Abstract
We assessed the intraspecific mass scaling of standard metabolic rate (SMR), maximum metabolic rate (MMR), excess post-exercise oxygen consumption (EPOC), and erythrocyte size in grass carp (Ctenopharyngodon idellus), with body masses ranging from 4.0 to 459 g. SMR and MMR scaled with body mass with similar exponents, but neither exponent matched the expected value of 0.75 or 1, respectively. Erythrocyte size scaled with body mass with a very low exponent (0.090), suggests that while both cell number and cell size contribute to the increase in body mass, cell size plays a smaller role. The similar slopes of MMR and SMR in grass carp suggest a constant factorial aerobic scope (FAS) as the body grows. SMR was negatively correlated with FAS, indicating a tradeoff between SMR and FAS. Smaller fish recovered faster from the exhaustive exercises, and the scaling exponent of EPOC was 1.075, suggesting a nearly isometric increase in anaerobic capacity. Our results provide support for the cell size model and suggest that variations of erythrocyte size may partly contribute to the intraspecific scaling of SMR. The scaling exponent of MMR was 0.863, suggesting that the metabolism of non-athletic fish species is less reliant on muscular energy expenditure, even during strenuous exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic rate (MR) typically scales with body mass (M) according to the function \( {\text{MR}} = aM^{b} \), where a is the constant and b is the scaling exponent. This relationship has been studied by many researchers because of its apparent law-like nature, and several hypotheses about metabolic scaling have recently been proposed (Oikawa and Itazawa 1984; West et al. 1997; Kozłowski et al. 2003; Agutter and Wheatley 2004; Glazier 2005, 2010; Kooijman 2010; Agutter and Tuszynski 2011). One of the hypotheses is the Metabolic theory of ecology (MTE), which predicts that the standard metabolic rate (SMR) of organisms scales inter- and intraspecifically to the three-quarter power of M (b = 0.75), as a result of the geometry of optimized resource distribution networks (West et al. 1997; Brown et al. 2004). The MTE hypothesis is supported by many studies (Savage et al. 2004; Farrell-Gray and Gotelli 2005). However, the intraspecific scaling exponent for SMR varies significantly among species and life histories, especially in fish (Clarke and Johnston 1999; Bokma 2004; Killen et al. 2007, 2010. Recently, Savage et al. analyzed the MTE hypothesis and suggested that the cell volume of quickly dividing cells (e.g., erythrocytes) was expected to be body size invariant, whereas cell metabolism was expected to be dependent on body size (Savage et al. 2007). According to this theory, erythrocyte size should be independent of M.
In addition, a “cell size” model has been proposed suggesting that cell size influences the allometric relationship between M and MR (Davison 1955, 1956; Szarski 1983; Kozłowski et al. 2003). Any substantial changes in body mass must be caused by an alteration of cell size or cell number or a combination of the two. While cell size increases, the cell surface area/volume ratio decreases, and a smaller fraction of metabolic effort is spent on maintaining ionic gradients across cell membranes (Davison 1955; Kozłowski et al. 2003). Thus, larger cells are less metabolically active due to their relatively smaller surface area/volume ratio (Davison 1955, 1956; Kozłowski et al. 2003). The cell size model predicts that MR should scale with body mass according to the exponent b = 0.67 if the variation in body mass is attributed entirely to changes in cell size; the exponent should be 1.0 if cell size remains unchanged and body mass variation is entirely due to differences in cell number (Davison 1955; Kozłowski et al. 2003). The cell size model has been supported by studies of interspecific metabolic scaling in mammals and birds (Kozłowski et al. 2003) and intraspecific metabolic scaling in ants (Chown et al. 2007), crayfish (Davison 1956), frogs (Davison 1955), and eyelid geckos (Starostová et al. 2013). Erythrocyte size was used as a proxy for the general cell size of an organism to test the cell size model because of the direct role of erythrocytes in oxygen transportation (Starostová et al. 2009; Maciak et al. 2011). A recent study of geckos suggests that the cell size model in vertebrates holds for erythrocytes only, rather than for cell types in general (Starostová et al. 2013). However, the correlation between erythrocyte size and body mass may vary among species (Starostová et al. 2009, 2013; Kozłowski et al. 2010; Maciak et al. 2011). Therefore, the cell size model requires testing in more species. Fish display the largest variation in erythrocyte area (approximately 30-fold) among species compared to other vertebrates (Gregory 2013). We found only limited studies examining the relationship between erythrocyte size and SMR in fish (Maciak et al. 2011; Huang et al. 2013). It is of interest to test whether the correlation between variation in erythrocyte size and metabolic scaling holds for different fish species.
