103
Robertson CE, Turko AJ, Jonz MG, Wright PA (2015) Hypercapnia and low pH induce neuroepithelial cell proliferation and emersion behaviour in the amphibious fish Kryptolebias marmoratus. J Exp Biol 218:2987–2990. https://doi.org/10.1242/jeb.123133
Rossi GS, Cochrane PV, Wright PA (2020) Fluctuating environments during early development can limit adult phenotypic flexibility: insights from an amphibious fish. J Exp Biol. https://doi.org/10.1242/jeb.228304
Rytkönen KT, Storz JF (2011) Evolutionary origins of oxygen sensing in animals. EMBO Rep 12:3–4. https://doi.org/10.1038/embor.2010.192
Rytkönen KT, Williams TA, Renshaw GM et al (2011) Molecular evolution of the metazoan PHD–HIF oxygen-sensing system. Mol Biol Evol 28:1913–1926. https://doi.org/10.1093/molbev/msr012
Rytkönen KT, Akbarzadeh A, Miandare HK et al (2013) Subfunctionalization of cyprinid hypoxia-inducible factors for roles in development and oxygen sensing. Evolution 67:873–882. https://doi.org/10.1111/j.1558-5646.2012.01820.x
Saltys HA, Jonz MG, Nurse CA (2006) Comparative study of gill neuroepithelial cells and their innervation in teleosts and Xenopus tadpoles. Cell Tissue Res 323:1–10. https://doi.org/10.1007/s00441-005-0048-5
Sardella B, Brauner C (2007) The osmo-respiratory compromise in fish: the effects of physiological state and the environment. Fish respiration and environment edition, 1st edn Imprint CRC Press
Sebastiani J, Sabatelli A, McDonald MD (2022) Mild hypoxia exposure impacts peripheral serotonin uptake and degradation in Gulf toadfish (Opsanus beta). J Exp Biol 225:jeb24064. https://doi.org/10.1242/jeb.244064
Shakarchi K, Zachar PC, Jonz MG (2013) Serotonergic and cholinergic elements of the hypoxic ventilatory response in developing zebrafish. J Exp Biol. https://doi.org/10.1242/jeb.079657
Shelton G, Jones DR, Milsom WK (1986) Control of breathing in ectothermic vertebrates. In: Handbook of Physiology section 3 The respiratory system. Cherniak NS and Widdicombe JG (eds) American Physiological Society, Bethesda control of breathing 2:857–909. https://doi.org/10.1002/cphy.cp030228
Shivaraju M, Chitta UK, Grange RMH et al (2021) Airway stem cells sense hypoxia and differentiate into protective solitary neuroendocrine cells. Science 371:52–57. https://doi.org/10.1126/science.aba0629
Smith DG, Johnson DW (1977) Oxygen exchange in a simulated trout gill secondary lamella. Am J Physiol Regul Integr Comp Physiol 233:R145–R161
Soitamo AJ, Råbergh CMI, Gassmann M et al (2001) Characterization of a hypoxia-inducible factor (HIF-1α) from rainbow trout. J Biol Chem 276:19699–19705. https://doi.org/10.1074/jbc.M009057200
Sollid J, Rissanen E, Tranberg HK et al (2006) HIF-1α and iNOS levels in crucian carp gills during hypoxia-induced transformation. J Comp Physiol B 176:359–369. https://doi.org/10.1007/s00360-005-0059-2
Stecyk JAW, Farrell AP (2006) Regulation of the cardiorespiratory system of common carp (Cyprinus carpio) during severe hypoxia at three seasonal acclimation temperatures. Physiol Biochem Zool 79:614–627. https://doi.org/10.1086/501064
Stensløkken K-O, Sundin L, Renshaw GMC, Nilsson GE (2004) Adenosinergic and cholinergic control mechanisms during hypoxia in the epaulette shark (Hemiscyllium ocellatum), with emphasis on branchial circulation. J Exp Biol 207:4451–4461. https://doi.org/10.1242/jeb.01291
Stensløkken K-O, Sundin L, Nilsson GE (2006) Endothelin receptors in teleost fishes: cardiovascular effects and branchial distribution. Am J Physiol Regul Integr Comp Physiol 290:R852–R860. https://doi.org/10.1152/ajpregu.00618.2004
Sundin L (1995) Serotonergic vasomotor control in fish gills. Braz J Med Biol Res 28(11–12):1217–1221. https://doi.org/10.1007/s003600050184
