Abstract
Spiders (Araneae) are unique regarding their respiratory system: they are the only animal group that breathe simultaneously with lungs and tracheae. Looking at the physiology of respiration the existence of tracheae plays an important role in spiders with a well-developed tracheal system. Other factors as sex, life time, type of prey capture and the high ability to gain energy anaerobically influence the resting and the active metabolic rate intensely. Most spiders have metabolic rates that are much lower than expected from body mass; but especially those with two pairs of lungs. Males normally have higher resting rates than females; spiders that are less evolved and possess a cribellum have lower metabolic rates than higher evolved species. Freely hunting spiders show a higher energy turnover than spiders hunting with a web. Spiders that live longer than 1 year will have lower metabolic rates than those species that die after 1 year in which development and reproduction must be completed. Lower temperatures and starvation, which most spiders can cope with, will decrease the metabolic rate as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spiders (Araneae) are air-breathing arthropods. Spider respiration is a topic that absorbed authors since more than 100 years. First morphologists and later on physiologists studied the respiratory organs and the metabolism of spiders (Bertkau 1876; Edwards 1946; Haller 1912; Kästner 1929; Lamy 1902; Purcell 1895, 1909, 1910; Simmons 1894). Respiration in spiders is a sophisticated topic: most spiders breathe with both diffusion lungs and tracheae, possess haemocyanin as their respiratory pigment, and meet their maximum metabolic demands via anaerobic pathways (Prestwich 1983a). Spiders are adapted to different physiological situations in which staying motionless, escaping and aggressive behavior against a threat is possible. Moreover, different ‘factors’ influence the respiration of spiders: lifestyle, temperature, starvation, sex, body size, developmental stage and reproductive condition, the entire life time, courtship behavior and prey capture will influence resting and activity metabolic rates (Fig. 1).
Eight main factors that influence the metabolism of spiders. Prey availability and temperature are exogenous factors, body mass and tracheae and the type of hunting, sex, phylogenetic status and life time are endogenous factors. Red arrows indicate an increase in metabolism if the condition is given that is written in the arrow
In this review the physiological aspect is in the focus. Nevertheless, the morphological part will be discussed at the beginning to better understand the whole story.
Short description of respiratory systems—lungs and tracheae
The Araneae comprise an animal group with a unique arrangement of respiratory organs: they can breathe with lungs and tracheae simultaneously (Hsia et al. 2013; Schmitz 2013). The book lungs are diffusion lungs, gas exchange is regulated by the spiracle entrance area, which is muscular controlled and acts as a diffusion controller (Fincke and Paul 1989; Paul et al. 1987). Basic spiders (Mesothelae and Mygalomorphae, some basic Araneomorphae = Paleocribellata and Austrochiloidea) possess two pairs of book lungs in the 2nd and 3rd opisthosomal segment (Fig. 2). Most modern spiders (Araneomorphae), however, are bimodal breathers, reduced one of the lung pairs, most often the second one, and replaced it by tracheae (Fig. 2). This higher evolved respiratory system is differently developed in haplogyne and entelegyne spiders but the level of development is not linked 1:1 to the systematic level of a spider family (Schmitz 2013). In haplogyne Araneomorphae two tracheal spiracles are situated directly behind the lung spiracles (Dysderoidea), tracheae lack completely (Tetrablemmidae, Pholcidae, Diguetidae, Plectreuridae, Sicariidae) (Fig. 2), or also the first lung pair is replaced by tracheae (sieve tracheae, e.g., Caponiidae) (Lamy 1902; Ramirez 2000). In most entelegyne Araneomorphae, however, the single tracheal spiracle is situated at the end of the opisthosoma just in front of the spinnerets (Fig. 2). From this spiracle four tube tracheae originate. The outer two tracheae (primary tracheae, lateral tracheae) are relicts of the lungs and can be connected to the lung extended lung atria. The inner two tracheae (secondary tracheae, median tracheae) are new structures and are hollowed apodemes elongated along the long axis of the spider (Forster 1980; Lamy 1902; Levi 1967, 1976; Purcell 1909, 1910). Tracheae may be elongated into the prosoma and may penetrate muscles, nervous system or the epithelia of other organs (Fig. 2) (Bromhall 1987b; Hsia et al. 2013; Schmitz 2013; Schmitz and Perry 2000, 2001, 2002; Strazny and Perry 1987).