A difference between the mass scaling of SMR and MMR in animals may result in changes in metabolic scope with increasing body mass. The majority of prior studies found that metabolic scope increases with body mass in many fish species (Beamish 1978; Armstrong et al. 1992; Post and Lee 1996; Killen et al. 2007). However, a mass-independent factorial aerobic scope (FAS) was found in Atlantic salmon (Salmo salar) (Cutts et al. 2002). Because metabolic scope, especially FAS, relates to the Darwinian fitness of an animal (Priede 1985) and reflects the ability to respond to environmental extremes or other challenges (Killen et al. 2007), it is relevant to examine the ways in which metabolic scope changes as the body grows.
Grass carp (Ctenopharyngodon idellus Valenciennes) is an active grazing herbivorous fish that is widely distributed throughout China. Some data have shown that the erythrocyte size of grass carp is smaller as compared to other species of the same family, Cyprinidae (Maciak and Kostelecka-Myrcha 2011), but the correlation between cell size and SMR in this species is unknown. It is of interest to examine the cell size model for metabolic scaling in grass carp. Recently, a study demonstrated that this species has relatively lower critical swimming speed and maximum metabolic rate (MMR) than many other carp species (Yan et al. 2012). However, the intraspecific scaling of the MMR of grass carp remains unclear. MMR was predicted to scale nearly isometrically with body mass (Calder 1984; Glazier 2005, 2010). However, most of the previous results on scaling of MMR were observed from athletic fish species (Brett 1965; Brett and Glass 1973; Wieser 1985; Goolish 1991). It would be interesting to determine whether the grass carp, a species with lower swimming performance, exhibits an isometric scaling of MMR. Thus, the first aim of this study was to determine the mass scaling of SMR and MMR in grass carp. Second, we used erythrocyte size as a proxy for general cell size to test the hypothesis that the coupling between cell size and body mass plays an important role in shaping metabolic scaling (Davison 1955; Szarski 1983; Kozłowski et al. 2003). Third, we examined whether individuals having a higher SMR tended to have a higher FAS.
Materials and methods
Animals
Grass carp were sourced from local fisheries in Xiema, Chongqing, China, and acclimated in a rearing system for at least 2 weeks before beginning the experiment. During this period, the fish were fed once daily (1 % of body mass) on a commercial diet, the chemical composition of which was 6.3 % moisture, 30.3 % protein, 2.9 % fat, and 10.0 % digestible carbohydrate. The temperature of the dechlorinated fresh water was maintained at 25.0 ± 1.0 °C, oxygen concentration was maintained above 90 % saturation, and the photoperiod was 14 L/10 Days. Animal handling and experiments complied with the ethical requirements and recommendations of the Animal Care of the Fisheries Science Institution of Southwest University, China.
Measurement of metabolic rate
The oxygen consumption rate of individual fish was measured using a continuous-flow respirometer (Wang et al. 2012). The dissolved oxygen concentration was measured at the outlet of the perspex chamber using an oxygen meter (HQ30, Hach Company, Loveland CO, USA). One chamber without fish was used as a control for measuring the background oxygen consumption. Different-sized chambers (0.13, 0.52, 0.86, and 1.20 L) were used depending on the body mass of the experimental fish. The flow rate of water through the respirometer chamber was obtained by collecting the outflow from each tube into a beaker over different time intervals (in minutes) as previously described by Wang et al. (2012). The water flow rate was adjusted to ensure that 95 % of the water was replaced within 1 min in the chamber (Steffensen 1989) and to ensure a >7 mg L−1 oxygen concentration in the outlet water to avoid physiological stress. The following formula was used to calculate oxygen consumption rate \( \dot{M}_{{{\text{O}}_{2} }} \) (mg O2 h−1):
where ΔO2 is the difference (mg O2 L−1) in oxygen concentration between an experimental chamber and the control chamber (chamber without fish) and v is the water flow rate in the experimental chamber (L h−1).
