Abstract
We investigated the body mass (M) scaling of resting metabolic rate (RMR), maximum metabolic rate (MMR), excess post-exercise oxygen consumption (EPOC), blood parameters, and organ masses of black carp (Mylopharyngoden piceus). The results showed that RMR scaled with M of the fish by an exponent (b) of 0.833 (bR), which was significantly larger than 0.75. MMR scaled with M by a power of 0.775 (bM), which was significantly lower than 1 and may be due to a small size proportion of red muscle. No difference between bR and bM or correlation between factorial aerobic scope and M was found. However, EPOC scaled positively with M by a power of 1.231, suggesting a constant aerobic capacity and an enhanced anaerobic capacity with fish growth. Mass of the inactive organs scaled with M by a power of 1.005, which was significantly larger than 1 and was negatively correlated with RMR, suggesting that the proportion of inactive organs increases with fish growth, which may contribute to the negative scaling of RMR. Red blood cell surface area (S) did not increase with increasing M, suggesting that the ontogenetic decrease in the surface area to volume ratio of cells may not contribute to the negative scaling of RMR. The predicted bR value (0.846) by the average S (1.746 µm²) differs by only 1.62% from the observed bR value using our previously reported S − bR function in carp, suggesting that the species-specific cell size, rather than its ontogenetic change, affects the metabolic scaling of a species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The allometric scaling relationship between metabolic rate (MR) and body mass (M), a fundamental issue in eco-physiology, can be described by a power function MR = aMb, where a is a constant and b is the scaling exponent. The mechanisms underlying metabolic scaling have attracted a large amount of research, and the allometric scaling of the metabolic rate has been suggested to be the consequence of multiple causes (Glazier 2014a), such as the surface area-related heat loss (Rubner 1883), the ontogenetic decrease in the size proportions of active organs (Itazawa and Oikawa 1983; Oikawa et al. 1992), the fractal resource distribution network (West et al. 1997; Brown et al. 2004), the ontogenetic change in cell size and thus in the relative surface area of cells (Davison 1955; Kozłowski et al. 2003; Starostová et al. 2013), and the constraints resulting from the relative importance of metabolic level and effects of surface area vs. volume-related metabolic processes of organisms (the metabolic level boundaries hypothesis, MLBH) (Glazier 2005, 2010, 2014b). According to the aforementioned mechanisms, resting metabolic rate (RMR) can scale with M by various b values, e.g., 0.67 (Rubner 1883), 0.75 (West et al. 1997; Brown et al. 2004), and varying values between 0.67 and 1 (Davison 1955; Kozłowski et al. 2003; Glazier 2005, 2010).
Among most fish species, the b value for RMR (bR) varies between 0.67 and 1 depending on lifestyle and environmental factors (Killen et al. 2007, 2010). Our recent studies quantifying metabolic scaling in several closely related species of cyprinids suggest that previous theories can only partially explain their metabolic scaling (Huang et al. 2013; Zhang et al. 2014; Luo et al. 2015). Data for additional species are necessary to test the previous theories of metabolic scaling. In recent work, we proposed a negative relationship between the red blood cell surface area (S) and bR among cyprinid species. This can be explained by a smaller surface to volume ratio of the cells in species with larger cell sizes (Luo et al. 2015), which may intensify the surface boundary limits on bR (Glazier 2005, 2010). It would be useful to validate the accuracy of this S − bR function in predicting the intra-specific bR values by S in additional closely related species.
We selected the black carp (Mylopharyngoden piceus), a carnivorous cyprinid, as the focus of this study. Previous studies have reported the inter-individual variation in metabolic level, growth, and swimming performance of the black carp under multiple conditions (Yan et al. 2013; Pang et al. 2015, 2016). The metabolic scaling of this species and its underlying mechanism remain unknown. This study aims to assess the mass scaling of both RMR and MMR and the effects of cell size and organ size on metabolic scaling and to validate the S − bR function by Luo et al. (2015) in the black carp.