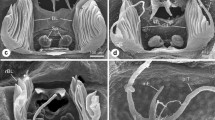
Anatomy of the respiratory organs in Araneae. a, b give the ultrastructure of the lungs of a jumping spider (Salticus scenicus). Bar is 100 µm in a and 5 µm in b. The Atrium (At) is situated in front of the lung lamellae. The lung lamella are lined by a cuticular (cu) and an epidermal layer (ep), the cuticle is adjacent to the air space (As), while the epidermis is adjacent the hemolymph space (Hl). c, d give two tracheae (Tr) in the nervous system (Ns) and between muscle fibers (Mu). Bars indicate 2 µm. The schematic drawings give some examples for the development of lungs (L) and tracheae (Tr). Mesothelae are not shown, they have four lungs and the spinnerets directly behind them. The schematic longitudinal section of a spider shows the relations in many Araneomorphae with one pair of lungs and four simple tube tracheae. NS nervous system, H heart, G gut. For more information on tracheal systems see Schmitz 2013
The ultrastructure of tracheae consists of an epidermal outer layer and a cuticular inner layer that builds taenidia for stabilization. Therefore, spider tracheae look very similar to insect tracheae. The ultrastructure of lungs was examined in a couple of species. In the mygalomorph Eurypelma californica, the thickness of the epidermal layer is about 0.02–0.1 µm, while the cuticular layer is down to 0.03 µm thin (Moore 1976; Reisinger et al. 1990, 1991). These dimensions are similar to the lungs of araneomorph spiders (Fig. 2a, b). E.g. epidermal and cuticular layers have similar thicknesses in wolf spiders Pardosa lugubris, cellar spiders Pholcus phalangioides and jumping spiders Salticus scenicus (both layers combined 0.16–0.19 µm), but about twice these values in Tegenaria (Agelenidae) (Schmitz 2015; Schmitz and Perry 2000, 2001, 2002; Strazny and Perry 1984). Both cuticle and epidermis of the tracheae constitute about the same proportion of the walls: the walls of the smallest tracheae have about the same thickness as the lungs (Schmitz and Perry 2001, 2002) (Fig. 2c, d).
Examples for well-developed tracheal systems are the sheetweb weavers (Linyphiidae), jumping spiders (Salticidae), crab spiders (Thomisidae), the hackled orb-weaver (Uloboridae), the tube dwelling spiders (Segestriidae), the woodlouse hunters (Dysderidae) (Fig. 2), the Dictynidae and the water spider Argyroneta (Blest 1976; Bromhall 1987b; Millidge 1986; Schmitz and Perry 2001). In Dysderidae, Segestriidae and in Argyroneta lungs are only little developed and the tracheal system is the main respiratory system (Braun 1931; Bromhall 1987b). In other spiders, lungs are the main respiratory organs as in the wolf spiders (Lycosidae) (Schmitz and Perry 2002) and the funnel web spiders (Agelenidae) (Strazny and Perry 1984). In some families, lungs and tracheae complement one another. This is, e.g., the case in jumping spiders where 25–30 % of the entire diffusing capacity lies in the tracheae (Schmitz and Perry 2000, 2001). In these spiders, the tracheal system was interpreted as being especially responsible for the function of the nervous system which is especially well supported by tracheae (Schmitz 2004, 2005). In the Uloboridae, it was demonstrated that when tracheae are well developed, lungs are less developed and vice versa. In this family, an extensive tracheal system that also reaches into the legs and is best developed in spiders which use their legs actively for net monitoring. Thus, tracheae are interpreted as adaptations to meet the greater O2 demands in active body parts as the legs (Opell 1979, 1987, 1989, 1990, 1998; Opell and Konur 1992).