At the end of the acclimation period, the fish were fasted for 24 h and were weighed to the nearest 0.1 g. Each fish was placed in the respirometer chamber and allowed to acclimate for another 24 h. During the acclimation period, the control chamber was adjusted to the same water conditions as the fish chamber. The oxygen consumption rate was then measured at 1-h intervals for 4 h, and the mean value of the last three measurements was used as the SMR. The standard deviation of the three SMR measurements was lower than 5 % of their mean value. In previous studies, MMR was typically measured in fish by gradually increasing swimming speed (Brett 1965). In the present study, however, MMR was measured by the chasing protocol as described by Wang et al. (2012), where the peak post-exercise \( \dot{M}_{{{\text{O}}_{ 2} }} \) following exhaustive exercise was used as a measure of maximum \( \dot{M}_{{{\text{O}}_{ 2} }} \)for fish (Reidy et al. 1995; Milligan 1996; Cutts et al. 2002; Fu et al. 2009a). After the SMR measurement, the fish were chased to exhaustion in a circle with a hand net (this process lasted for 5 min) and were then immediately placed back into the respirometer chambers. The oxygen consumption rates were measured at 1-min intervals for the first 10 min post-exercise and then at 15, 20, 30, 40, 60, 80, 100, and 120 min, until the oxygen consumption rate returned to within 120 % of the SMR. MMR was usually observed immediately after a fish was placed into the chamber. FAS was calculated as the ratio of MMR to SMR. Excess post-exercise oxygen consumption (EPOC) was calculated as the magnitude of excess oxygen consumption above SMR during the recovery phase. EPOC duration (h) was calculated as the time from exercise to when \( \dot{M}_{{{\text{O}}_{ 2} }} \) returned to within 120 % of the SMR in a given fish. A total of 81 individuals were used for metabolic rate analysis.
Parameters of erythrocytes
As soon as the oxygen consumption rate recovered from exercise, the fish was anesthetized by adding tricaine methanesulfonate (MS-222) to a concentration of 0.15 g L−1 in the water for blood and organ sampling. Blood was taken from the caudal vessels with an anti-freezing syringe (sodium fluoride: potassium oxalate = 1:3 g g−1) and transferred to an anti-freezing centrifuge tube placed on ice. The sampling was usually completed within 1 min of blood withdrawal. Erythrocytes were counted using a Neubauer hemocytometer after 1:200 dilution in 0.65 % NaCl. Fresh blood smears were air-dried and stained with Wright’s–Giemsa fluid (Gao et al. 2007). A compound light microscope mounted with a video camera linked to computer image analysis software (EV5680B, Aigo Company, China) was used to measure the length (LC) and width (WC) of 20 randomly selected erythrocytes from each slide. The erythrocyte was assumed as ellipsoid in shape and its surface area (S) was calculated using the following formula: S = LC × WC × π/4. Because five individuals were too small to sample enough blood, the final sample size for S was 76.
Data analysis
Data analysis was conducted using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). Decadic logarithms (log) of SMR, MMR, EPOC, recovery duration, erythrocyte size, and body mass data were used for correlation or regression analysis. Ordinary least square (OLS) regression was used to analyze the relationship between M and each of the other parameters, and an ANCOVA was used to compare the slopes of the regressions using M as a covariate. The residual value of each measurement was calculated as the difference between the log-observed value and the log-predicted value by the corresponding allometric equation. Regression analysis was used to test the mass-independent correlations between residual SMR (rSMR) and MMR (rMMR) or FAS (rFAS). Mass-scaling exponents for SMR, MMR, EPOC, and erythrocyte size were also analyzed by reduced major axis (RMA) regression using RMA software version 1.17 (Bohonak and van der Linde 2004; available at http://www.bio.sdsu.edu/pub/andy/rma.html). RMA regression led to a similar pattern, although the scaling exponents by RMA tended to be higher than those by OLS regression. The RMA regression values are listed in Table 1. All of the allometric exponents include ±95 % confidence intervals (CI). p values <0.05 were considered statistically significant.