Sundin L, Nilsson GE (1997) Neurochemical mechanisms behind gill microcirculatory responses to hypoxia in trout: in vivo microscopy study. Am J Physiol Regul Integr Compar Physiol 272:R576–R585. https://doi.org/10.1152/ajpregu.1997.272.2.R576
Sundin L, Nilsson GE (1998) Endothelin redistributes blood flow through the lamellae of rainbow trout gills. J Comp Physiol [b] 168:619–623. https://doi.org/10.1007/s003600050184
Sundin L, Nilsson S (2002) Branchial innervation. J Exp Zool 293:232–248. https://doi.org/10.1002/jez.10130
Sundin L, Davison W, Forster M, Axelsson M (1998a) A role of 5-HT2 receptors in the gill vasculature of the antarctic fish Pagothenia borchgrevinki. J Exp Biol 201:2129–2138. https://doi.org/10.1242/jeb.201.14.2129
Sundin L, Holmgren S, Nilsson S (1998b) The oxygen receptor of the teleost gill? Acta Zool 79:207–214. https://doi.org/10.1111/j.1463-6395.1998.tb01159.x
Sundin L, Reid SG, Rantin FT, Milsom WK (2000) Branchial receptors and cardiorespiratory reflexes in a neotropical fish, the tambaqui (Colossoma macropomum). J Exp Biol 203:1225–1239. https://doi.org/10.1242/jeb.203.7.1225
Sundin L, Burleson ML, Sanchez AP et al (2007) Respiratory chemoreceptor function in vertebrates comparative and evolutionary aspects. Integr Comp Biol 47:592–600. https://doi.org/10.1093/icb/icm076
Thomsen MT, Wang T, Milsom WK, Bayley M (2017) Lactate provides a strong pH-independent ventilatory signal in the facultative air-breathing teleost Pangasianodon hypophthalmus. Sci Rep 7:6378. https://doi.org/10.1038/s41598-017-06745-4
Thomsen MT, Lefevre S, Nilsson GE et al (2019) Effects of lactate ions on the cardiorespiratory system in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Compar Physiol 316:R607–R620. https://doi.org/10.1152/ajpregu.00395.2018
Timón-Gómez A, Scharr AL, Wong NY et al (2022) Tissue-specific mitochondrial HIGD1C promotes oxygen sensitivity in carotid body chemoreceptors. Elife 11:e78915. https://doi.org/10.7554/eLife.78915
Torres-Torrelo H, Ortega-Sáenz P, Macías D et al (2018) The role of Olfr78 in the breathing circuit of mice. Nature 561:E33–E40. https://doi.org/10.1038/s41586-018-0545-9
Torres-Torrelo H, Ortega-Sáenz P, Gao L, López-Barneo J (2021) Lactate sensing mechanisms in arterial chemoreceptor cells. Nat Commun 12:4166. https://doi.org/10.1038/s41467-021-24444-7
Tresguerres M, Milsom WK, Perry SF (2019) CO2 and acid-base sensing. In: Fish physiology. Elsevier, London, pp 33–68
Tzaneva V, Perry SF (2010) The control of breathing in goldfish (Carassius auratus) experiencing thermally induced gill remodelling. J Exp Biol 213:3666–3675. https://doi.org/10.1242/jeb.047431
Tzaneva V, Perry SF (2014) Heme oxygenase-1 (HO-1) mediated respiratory responses to hypoxia in the goldfish, Carassius auratus. Respir Physiol Neurobiol 199:1–8. https://doi.org/10.1016/j.resp.2014.04.006
Tzaneva V, Perry SF (2016) Role of endogenous carbon monoxide in the control of breathing in zebrafish (Danio rerio). Am J Physiol Regul Integr Compar Physiol 311:R1262–R1270. https://doi.org/10.1152/ajpregu.00094.2016
Tzaneva V, Bailey S, Perry SF (2011) The interactive effects of hypoxemia, hyperoxia, and temperature on the gill morphology of goldfish (Carassius auratus). Am J Physiol Regul Integr Compar Physiol 300:R1344–R1351. https://doi.org/10.1152/ajpregu.00530.2010
Varas R, Wyatt CN, Buckler KJ (2007) Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J Physiol 583:521–536. https://doi.org/10.1113/jphysiol.2007.135657
Voshol H, CarolWEM VZ, Orberger G et al (1996) Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. J Biol Chem 271:22957–22960. https://doi.org/10.1074/jbc.271.38.22957
Vulesevic B, McNeill B, Perry SF (2006) Chemoreceptor plasticity and respiratory acclimation in the zebrafish Danio rerio. J Exp Biol 209:1261–1273. https://doi.org/10.1242/jeb.02058