One of the most interesting questions regarding respiratory biology of spiders is why these animals reduced the existing and well-functioning lungs and developed tracheae (Anderson and Prestwich 1975; Ellis 1944; Levi 1967, 1976; Schmitz 2005). Hypotheses are: reduction of water loss, increase in general metabolism, increase in local O2 demand, and the loss of the hydraulic separation of pro- and opisthosoma by tracheae running through the petiolus (Anderson and Prestwich 1975; Levi 1967, 1976; Schmitz 2013). Tracheae evoke a conflict in gas exchange as lungs are designed to work together with the hemolymph and the hemocyanin herein (see below) and tracheae are most effective when used for diffusion at their endings (terminal diffusion). But tracheae enable spiders to become more flexible in their respiratory behavior, because their tracheae can function as tracheal lungs (general increase in respiratory surface area) or use terminal diffusion (local O2 demands) even in the same animal depending on the exact position of tracheae. As tracheal lungs, their entire surface may be used as gas exchanger with haemocyanin within the hemolymph. In terminal diffusion tracheae reach into the epithelia of special organs (e.g., nervous system, muscle of the legs) in which gas exchange takes place (Opell 1979, 1987, 1989, 1990, 1998; Opell and Konur 1992; Schmitz 2004, 2005). More information about tracheal systems in spiders is given in Schmitz (2013).
In Cupiennius salei, mitochondria comprise only 0.1 % of leg muscle mass (Linzen and Gallowitz 1975). Even if the mitochondria mass of prosoma muscles were never measured, anaerobic metabolism must be the standard strategy of a spider during extreme activity in which movement of the legs is necessary. In most spiders, this seems to be not generally changed when animals developed tracheae. But species of the Uloboridae with a more active web-monitoring tactic (using their legs) and greater tracheal supply have more mitochondria (counted numbers per cell) in the leg muscles (Opell 1987; Opell and Konur 1992). More studies are necessary to show the mitochondria mass in the muscles of more spider species.
The circulatory system
The construction and the physiology of the circulatory system and the haemocyanin as respiratory pigment are of importance. The tubular heart is situated dorso-medially in the anterior part of the opisthosoma. The anterior aorta supplies the prosoma, while the heart runs out in the posterior aorta (Wirkner and Huckstorf 2013). Spiders have an open circulatory system without capillaries in which at least in the legs gas exchange has to take place along the open portion of the circulatory system. In four-lunged spiders, anterior and posterior circulation is separated, thus that hemolymph from the prosoma passes only through the anterior lung pair and hemolymph from the opisthosoma passes through the posterior lungs (Paul et al. 1989b).
In the four-lunged Eurypelma californicum (Mygalomorphae) in resting animals the arterial O2 pressure (P aO2) is 3.7 kPa, stays constant during walking, increases during the recovery phase and is maximum at the end of this phase (about 9.8 kPa) (Angersbach 1978). The crucial variable for O2 transport in the haemolymph is the arterious–venous pressure difference (∆P avO2) because a big difference causes a more effective uptake of O2 from the outside. During rest spiracles are nearly closed. Only small amounts of O2 were uptaken and little O2 is used; therefore, the ∆P avO2 is small. During recovery after an exhaustive run spiracles are open and the ∆P avO2 increases because of an increase in P aO2. Together with an increase in heart rate, this results in a more intensive use of haemocyanin in respiration (Paul et al. 1994b).