Materials and methods
Black carp specimens were collected from local fisheries in Xiema, Chongqing, China, and acclimated in a rearing system for at least 2 weeks prior to the experiment. During acclimation, the water temperature was maintained at 25 ± 1 °C with a 12 h:12 h light: dark photoperiod. The oxygen concentration was above 90% saturation, and the ammonia concentration was less than 0.015 mg L−1. The fish were fed with a commercial diet once daily. The sample size of fish was 30. Animal handling and experiments followed the ethical requirements and recommendations of the Animal Care of the Fisheries Science Institution of Southwest University, China. All data generated or analyzed during this study are included in the supplementary information files (Online Resource 1).
When the acclimation was completed, the fish were starved for 48 h and weighed to 0.01 g. The metabolic rates of individual fish were measured by a continuous flow respirometer (Wang et al. 2012). According to the body mass of the fish, respiratory chambers of different sizes were chosen (0.03, 0.13, 0.52, 0.86, and 1.20 L). The fish were transferred individually into chambers, and the metabolic rate was measured after 12 h. Fourteen individuals were measured at the same time. One chamber without fish was set as a control. The flow rate was adjusted to ensure that the difference in oxygen concentration between the control chamber and the fish chamber was within 0.5–1.0 mg L−1. An oxygen meter (HQ30D, HACH Company, Loveland, CO, USA) was used to determine the oxygen concentration of the chamber outlets. The following formula was used to calculate the oxygen consumption rate (\({{M}_{{{\text{O}}_2}}}\), mg O2 h−1):
where ΔO2 is the difference in the oxygen concentration (mg O2 L−1) between the fish chamber and the control chamber and v (L h−1) is the flow rate through the chamber. \({{M}_{{{\text{O}}_2}}}\) was measured hourly for 6 h, and the lowest two values was averaged as the RMR of the fish. The maximum metabolic rate (MMR, mg O2 h−1) was determined by the chasing protocol as described in Wang et al. (2012). After the RMR measurement, the flow rate was adjusted to ensure that 95% of the water in the chamber be refreshed in 1 min. The fish were transferred individually into the circle device and chased to exhaustion using a hand net (this process lasted for approximately 10 min). The fish were then rapidly returned to the respiratory chamber, and \({{M}_{{{\text{O}}_2}}}\) was measured at 1 min intervals for the first 10 min post-exercise and then at 20, 30, 40, 50, 60, 80, 100, and 120 min until the \({{M}_{{{\text{O}}_2}}}\) declined to within 1.2-fold of RMR. The peak \({{M}_{{{\text{O}}_2}}}\) post-exercise was used as MMR. Factorial aerobic scope (FAS) was calculated as the ratio of MMR to RMR. Excess post-exercise oxygen consumption (EPOC, mg O2) was calculated as the magnitude of excess oxygen consumption above RMR during the recovery phase.
After measuring the MMR, the fish were put into the anesthetic solution (MS − 222, 0.15 g L−1) for blood and organ sampling. Blood was collected via caudal artery puncture using a 1 mL syringe containing 0.04 g L−1 anticoagulant (1 g sodium fluoride:3 g potassium oxalate) (Huang et al. 2013). The Hb: hemoglobin concentration was measured with a spectrophotometer (752, Modern Science Company, Shanghai, China) at a wavelength of 540 nm. The red blood cell count (RBCC, 109 mL−1) was measured by a Neubauer hemocytometer after dilution to 1:200 in 0.65% NaCl. The blood smear was dried and stained with Wright’s–Giemsa fluid (Gao et al. 2007; Zhang et al. 2014). Photos were then taken using a microscope (EV5680B, Aigo Company, China). The red blood cell length (LC, µm) and red blood cell width (WC, µm) were determined using software (Image-pro Plus). The red blood cell was assumed as ellipsoid in shape, and the red blood cell surface area (S, µm2) was calculated as follows: S = LC × WC × π/4.