Watanabe S (2016) Flash-and-freeze: coordinating optogenetic stimulation with rapid freezing to visualize membrane dynamics at synapses with millisecond resolution. Front Synaptic Neurosci 8:24. https://doi.org/10.3389/fnsyn.2016.00024
Weir EK, López-Barneo J, Buckler KJ, Archer SL (2005) Acute oxygen-sensing mechanisms. N Engl J Med 353:2042–2055. https://doi.org/10.1056/NEJMra050002
Wicheser J, Kazemi H (1974) Ammonia and ventilation: site and mechanism of action. Resp Physiol 20:393–406. https://doi.org/10.1016/0034-5687(74)90035-8
Williams SEJ, Wootton P, Mason HS et al (2004) Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306:2093–2097. https://doi.org/10.1126/science.1105010
Wilson JM, Laurent P (2002) Fish gill morphology: inside out. J Exp Zool 293:192–213. https://doi.org/10.1002/jez.10124
Wood CM, Eom J (2021) The osmorespiratory compromise in the fish gill. Comp Biochem Physiol A Mol Integr Physiol 254:110895. https://doi.org/10.1016/j.cbpa.2021.110895
Wood CM, Simmons H (1994) The conversion of plasma HCO3- to CO2 by rainbow trout red blood cells in vitro: adrenergic inhibition and the influence of oxygenation status. Fish Physiol Biochem 12:445–454. https://doi.org/10.1007/BF00004447
Wright PA, Wood CM (2012) Seven things fish know about ammonia and we don’t. Respir Physiol Neurobiol 184:231–240. https://doi.org/10.1016/j.resp.2012.07.003
Wright PA, Steele SL, Huitema A, Bernier NJ (2007) Induction of four glutamine synthetase genes in brain of rainbow trout in response to elevated environmental ammonia. J Exp Biol 210:2905–2911. https://doi.org/10.1242/jeb.003905
Yeger H, Pan J, Cutz E (2009) Precursors and stem cells of the pulmonary neuroendocrine cell system in the developing mammalian lung. In: Zaccone G, Cutz E, Adriaensen D, Nurse CA, Mauceri A (eds) Airway chemoreceptors in the vertebrates. Science Publishers, Enfield, pp 235–268
Youngson C, Nurse C, Yeger H, Cutz E (1993) Oxygen sensing in airway chemoreceptors. Nature 365:153–155. https://doi.org/10.1038/365153a0
Zaccone G, Ainis L, Fasulo S, Lo Cascio P, Maucer A, Licata A (1989a) Merkel cells detected by immunocytochemistry in the epidermis of the freshwater Heteropneustes fossilis (Bloch) Zoo]. lb. Anat I 1(9):129–134
Zaccone G, Ainis L, Fasulo S, Lo Cascio P, Maucer A, Licata A (1989b) Localization of calmodulin positive immunoreactivity in the surface epidermis of the brown trout, Salmo trutta. Histochemistry 91:13–16
Zaccone G, Lauweryns JM, Fasulo S et al (1992) Immunocytochemical localization of serotonin and neuropeptides in the neuroendocrine paraneurons of teleost and lungfish gills. Acta Zool 73:177–183. https://doi.org/10.1111/j.1463-6395.1992.tb01185.x
Zaccone G, Fasulo S, Ainis L (1994) Distribution patterns of the paraneuronal endocrine cells in the skin, gills and the airways of fishes as determined by immunohistochemical and histological methods. Histochem J 26:609–629. https://doi.org/10.1007/BF00158286
Zaccone G, Fasulo S, Ainis L, Licata A (1997) Paraneurons in the gills and airways of fishes. Microsc Res Tech 37:4–12. https://doi.org/10.1002/(SICI)1097-0029(19970401)37:1%3C4::AID-JEMT2%3E3.0.CO;2-R
Zaccone G, Ainis L, Mauceri A et al (2003) NANC nerves in the respiratory air sac and branchial vasculature of the indian catfish, Heteropneustes fossilis. Acta Histochem 105:151–163. https://doi.org/10.1078/0065-1281-00695
Zaccone G, Mauceri A, Fasulo S (2006) Neuropeptides and nitric oxide synthase in the gill and the air-breathing organs of fishes. J Exp Zool 305A:428–439. https://doi.org/10.1002/jez.a.267
Zaccone G, Mauceri A, Maisano M et al (2008) Neurotransmitter localization in the neuroepithelial cells and unipolar neurons of the respiratory tract in the bichir, Polypterus bichir bichir G. ST-HIL. Acta Histochem 110:143–150. https://doi.org/10.1016/j.acthis.2007.09.002