In araneomorph spiders the correlation of heart rates and the equipment with tracheae was tested. Spiders with prosomal tracheae have significantly lower maximum heart rates than spiders with tracheae limited to the opisthosoma. In addition, the return to normal heart rates in recovery phases after running is faster in spiders with prosomal tracheae (Bromhall 1987a). Forced fast running was associated with a lowering of the heart-rate: a rise occurred when activity ceased (Bromhall 1987a). Carrel and Heathcote (1976), however, stated that resting heart rate is a measure of standard metabolic rate and independent of the respiratory organs (lungs and/or tracheae). Other authors (Carrel 1987; Carrel and Heathcote 1976; Greenstone and Bennett 1980) correlated the heart rate with lifestyle and not with tracheal supply to the prosoma. In these studies, resting heart rates of spiders were found to be primarily a function of body size and can be used as a measure for metabolism. The less active an animal is, the lower is the resting heart rate and the tracheal development thus reflecting fundamental differences in foraging strategies among spiders. Some authors have proposed that spitting spiders (Scytodidae) and brown spiders (Loxoscelidae) (so-called primitive hunters and weavers with sticky nets or catching prey by squirting them with a gluey secretion) do not have lower metabolic rates but lower heart rates compared with salticid spiders which are more active for hunting prey (Carrel 1987; Carrel and Heathcote 1976; Greenstone and Bennett 1980).
Haemocyanin as respiratory pigment
Almost all spiders possess haemocyanin in the haemolymph which is most effective with lung breathing because lungs are placed only in the opisthosoma and therefore a blood carrying pigment is appropriate. Haemocyanin concentrations of up to 120 mg ml−1 were reported (Mangum 1985). The O2-binding capacities of haemocyanin are similar between different spiders but depend on various effectors, e.g. temperature, allosteric effectors, and pH (Bohr effect). The O2-binding curve is typically a sigmoid curve as known from the vertebrate hemoglobin (Paul et al. 1994a; van Holde and Miller 1995). Tracheae evoke a modified use of the haemocyanin in the hemolymph. Comparison of the hemocyanin in differently tracheated spiders revealed a higher affinity and lower concentration of the haemocyanin in spiders with a well-developed tracheal system. Even if this point needs further investigation, in tracheal spiders the haemocyanin may work more efficiently in O2 storage while in lung spiders it works as a transporter for O2 (Schmitz and Paul 2003).
The structure of the haemocyanin is highly conserved. It is a protein which is built by hexamers or oligo-hexamers of subunits. Each subunit has a molecular weight of 70–85 kDa and can bind one O2 molecule by the means of two Cu+ ions (Markl and Decker 1992; van Holde and Miller 1995). Many spiders possess a 4 × 6-mer haemocyanin (e.g., Mygalomorphae, Araneidae, Nephilidae, Pholcidae, many Entelegynae). Other spiders possess a mixture of a 2 × 6-mer and a 1 × 6-mer haemocyanin (e.g., Agelenidae, Cupiennius, Salticidae, Lycosidae, Thomisidae) (Burmester 2013; Markl 1986; Markl et al. 1986). It was speculated that the haemocyanin structure, the loss of the 4 × 6-mer haemocyanin, is interconnected with the development of a tracheal system. In a first step a 1 × 6-mer molecule would have been acted as a high-affinity O2 storage protein instead of being an O2 carrier (Ballweber et al. 2002). Later in evolution the increasing size of the spiders may have made simple tracheal systems ineffective and the 2 × 6-mer haemocyanin evolved which allows a more efficient O2 transport by allosteric interaction. Another hypothesis is that the rebuilding of the haemocyanin was caused by a decrease in O2 levels in the atmosphere in the Permian period (Berner et al. 2007). Interestingly, in one species of spiders with a very well-developed tracheal system (Dysdera) no hemocyanin could be found (Rehm et al. 2012).