Results
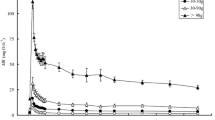
After exhaustive exercise, the specimens’ MR values immediately increased to their peak values and then recovered to the pre-exercise values (Fig. 1). Smaller individuals recovered faster, and the recovery periods ranged from 0.3 to 2.7 h, which was dependent on M (r 2 = 0.166, n = 81, p < 0.0001). As the M of the studied individuals increased 115-fold, whole-animal SMR varied 43-fold while MMR increased 75-fold.
Both SMR and MMR scaled with M of grass carp by exponents; however, the exponents were not the expected 0.75 or 1 values, respectively (Fig. 2). Similar slopes were found in SMR (bs) and MMR (bm) using ANCOVA analyses (F 1, 160 = 0.855, p = 0.357); as a result, FAS did not increase as M increased (r 2 = 0.014, n = 81, p = 0.286). Individuals with a higher SMR tended to have a relatively higher MMR (r 2 = 0.067, n = 81, p = 0.020) (Fig. 3a) but a lower FAS (r 2 = 0.239, n = 81, p < 0.0001) (Fig. 3b). Individuals with a higher MMR tended to have a higher FAS (r 2 = 0.338, n = 81, p < 0.0001) (Fig. 3c). EPOC increased as body mass increased by an exponent of 1.075 (Fig. 4).
Only a 1.8-fold variation was found in S among all individuals. As M increased, S increased weakly but significantly by an exponent of 0.090 (Fig. 5). A negative correlation was found between S and both mass-specific SMR (Fig. 6a) and mass-specific MMR (Fig. 6b). However, mass-corrected S was not significantly correlated with either mass-specific SMR (r 2 = 0.014, n = 76, p = 0.155) or mass-specific MMR (r 2 = 0.009, n = 76, p = 0.108), while only accounting for a limited variation in erythrocyte size remaining after mass correction. There was no significant correlation between S and FAS (r 2 = 0.023, n = 76, p = 0.093).
Discussion
The MR and hematological parameters examined in this study were all similar to previous results for grass carp of comparable size (Fu et al. 2009a; Maciak and Kostelecka-Myrcha 2011). Our results are within the range for the intraspecific b s previously reported for most fish species (0.65–0.95) (Clarke and Johnston 1999; Bokma 2004; Killen et al. 2010). The b s of grass carp does not agree with the exponential value of 0.75 proposed by the MTE hypothesis (West et al. 1997; Savage et al. 2004). An important assumption of the MTE is that the physical properties of the terminal capillaries of the supply systems are invariant with body size (West et al. 1997). Dictated by MTE, Savage et al. (2007) proposed that cell metabolic rate and cell size should roughly follow two strategies: the MR of quickly dividing cells, e.g., erythrocytes, should be body size dependent, whereas cell size should be body size invariant. On the contrary, cell metabolism should be roughly invariant, whereas the cell size of slowly dividing cells should be body size dependent. According to the MTE hypothesis, erythrocyte size should be independent of body size (Savage et al. 2007). In the present study, however, the erythrocyte size of grass carp increased with body mass (Fig. 5), which again rejects the MTE hypothesis.