Following blood sampling, the total mass of active organs (MActive organs) was determined, including the brain, gills, red muscle, heart, hepatopancreas, digestive tract, spleen, gonads, kidneys, skin, and scales. The total mass of inactive organs (MInactive organs), including white muscle, abdominal adipose, fins, and bone, was calculated as the difference between M and MActive organs.
Statistical analysis
The experimental data were analyzed using Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) and SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The log10 transformed data of RMR, MMR, EPOC, S, Hb, RBCC, and M were used for the ordinary least square regression. Estimates of the scaling exponents were described with 95% confidence intervals (CIs). The differences between scaling exponents were analyzed using a general linear model (GLM) with M as a covariate. A t test was used to compare the difference between b values and 0.75 or 1. The relationships between RMR and MMR, RMR and MInactive organs, RMR and S were analyzed using residual values. p values < 0.05 were considered statistically significant. Data are presented as the means ± standard error.
Results
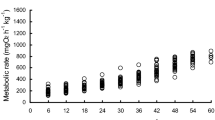
M of the experimental fish ranged from 3.7 to 202.1 g (n = 30). After exhaustive exercise, the metabolic rate values rose rapidly to a maximum followed by recovery to pre-exercise levels, and the recovery time ranged between 8 and 120 min dependent on M (Fig. 1). Whole animal RMR increased 21.9-fold with increasing M, ranging from 0.91 to 19.94 mg O2 h−1, while MMR increased 21.5-fold, ranging from 6.92 to 149.12 mg O2 h−1.
RMR and MMR scaled with M of the fish by powers of 0.833 (bR) and 0.775 (bM), respectively (Fig. 2). The bR value was significantly larger than 0.75 (t = 2.936, p = 0.007), while bM was not significantly different from 0.75 (t = 0.592, p = 0.559). Both bR (t = 5.950, p < 0.0001) and bM (t = 5.277, p < 0.0001) were significantly less than 1. There was no significant difference between bR and bM (F = 1.264, p = 0.266). No significant correlation was found between FAS and M (r2 = 0.074, p = 0.147) (Online Resource 2). MMR and RMR were positively correlated when controlling for M (Fig. 3). EPOC increased with M by a scaling exponent of 1.231 (Fig. 4).
The scaling exponent value of the MInactive organs (1.005) was significantly larger than 1 (t = 2.568, p = 0.016), while the scaling exponent value of the MActive organs (0.953) was not significantly different from 1 (t = 1.949, p = 0.061) (Fig. 5). RMR was negatively correlated with the MInactive organs when controlling for M (r2 = 0.190, p = 0.016) (Fig. 6).
No obvious correlations were found between M and any of the blood parameters, including S (r2 = 0.0045, p = 0.726), RBCC (r2 = 0.0454, p = 0.484), and Hb (r2 = 0.0554, p = 0.221) (Online Resource 3). No significant correlation between S and RMR was found when controlling for M (Online Resource 4).
Discussion
Our results indicate that the bR value of the black carp is within the previously reported range of intra-specific scaling exponents (0.65–0.95) for most teleost fishes (Clarke and Johnston 1999; Bokma 2004; Killen et al. 2010; Luo et al. 2015). Consistent with previous studies of several closely related carp species (Zhang et al. 2014; Luo et al. 2015), the bR value of the black carp does not agree with the scaling exponent of 0.75 proposed by the fractal resource distribution network theory (West et al. 1997; Brown et al. 2004).