Physiology of respiration
Anderson (1970) was the first to state that spiders have low resting metabolic rates compared with other animals. They are mainly sit-and-wait predators that live in fluctuating environments with variable prey availability and therefore a great ability to starvation accompanied with a reduction of metabolism. Moreover, they have low energetic needs in prey capture because of venom use, a high anaerobic capacity and an extension of the legs by hydrostatic pressure (Anderson 1970, 1974; Anderson and Prestwich 1982; Canals et al. 2007, 2015a, b; Carrel and Heathcote 1976; Prestwich 1983a, b). To say it in more detail, the resting metabolic rate of spiders is 50–80 % of that expected in poikilotherms and follow the equation VO2/t = 0.33 M 0.80 (M = body mass). Other poikilotherms follow the Hemmingsen’s prediction according to body size VO2/t = 0.82 M 0.75 (Anderson 1996; Greenstone and Bennett 1980; Hemmingsen 1960). The RQ of resting rates in spiders is 0.7, measured for the first time in Eurypelma (Paul 1992) and is due to their prey of other small arthropods. It stands for aerobic fat breakdown and aerobic energy generation as it is the case in rest in spiders.
Low metabolic rates in spiders need further research, especially to determine whether this pattern is a generalized characteristic of the animal group, or an adaptation for certain modes of life.
To understand the physiology of respiration in spiders authors use O2 uptake and CO2 release measurements. CO2 measurements might be difficult in some cases as the increase in CO2 can also derive from buffering of hydrogen ions which are generated anaerobically by bicarbonate forming CO2. Most of the data are also summarized in Table 1.
Resting metabolic rates
Resting metabolic rates correlate with temperature, lifestyle, life spans, body masses, behavior, sex, lifestyle, ecology, and developmental stage (Canals et al. 2015a; Foelix 1992). Spiders that live longer (more than 1 year) or use webs for prey capture have lower metabolic rates than prey-stalking spiders (e.g., wolf spiders, jumping spiders) or species that complete their life cycle within 1 year (many araneid or theriid spiders). In the latter high rates of metabolism are related to the high rates of growth and reproduction occurring synchronously with seasonal pulses of insect prey. Those species have metabolic rates equal to or even greater than other poikilotherms (Anderson 1994; Anderson and Prestwich 1982). Moreover, low metabolic rates were found in primitive hunters and weavers (50–60 % of expected values in Sicariidae and Scytodidae). These results were related to the lifestyle, independent of respiratory organ and higher metabolic rates were expected in higher evolved species (e.g. Pholcus phalangioides) (Canals et al. 2015a). This coincides with the fact that higher evolved species, bimodal breathers, e.g., jumping spiders, or P. phalangioides which has no tracheae, have higher resting rates than pure and lower evolved lung breathers, e.g., mygalomorph spiders (Anderson 1970). In spiders that have none or minor developed tracheae, e.g., mygalomorph spiders and wolf spiders, the resting rate was found to be proportional to the respiratory surface area of the lungs (Anderson 1970; Anderson and Prestwich 1982; Prestwich 1983b).
Paraphysa parvula (Theraphosidae) has a very low resting metabolism (only about 20 % of the expected value looking at the body mass) (Figueroa et al. 2010). This coincides with the hypothesis that an important aspect of their metabolic efficiency includes very low resting metabolic rates as the consequence of the anatomical characteristics of their respiratory system (lung volume and respiratory surface area) (Figueroa et al. 2010). Another example are the crab spiders Mecaphesa asperata and Misumenoides formosipes (Thomisidae) which have resting rates of 1.75–2.3 nmol s−1 g−1 O2 uptake (Schmalhofer 2011). As these animals were starved, the lower metabolic rates compared with other spider species result presumably from this starvation (Schmalhofer 2011). In Salticidae, Lycosidae and Pholcidae resting rates of O2 uptake of 2–2.6 nmol s−1 g−1 were measured (Schmitz 2004, 2015). This is 70–90 % of the expected values according to body mass (Anderson 1970, 1994, 1996). It can be hypothesized that low metabolism might result from the life time of these spiders that might last up to 3 years and decreases the metabolic rate compared with species that live for shorter time periods. The low mitochondrial content of the leg muscles (Linzen and Gallowitz 1975) might limit the metabolic rate as well; a statement which was already given by Prestwich (1988b).