Our results provide support for the cell size model and suggest that the variation of erythrocyte size may contribute to the intraspecific scaling of SMR. A similar increase in erythrocyte size with body mass was found in another fish species, the spined loach (Cobitis taenia) (Maciak et al. 2011), and in several species of geckos (Starostová et al. 2013). The erythrocyte size of grass carp scaled with body mass by a very low exponent of 0.090 (Fig. 5), suggests that while both cell number and cell size contribute to the increase in body mass, cell size plays only a minor role. According to the cell size model, SMR scales isometrically when cell size scales as M 0 (Davison 1955; Szarski 1983; Kozłowski et al. 2003). The scaling exponent (0.090) for erythrocyte size should predict a scaling exponent for SMR close to 1 (0.97 when calculated as described in Kozłowski et al. 2010). However, the observed scaling exponent for SMR of grass carp was 0.831, significantly lower than 1 or 0.97. Our results suggest that variation in erythrocyte size only partly explains the metabolic scaling. Several potential reasons may explain this finding. First, even though erythrocyte size is correlated well with the size of other cell types in birds and amphibians, this relationship does not hold for many mammals (Kozłowski et al. 2010); whether erythrocyte size can be used as a proxy for general cell size in fish is unknown. Second, metabolic differences may be only partially determined by the cell surface/volume ratio; other body size-related factors, such as membrane permeability and the density and activity of mitochondria, may play a role in these differences (Kozłowski et al. 2010). Third, the metabolic activity of cells may decrease as the body grows, which can affect allometric scaling of SMR (Porter and Brand 1995; Savage et al. 2007).
Interestingly, the b m value of grass carp in our results was 0.863 and significantly lower than 1 (Fig. 2). The scaling exponent of the MR is expected to increase as the exercise level increases (Brett 1965), and it is assumed that MMR scales approximately isometrically with body mass (Brett and Glass 1973; Calder 1984; Wieser 1985; Goolish 1991). As activity increases, an animal’s metabolism becomes increasingly devoted to muscular energy expenditure, which scales in direct proportion to muscle mass; this in turn scales as M 1 (Glazier 2005, 2009). The nearly isometric metabolic scaling during strenuous exercise may benefit fitness-related activities, such as escape from predators and capture of prey (Lane 2005). However, most of the previous results pertaining to scaling of MMR were from studies on athletic fish species (Brett 1965; Brett and Glass 1973; Wieser 1985; Goolish 1991). The grass carp in our study cannot be considered an athletic species, as it exhibits relatively lower critical swimming speed (~5 BL s−1 at 25 °C for 8.6-cm fish) compared to many other carp species (Yan et al. 2012). Our results support the argument that b m data of fish vary depending on a species’ lifestyle and tends to be higher in athletic species (Killen et al. 2007; Glazier 2008, 2010). Our results suggest that metabolism is less reliant on muscular energy expenditure even during strenuous exercise in non-athletic fish. In addition, the majority of fish muscle is white muscle, which contributes less to aerobic metabolism, while the metabolically active red muscle occupies only a small proportion of the total muscle (Luo et al. 2013). In fact, the average red muscle somatic index of grass carp was only approximately 1 % in our study.
Values of b m are generally higher than b s, which results in increased FAS with body mass in many fish species, including sockeye salmon (Oncorhynchus nerka) (Brett 1965), charr (Salvelinus fontinalis) (Beamish 1978), northern pike (Esox lucius) (Armstrong et al. 1992), rainbow trout (Oncorhynchus mykiss) (Post and Lee 1996), ocean pout (Macrozoarces americanus), lumpfish (Cyclopterus lumpus), and shorthorn sculpin (Myoxocephalus scorpius) (Killen et al. 2007). Interestingly, b m was close to b s in grass carp in the present study, and as a result, FAS did not increase with body mass; indicating no increase in aerobic scope as its body grows. The mean value of FAS (4.19) may be adequate for the grass carp, particularly because it is a non-athletic species.
A positive correlation was found between MMR and SMR in grass carp (Fig. 3a), suggesting that there is a cost to maintain the machinery that supports high aerobic capacity. It has been speculated that evolutionary selection for a high MMR will generate a concomitant increase in SMR (Bennett and Ruben 1979). Animals with higher locomotory performance usually have a higher SMR (Reinhold 1999). This relationship would be reflected in a greater size of the organs responsible for energy turnover, making such organs more efficient at processing food but at the same time more expensive to maintain, thereby increasing SMR (Daan et al. 1990; Chappell et al. 1999). Similar positive relationships between SMR and MMR were found in rainbow trout (Wieser 1985) and brown trout (Salmo trutta) (Norin and Malte 2012).