The cell size theory predicts that the bR value should equal 1.0 if an increase in body size is entirely due to an increase in cell number rather than cell size and suggests that larger cells have relatively lower MR (Davison 1955; Kozłowski et al. 2003; Starostová et al. 2013). This theory has been supported by the negative correlations between RMR with S in the spined loach (Cobitis taenia) and the crucian carp (Carassius auratus) (Maciak et al. 2011; Huang et al. 2013). However, no negative correlations between RMR with S were found in the black carp in the present study and several other species of carp in previous studies (Zhang et al. 2014; Luo et al. 2015), suggesting no general roles of cell size on RMR across different fishes. In addition, the present results showed that S of the black carp did not increase with increasing M (Online Resource 3), suggesting a constant cell size and thus a constant surface area to volume ratio of cells. Therefore, the ontogenetic decrease in the surface area to volume ratio of cells may not contribute to the negative scaling of RMR of the black carp. A similar unchanged erythrocyte size has also been reported in a closely related species, the crucian carp (Huang et al. 2013). A potential explanation may be that MR is determined by factors other than ontogenetic change in the cell surface area to volume ratio, such as body size-dependent changes in the membrane permeability and metabolic activity of cells (Savage et al. 2007; Kozłowski et al. 2010). Alternatively, it has been proposed that the species-specific cell size, rather than its ontogenetic change, affects metabolic scaling. Species with a larger cell size may have a smaller surface area to volume ratio (Luo et al. 2015), which may intensify the surface boundary limits on bR (Glazier 2005, 2010). Thus, a larger cell size results in a smaller bR of a species. The negative relationship between bR and S among several closely related species of carps can be expressed by bR = − 0.260 log S + 1.30 (Luo et al. 2015). By this function, a bR of 0.846 can be predicted by the logarithmic average S (1.746 µm2) of the black carp, which differs by only 1.62% from the observed bR value (0.833). The results suggest that the function described by Luo et al. (Luo et al. 2015) can effectively predict the intra-specific scaling exponent of RMR in carps.
According to the MLBH, a negative relationship exists between metabolic level (L) and bR (Glazier 2005, 2010, 2014b). Killen et al. (2010) proposed an equation between the metabolic level and bR among 89 species of teleost fish: bR = − 0.145 ln L + 1.377. Using this equation, a recent study showed that the predicted bR was only 7.5% deviated from the observed value in the crucian carp (Huang et al. 2013). In the present results, the natural logarithmic mass-specific RMR (4.88 mg O2 kg−1 h−1) of the black carp at the midpoint of the regression (ln M = 3.31) predicts a bR of 0.669, which is 19.6% deviated from the observed bR (0.833). The results cannot be explained according to the MLBH as that the black carp has an intermediate metabolic level in compared to that of those teleost species, which should entail an intermediate metabolic scaling exponent within the limited range (0.67–1) (Glazier 2005; 2010, 2014b).
It had been suggested that the allometric scaling of MR is attributed to a gradual decrease in the proportion of the MActive organs while increasing the proportion of the MInactive organs with increasing M (Itazawa and Oikawa 1983; Oikawa et al. 1992). Consistently, the positive scaling of MInactive organs with increasing M of the black carp suggests that the proportion of MInactive organs increases as the fish grows (Fig. 5), which can contribute to the negative scaling of RMR of the black carp. In addition, the negative correlation observed between MInactive organs and RMR suggests that the variation of RMR among individuals may be due to intra-specific differences of MInactive organs (Fig. 6). Because the scaling exponent of MInactive organs (1.005) was very close to 1 and the r2 for the correlation between RMR and MInactive organs was small (0.19), this suggests that the intra-specific metabolic scaling of the fish can only partly be explained by the size changes of organs, and therefore other organ characteristics (e.g., organ metabolic level) may be involved.
The bM value of the black carp was less than 1, suggesting that MMR may not scale isometrically with M. In many salmonids, MMR scales approximately isometrically (Brett 1965; Wieser 1985), which is attributed to the increasing importance of volume-related muscular energy expenditure on metabolism during exercise and the linear increase in muscle mass in proportion to M (Glazier 2005, 2009). However, in several species of carp, MMR scales negatively with M, suggesting that muscular energy expenditure has a limited contribution to whole-body metabolism in carp (Huang et al. 2013; Zhang et al. 2014; Luo et al. 2015). A possible explanation is that only a small portion of the carp’s body contains red muscle. Indeed, the maximal locomotion speeds of carp are also very low (Yan et al. 2013).