In two spider species (Zosis geniculata, Uloboridae, cribellate) and (Metazygia rogenhoferi, Araneidae, ecribellate) the influence of the cribellum and of the form of web-building on the metabolism was tested (Kawamoto et al. 2011). An ecribellate web is adhesive while the cribellate silk must be repeatedly combed to produce the capture spiral (Peters 1987). The results revealed that M. rogenhoferi (4.7–5 nmol s−1 g−1 O2 consumption) has about three times the resting rate of Z. geniculata, which indicates that the absence of the cribellum is associated with a higher resting metabolic rate (Kawamoto et al. 2011). In other primitive spiders lower metabolic rates than in higher developed ones were shown as well (Canals et al. 2015a). If these results prove to be a general rule among spiders, the radiation of Araneoidea could be connected to a more energy-consuming lifestyle.
In most publications spiders are starved to get the real resting rate. But the direct effect of starvation and of food quality is dealt with only in some papers: sit-and-wait predators with unsteady food availability will have low metabolic rates to sustain starvation periods. During food deprivation, metabolism is low but aerobic (Anderson 1974; Canals et al. 2011; Jensen et al. 2010; Nakamura 1987; Tanaka and Itô 1982; Tanaka et al. 1985). In the first 5 days of starvation a wolf spider (Lycosa t-insignata) considerably decreased CO2 release (presumably postprandial effects) and stabilizes its metabolic rate afterwards (Miyashita 1969). In mygalomorph spiders after a 3 weeks starvation period a reduction in metabolism was found as well (Canals et al. 2007). This additional reduction results in longer survival than expected from standard metabolic rates. In the wolf spider Pardosa prativaga the diet composition (lipid-protein composition) itself did not affect the resting and maximal metabolic response (Jensen et al. 2010).
Metabolism increases after feeding. In a tarantula (Euathlus truculentus), it was measured to be about 6.6 times the standard metabolic rate during about 45 min after prey catching and it lasted 8 h to get back to standard rates (Nespolo et al. 2011). This fact suggests that spiders spend most of the energy for digestion in a short period after prey capture, which could be a consequence of their external digestion (Nespolo et al. 2011). Temperature and hunger influence the metabolism of crab spiders as well. M. formosipes and M. asperata were tested under increasing temperature (between 10 and 40 °C) and under starvation which both strongly affected the resting rates (Schmalhofer 2011).
Males and females—courtship behavior
In general the density of available females and rival males is likely to change a male’s lifetime energetic demands. The low standard or resting metabolic rates of spiders (Anderson 1970) are of importance for males when females are rare. This would allow males to reduce energy consumption and therefore live longer to ensure that they will have got contact to females before they die. When sufficient males and females are available, males may have higher energetic costs for competitive courtship and for mate searching (Shillington 2005). In the Australian redback spider (Latrodectus hasselti, Theridiidae) resting metabolic rate was stated to be lowest if animals were not influenced by conspecifics—males or females (Stoltz et al. 2012). The routine metabolic rate decreased with decreasing resource abundance, but was positively correlated to the density of male rivals and was not correlated to body conditions at maturity or to size (Stoltz et al. 2012). In Nephila plumipes (Nephilidae) it was shown that males that are closer to females have higher active metabolic rates than males further from females (Kasumovic and Seebacher 2013). This higher metabolic activity is paralleled by increased citrate synthase activity in the whole body and might be due to greater mitochondrial densities (Kasumovic and Seebacher 2013). In the Texas tarantula Aphonopelma anax (Theraphosidae) higher resting rates are an adaptive strategy to support higher energetic demands for males during the mating season (active, locomotory search for females) (Shillington 2005). However, no intersexual differences in maximum rates and factorial scopes were found in the same species (Shillington 2005). This shows a trade-off in metabolic rate between the individuals and the competitive environment.