The present results (Fig. 3b) suggest that grass carp with a relatively higher SMR have a reduced metabolic capacity for activity, a tradeoff between SMR and FAS. Similarly, a previous study found that Atlantic salmon with a relatively high SMR had small metabolic scope (Cutts et al. 2002). However, in many other species, FAS increases with SMR, and a greater SMR has been considered a consequence of greater FAS (Priede 1985; Metcalfe et al. 1995). Our results indicate that the correlation between FAS and SMR may vary according to species’ life-history strategies. A lower SMR could be beneficial for fish because it allows a greater scope of power allocation for some important behaviors (Post and Lee 1996; Killen et al. 2007; Fu et al. 2009a). By contrast, a larger SMR allows fish to expend a greater amount of energy on avoiding predators and on grazing food (Fu et al. 2009b).
Like all other vertebrates, fish need time to recover after exhaustive activity. The elevated oxygen consumption rate following exhaustive exercise, EPOC, has been used as an important index of anaerobic capacity in animals (Gaesser and Brooks 1984; Hancock and Gleeson 2002). It has been suggested that animals have a lower aerobic capacity but a higher anaerobic capacity with increasing body mass (Goolish 1991; Kieffer et al. 1996). The mass-scaling exponent of EPOC was 1.075 in grass carp (Fig. 4), suggesting a nearly isometric increase in its anaerobic capacity. The duration of EPOC also has important ecological relevance because the time required for exercise-induced disturbances to return to baseline levels may affect the characteristics of physiological ecology and fitness, such as repeated burst swimming and survival (Goolish 1991; Gingerich and Suski 2012). The duration of EPOC is body mass dependent in grass carp, and smaller fish recovered faster (Fig. 1). The accelerated recovery rates of metabolites in smaller fish have been attributed to smaller individuals having a greater per-gram MR than larger individuals, an increased reliance on aerobic processes during burst swimming, or a faster lactic acid removal rate, thereby resulting in an accelerated time of return to homeostasis (Wakefield et al. 2004; Ohlberger et al. 2005; Gingerich and Suski 2012).
In conclusion, our results support the cell size model that states that erythrocyte size can partially account for the observed allometric patterns in SMR. In addition, Grass carp with a relatively higher MMR tended to have a higher SMR, suggesting that more active individuals have larger metabolic machinery. Finally, similar scaling exponents for MMR and SMR suggest that metabolism during strenuous exercise is less reliant on muscular energy expenditure in non-athletic fish, such as the grass carp, than in more athletic fish that show significantly steeper scaling exponents for MMR.
References
Agutter P, Tuszynski J (2011) Analytic theories of allometric scaling. J Exp Biol 214:1055–1062
Agutter P, Wheatley D (2004) Metabolic scaling: consensus or controversy? Theor Biol Med Model 1:13
Armstrong JD, Priede IG, Lucas MC (1992) The link between respiratory capacity and changing metabolic demands during growth of northern pike, Esox lucius L. J Fish Biol 41:65–75
Beamish FWH (1978) Swimming capacity. In: Hoar WS, Randall DJ (eds) Fish physiology, vol VII. Academic Press, London
Bennett AF, Ruben JA (1979) Endothermy and activity in vertebrates. Science 206:649–654
Bohonak AJ, van der Linde K (2004) Software for reduced major axis regression, V.1.17. San Diego State University, San Diego, USA
Bokma F (2004) Evidence against universal metabolic allometry. Funct Ecol 18:184–187
Brett JR (1965) The relation of size to rate of oxygen consumption and sustained swimming speed of sockeye salmon (Oncorhynchus nerka). J Fish Res B Can 22:1491–1501
Brett JR, Glass NR (1973) Metabolic rates and critical swim speeds of sockeye salmon (Oncorhynchus nerka). J Fish Res B Can 30:379–387