The difference of bM and bR may determine a change in FAS, a species aerobic capacity-related parameter, as organisms grow. The lower bM value than bR value of the black carp results in no significant change in FAS with increasing M (Figs. 2, S1). This suggests that, in contrast to the general observations in salmonids (Brett 1965; Beamish 1978), the aerobic capacity of the black carp may not increase as their bodies grow. Accordingly, several oxygen capacity-related blood parameters, including Hb, RBCC, and S, are also independent of M (Online Resource 3). In contrast, we found that EPOC scaled positively with M by a scaling exponent of 1.231 (Fig. 4), suggesting a higher metabolic consumption level restoring the RMR physiological state in larger fish.
MMR of the black carp correlated positively with RMR after controlling for body size (Fig. 3). This is consistent with the intra-specific results of the same species (Pang et al. 2015), as well as the inter-specific results among teleost fish species (Killen et al. 2016) and the intra- and inter-specific results of vertebrates (Auer et al. 2017), suggesting that there is a cost of maintaining the machinery that supports the maximal aerobic capacity. Similar positive relationships between RMR and MMR were found in the rainbow trout (Salmo gairdneri) (Wieser 1985), the brown trout (Salmo trutta) (Norin and Malte 2012), the crucian carp (Huang et al. 2013), and the grass carp (Ctenopharyngodon idellus) (Zhang et al. 2014).
In conclusion, the present study quantified the allometric scaling of the metabolic rate of black carp. The results suggest that the negative intra-specific scaling of RMR of the fish can be partly explained by the size changes of active/inactive organs. It suggests that mass-specific metabolic rate of active organs likely decreases with body mass.
Abbreviations
- M :
-
Body mass
- MR:
-
Metabolic rate
- RMR:
-
Resting metabolic rate
- MMR:
-
Maximum metabolic rate
- FAS:
-
Factorial aerobic scope
- EPOC:
-
Excess post-exercise oxygen consumption
- b :
-
Scaling exponent
- b R :
-
Scaling exponent of RMR
- b M :
-
Scaling exponent of MMR
- \({{M}_{{{\text{O}}_2}}}\) :
-
Oxygen consumption rate
- Hb:
-
Hemoglobin concentration
- RBCC:
-
Red blood cell count
- S :
-
Red blood cell surface area
- M Active organs :
-
Total mass of active organs
- M Inactive organs :
-
Total mass of inactive organs
References
Auer SK, Killen SS, Rezende EL (2017) Resting vs. active: a meta-analysis of the intra- and inter-specific associations between minimum, sustained, and maximum metabolic rates in vertebrates. Funct Ecol 31:1728–1738
Beamish FWH (1978) Swimming capacity. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VII. Academic Press, London
Bokma F (2004) Evidence against universal metabolic allometry. Funct Ecol 18:184–187
Brett JR (1965) The relation of size to rate of oxygen consumption and sustained swimming speed of sockeye salmon (Oncorhynchus nerka). J Fish Res Board Can 22:1491–1501
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Davison J (1955) Body weight, cell surface and metabolic rate in anuran Amphibia. Biol Bull 109:407–419
Gao ZX, Wang WM, Abbas K, Zhou XY, Yang Y, Diana JS, Wang HP, Wang HL, Li Y, Sun YH (2007) Haematological characterization of loach Misgurnus anguillicaudatus: comparison among diploid, triploid and tetraploid specimens. Comp Biochem Physiol A 147:1001–1008
Glazier DS (2005) Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev 80:611–662
Glazier DS (2009) Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. J Comp Physiol B 179:821–828
Glazier DS (2010) A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev 85:111–138
Glazier DS (2014a) Metabolic scaling in complex living systems. Systems 2:451–540
Glazier DS (2014b) Scaling of metabolic scaling within physical limits. Systems 2:425–450
Huang QD, Zhang YR, Liu ST, Wang W, Luo YP (2013) Intraspecific scaling of the resting and maximum metabolic rates of the crucian carp (Carassius auratus). PLoS One 8:e82837