Zaccone G, Garcia LMP, Beltran-Frutos E (2009) Neuroendocrine system of the reptilian respiratory tract. In: Zaccone G, Cutz E, Adriaensen D, Nurse CA, Mauceri A (eds) Airway chemoreceptors in the vertebrates. Science Publishers, Enfield, pp 235–268
Zaccone D, Gopesh A, Anastasi G et al (2012) Localization of neurotransmitters, peptides and nNOS in the pseudobranchial neurosecretory cell system and associated carotid labyrinth of the catfish, Clarias batrachus. Acta Histochemica 114:62–67. https://doi.org/10.1016/j.acthis.2011.02.005
Zaccone G, Lauriano ER, Kuciel M et al (2017) Identification and distribution of neuronal nitric oxide synthase and neurochemical markers in the neuroepithelial cells of the gill and the skin in the giant mudskipper, Periophthalmodon schlosseri. Zoology 125:41–52. https://doi.org/10.1016/j.zool.2017.08.002
Zaccone G, Lauriano ER, Capillo G, Kuciel M (2018) Air-breathing in fish: air-breathing organs and control of respiration. Acta Histochem 120:630–641. https://doi.org/10.1016/j.acthis.2018.08.009
Zaccone G, Cupello C, Capillo G et al (2020) Expression of acetylcholine- and G protein coupled Muscarinic receptor in the Neuroepithelial cells (NECs) of the obligated air-breathing fish, Arapaima gigas (Arapaimatidae: Teleostei). Zoology 139:125755. https://doi.org/10.1016/j.zool.2020.125755
Zaccone G, Capillo G, Aragona M et al (2022a) Gill structure and neurochemical markers in the African bonytongue (Heterotis niloticus): a preliminary study. Acta Histochem 124:151954. https://doi.org/10.1016/j.acthis.2022.151954
Zaccone G, Capillo G, Fernandes JMO et al (2022b) Expression of the antimicrobial peptide piscidin 1 and neuropeptides in fish gill and skin: a potential participation in neuro-immune interaction. Mar Drugs 20:145. https://doi.org/10.3390/md20020145
Zachar PC, Jonz MG (2012) Neuroepithelial cells of the gill and their role in oxygen sensing. Respir Physiol Neurobiol 184:301–308. https://doi.org/10.1016/j.resp.2012.06.024
Zachar PC, Pan W, Jonz MG (2017) Distribution and morphology of cholinergic cells in the branchial epithelium of zebrafish (Danio rerio). Cell Tissue Res 367:169–179. https://doi.org/10.1007/s00441-016-2531-6
Zhang L, Wood CM (2009) Ammonia as a stimulant to ventilation in rainbow trout Oncorhynchus mykiss. Respir Physiol Neurobiol 168:261–271. https://doi.org/10.1016/j.resp.2009.07.011
Zhang L, Nawata CM, Wood CM (2013) Sensitivity of ventilation and brain metabolism to ammonia exposure in rainbow trout, Oncorhynchus mykiss. J Exp Biol 216:4025–4037. https://doi.org/10.1242/jeb.087692
Zhang L, Nurse CA, Jonz MG, Wood CM (2011) Ammonia sensing by neuroepithelial cells and ventilatory responses to ammonia in rainbow trout. J Exp Biol 214:2678–2689. https://doi.org/10.1242/jeb.055541
Zhang L, Michele Nawata C, De Boeck G, Wood CM (2015) Rh protein expression in branchial neuroepithelial cells, and the role of ammonia in ventilatory control in fish. Comp Biochem Physiol A Mol Integr Physiol 186:39–51. https://doi.org/10.1016/j.cbpa.2014.10.004
Zhang X-L, Sun Y-W, Chen J et al (2017) Gene duplication, conservation and divergence of Heme oxygenase 2 genes in blunt snout bream (Megalobrama amblycephala) and their responses to hypoxia. Gene 610:133–139. https://doi.org/10.1016/j.gene.2017.02.017
Acknowledgements
We would like to thank Jocelyn Shu for creating the illustration of zebrafish innervation pattern. We would like to acknowledge funding from the Natural Sciences and Engineering Research Council of Canada Grants to EML (RGPIN-2023-05466), CSP (RGPIN-2021-03509), and WKM (GR010285).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernd Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leonard, E.M., Porteus, C.S., Brink, D. et al. Fish gill chemosensing: knowledge gaps and inconsistencies. J Comp Physiol B (2024). https://doi.org/10.1007/s00360-024-01553-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00360-024-01553-5