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Calder WA (1984) Size, function, and life history. Harvard University Press, Cambridge
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Chown SL, Marais E, Terblanche JS, Klok CJ, Lighton JRB, Blackburn TM (2007) Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct Ecol 21:282–290
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Cutts CJ, Metcalfe NB, Taylor AC (2002) Juvenile Atlantic salmon (Salmo salar) with relatively high standard metabolic rates have small metabolic scopes. Funct Ecol 16:73–78
Daan S, Masman D, Groenewold A (1990) Avian basal metabolic rate: their association with body composition and energy expenditure in nature. Am J Physiol 259:R333–R340
Davison J (1955) Body weight, cell surface and metabolic rate in anuran Amphibia. Biol Bull 109:407–419
Davison J (1956) An analysis of cell growth and metabolism in the cryfish (Procambarus alleni). Biol Bull 110:264–273
Farrell-Gray CC, Gotelli NJ (2005) Allometric exponents support a 3/4-power scaling law. Ecology 86:2083–2087
Fu SJ, Zeng LQ, Li XM, Pang X, Cao ZD, Peng JL, Wang YX (2009a) Effect of meal size on excess post-exercise oxygen consumption in fishes with different locomotive and digestive performance. J Comp Physiol B 179:509–517
Fu SJ, Zeng LQ, Li XM, Pang X, Cao ZD, Peng JL, Wang YX (2009b) The behavioural, digestive and metabolic characteristics of fishes with different foraging strategies. J Exp Biol 212:2296–2302
Gaesser GA, Brooks GA (1984) Metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc 16:29–43
Gao ZX, Wang WM, Abbas K, Zhou XY, Yang Y, Diana JS, Wang HP, Wang HL, Li Y, Sun YH (2007) Haematological characterization of loach Misgurnus anguillicaudatus: Comparison among diploid, triploid and tetraploid specimens. Comp Biochem Physiol A 147:1001–1008
Gingerich AJ, Suski CD (2012) The effect of body size on post-exercise physiology in largemouth bass. Fish Physiol Biochem 38:329–340
Glazier DS (2005) Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev Camb Philos 80:611–662
Glazier DS (2008) Effects of metabolic level on the body-size scaling of metabolic rate in birds and mammals. Proc R Soc Lond B 275:1405–1410
Glazier DS (2009) Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. J Comp Physiol B 179:821–828
Glazier DS (2010) A unifying explanation for diverse metabolic scaling in animals and plants. Biol Rev 85:111–138
Goolish EM (1991) Aerobic and anaerobic scaling in fish. Biol Rev 66:33–56
Gregory TR (2013) Animal genome size database. http://www.genomesize.com
Hancock TV, Gleeson TT (2002) Metabolic recovery in the desert iguana (Dipsosaurus dorsalis) following activities of varied intensity and duration. Funct Ecol 16:40–48
Huang QD, Zhang YR, Liu ST, Wang W, Luo YP (2013) Intraspecific scaling of the resting and maximum metabolic rates of the crucian carp (Carassius auratus). PLoS One 8:e82837
Kieffer JD, Ferguson RA, Tompa JE, Tufts BL (1996) Relationship between body size and anaerobic metabolism in brook trout and largemouth bass. Trans Am Fish Soc 125:760–767
Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc R Soc Lond B 274:431–438
Killen SS, Atkinson D, Glazier DS (2010) The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol Lett 13:184–193
Kooijman SALM (2010) Dynamic energy budget theory for metabolic organisation. Cambridge University Press, Cambridge
Kozłowski J, Konarzewski M, Gawelczyk AT (2003) Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci USA 100:14080–14085
Kozłowski J, Czarnołęski M, François-Krassowska A, Maciak S, Pis T (2010) Cell size is positively correlated between different tissues in passerine birds and amphibians, but not necessarily in mammals. Biol Lett 6:792–796
Lane N (2005) Power, sex, suicide: mitochondria and the meaning of life. Oxford University Press, Oxford
Luo YP, Wang W, Zhang YR, Huang QD (2013) Effect of body size on organ-specific mitochondrial respiration rate of the largemouth bronze gudgeon. Fish Physiol Biochem 39:513–521