Itazawa Y, Oikawa S (1983) Metabolic rates in excised tissues of carp. Experientia 39:160–161
Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc R Soc B 274:431–438
Killen SS, Atkinson D, Glazier DS (2010) The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol Lett 13:184–193
Killen SS, Glazier DS, Rezende EL, Clark TD, Atkinson D, Willener AS, Halsey LG (2016) Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am Nat 187:592–606
Kozłowski J, Konarzewski M, Gawelczyk AT (2003) Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci USA 100:14080–14085
Kozłowski J, Czarnołęski M, Françoiskrassowska A, Maciak S, Pis T (2010) Cell size is positively correlated between different tissues in passerine birds and amphibians, but not necessarily in mammals. Biol Lett 6(6):792–796
Luo YP, He DC, Li G, Xie H, Zhang RY, Huang QD (2015) Intraspecific metabolic scaling exponent depends on red blood cell size in fishes. J Exp Biol 218(10):1496–1503
Maciak S, Janko K, Kotusz J, Choleva L, Boron A, Juchno D, Kujawa R, Kozłowski J, Konarzewski M (2011) Standard metabolic rate (SMR) is inversely related to erythrocyte and genome size in allopolyploid fish of Cobitis taenia hybrid complex. Funct Ecol 25:1072–1078
Norin T, Malte H (2012) Intraspecific variation in aerobic metabolic rate of fish: relations with organ size and enzyme activity in brown trout. Physiol Biochem Zool 85:645–656
Oikawa S, Takemori M, Itazawa Y (1992) Relative growth of organs and parts of a marine teleost, the porgy, Pagrus major, with special reference to metabolism size relationships. Jap J Ichthyol 39:243–249
Pang X, Fu SJ, Zhang YG (2015) Individual variation in metabolism and swimming performance in juvenile black carp (Mylopharyngodon piceus) and the effects of hypoxia. Mar Freshw Behav Physiol 48(6):431–443
Pang X, Fu SJ, Li XM, Zhang YG (2016) The effects of starvation and re-feeding on growth and swimming performance of juvenile black carp (Mylopharyngodon piceus). Fish Physiol Biochem 42(4):1203
Rubner M (1883) Über den einfluss der körpergrösse auf stoff-und kraftwechsel. Zeit Biol 19:535–562
Savage VM, Allen AP, Brown JH, Gillooly JF, Herman AB, Woodruff WH, West GB (2007) Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc Natl Acad Sci USA 104:4718–4723
Starostová Z, Konarzewski M, Kozłowski J, Kratochvĺl L (2013) Ontogeny of metabolic rate and red blood cell size in eyelid geckos: species follow different paths. PLoS One 8(5):e64715
Wang QQ, Wang W, Huang QD, Zhang YR, Luo YP (2012) Effect of meal size on the specific dynamic action of the juvenile snakehead (Channa argus). Comp Biochem Physiol A 161:401–405
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Wieser W (1985) Developmental and metabolic constraints of the scope for activity in young rainbow trout (Salmo gairdneri). J Exp Biol 118:133–142
Yan GJ, He XK, Cao ZD, Fu SJ (2013) An interspecific comparison between morphology and swimming performance in cyprinids. J Evolution Biol 26:1802–1815
Zhang YR, Huang QD, Liu ST, He DC, Wei G, Luo YP (2014) Intraspecific mass scaling of metabolic rates in grass carp (Ctenopharyngodon idellus). J Comp Physiol B 184:347–354
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31672287) and the Start-up Research Grant from Qinzhou University (no. 2017KYQD107). We thank Mr. Bo Zhang for his help in fish collection and thank the anonymous reviewers for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
360_2018_1154_MOESM3_ESM.doc
Supplementary material 3 ESM_3. Relationship between the body mass (M, g) and several hematological parameters in the black carp. A: red blood cell surface area, S (μm2); B: red blood cell count, RBCC (109 mL-1); C: hematological concentration, Hb (mg mL-1) (DOC 33 KB)
Rights and permissions
About this article
Cite this article
Lv, X., Xie, H., Xia, D. et al. Mass scaling of the resting and maximum metabolic rates of the black carp. J Comp Physiol B 188, 591–598 (2018). https://doi.org/10.1007/s00360-018-1154-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-018-1154-5