Maciak S, Kostelecka-Myrcha A (2011) Regularities of variation of the red blood indices characterizing the respiratory function of blood in selected fish. Zool Pol 56:35–48
Maciak S, Janko K, Kotusz J, Choleva L, Boron A, Juchno D, Kujawa R, Kozłowski J, Konarzewski M (2011) Standard metabolic rate (SMR) is inversely related to erythrocyte and genome size in allopolyploid fish of Cobitis taenia hybrid complex. Funct Ecol 25:1072–1078
Metcalfe NB, Taylor AC, Thorpe JE (1995) Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim Behav 49:431–436
Milligan CL (1996) Metabolic recovery from exhaustive exercise in rainbow trout. Comp Biochem Physiol A 113:51–60
Norin T, Malte H (2012) Intraspecific variation in aerobic metabolic rate of fish: relations with organ size and enzyme activity in brown trout. Physiol Biochem Zool 85:645–656
Ohlberger J, Staaks G, van Dijk PLM, Hölker F (2005) Modeling energetic costs of fish swimming. J Exp Zool A 303:657–664
Oikawa S, Itazawa Y (1984) Relative growth of organs and parts of the carp, Cyprinus carpio, with special reference to the metabolism-size relationship. Copeia 3:800–803
Porter RK, Brand MD (1995) Cellular oxygen consumption depends on body mass. Am J Physiol Regul Integ Comp Physiol 269:226–228
Post JR, Lee JA (1996) Metabolic ontogeny of teleost fishes. Can J Fish Aquat Sci 53:910–923
Priede IG (1985) Metabolic scope in fishes. In: Tytler P, Calow P (eds) Fish energetics: new perspectives, Croom-Helm, London, pp. 33–64
Reidy SP, Nelson JA, Tang Y, Kerr SR (1995) Post exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J Fish Biol 47:377–387
Reinhold K (1999) Energetically costly behaviour and the evolution of resting metabolic rate in insects. Funct Ecol 13:217–224
Savage VM, Gillooly JF, Woodruff WH, West GB, Allen AP, Enquist BJ, Brown JH (2004) The predominance of quarter-power scaling in biology. Funct Ecol 18:257–282
Savage VM, Allen AP, Brown JH, Gillooly JF, Herman AB, Woodruff WH, West GB (2007) Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc Natl Acad Sci USA 104:4718–4723
Starostová Z, Kubička L, Konarzewski M, Kozłowski J, Kratochvíl L (2009) Cell size but not genome size affects scaling of metabolic rate in eyelid geckos. Am Nat 174:E100–E105
Starostová Z, Konarzewski M, Kozłowski J, Kratochvíl L (2013) Ontogeny of metabolic rate and red blood cell size in eyelid geckos: species follow different paths. PLoS One 8:e64715
Steffensen JF (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol Biochem 6:49–59
Szarski H (1983) Cell size and the concept of wasteful and frugal evolutionary strategies. J Theor Biol 105:201–209
Wakefield AM, Cunjak RA, Kieffer JD (2004) Metabolic recovery in Atlantic salmon fry and parr following forced activity. J Fish Biol 65:920–932
Wang QQ, Wang W, Huang QD, Zhang YR, Luo YP (2012) Effect of meal size on the specific dynamic action of the juvenile snakehead (Channa argus). Comp Biochem Physiol A 161:401–405
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Wieser W (1985) Developmental and metabolic constraints of the scope for activity in young rainbow trout (Salmo gairdneri). J Exp Biol 118:133–142
Yan GJ, He XK, Cao ZD, Fu SJ (2012) The trade-off between steady and unsteady swimming performance in six cyprinids at two temperatures. J Therm Biol 37:424–431
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31000958) and the Natural Science Foundation Project of CQ (CSTC2013jcyjA80023). We thank Mr. Bo Zhang for his help in fish collection. We sincerely thank the anonymous reviewers for the suggestions and comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Zhang, Y., Huang, Q., Liu, S. et al. Intraspecific mass scaling of metabolic rates in grass carp (Ctenopharyngodon idellus). J Comp Physiol B 184, 347–354 (2014). https://doi.org/10.1007/s00360-014-0802-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0802-7