More examples are Linyphia litigiosa and Pardosa astrigera in which males had about 60 % higher resting rates than females (Tanaka and Itô 1982; Watson and Lighton 1994). Moreover, in the wolf spiders Pardosa milvana (not sexual dimorphic) and Hogna helluo (strongly sexual dimorphic), differences in the influence of sex was found. While males of P. milvana had higher metabolic rates than females (Walker and Irwin 2006), in H. helluo no sexual differences in metabolism were found. This is explained as Pardosa is actively foraging while Hoga is a sit-and-wait predator (Walker and Irwin 2006). Moreover, in many species females maintain a larger body size over a longer life span than do males and also have higher energetic costs associated with gamete production (Foelix 1992). In P. phalangioides, males had a slightly higher metabolic rate during rest and after a 120-s stimulation. Resting rates were presumably higher because of the lower body mass (sexual dimorphism) and therefore a higher relation of lungs to body mass in males while the difference in metabolism during activity was mainly due to a higher activity of the males (Schmitz 2015).
As in H. helluo also in other spider species no clear pattern in male–female comparison of resting metabolic rates has been demonstrated in (Canals et al. 2015a; Humphreys 1977; Kotiaho 1998; Watson and Lighton 1994). But in Loxosceles laeta (Sicariidae) and in Scytodes globula (Scytodidae) this lacking differences was referred to the non breeding season of metabolic measurements (Canals et al. 2015a). In Hygrolycosa rubrofasciata (Lycosidae) males had even lower resting rates than females (Kotiaho 1998). In this study, however, males and females might have been in different physiological states (Kotiaho 1998).
Energetic costs of courtship behavior were measured for two sympatric wolf spiders. Schizocosa ocreata and S. rovneri have different signaling modes and activity levels. Peak CO2 output while standing or walking was similar between species while the courtship behavior of S. ocreata has a significantly greater peak CO2 release than that of S. rovneri. Differing courtship behavior may serve as a criterion for the mate choice in both species and isolate these species reproductively (Cady et al. 2011).
Low and high activity
During phases in which low and medium activity is necessary, such as web-building or egg production, the partition between aerobic and anaerobic metabolism depends on the actual ATP needs and the capability of the species specific respiratory organs. For example the energetic costs of web-building in Zygiella x-notata are closely related to body mass and to web-building activity. For compensation, these spiders reduce the amount of silk used per web when the body mass increases and increase their foraging effort (Venner et al. 2003). In free living spiders, running activity and respiratory surface are so correlated that anaerobic activity is normally very low (Prestwich 1988a). Even free hunting species (such as wolf spiders or jumping spiders) use a sit-and-wait strategy and are dependent on anaerobic capacities for running in short spurts or jumping after slowly sneaking up on the prey. Such behavior does not require prolonged high metabolic rates. During short phases of high activity, anaerobic metabolism predominates. d-lactate is the major anaerobic by-product and the legs and the prosoma are the main site of lactate accumulation (Prestwich 1983a).
Most spiders are completely exhausted after 1–2 min of maximum activity because an O2 debt is caused by this activity. This could happen, e.g., during being chased. This behavior is difficult to test as most spiders cannot be forced to run continuously and continuous running in nature is normally shorter than 2 min. Therefore, a treadmill is a good solution once the spiders have to run on the wheel and have to use the preset velocity (Schmitz 2005) (Fig. 3a, b). Moreover, maximum metabolic rates are often accomplished when spiders were shaken in a little vessel and try to escape this situation (Fig. 3c). Movement in such a way requires low prosomal hemolymph pressures and thus permit constant circulation and exchange of O2 (Prestwich 1983b; Schmitz 2015). The length of recovery depends on the duration of anaerobiosis and body mass. Complete lactate removal requires 30–45 min in small spiders and several hours in large species, e.g., mygalomorph spiders (Paul 1986; Schmitz 2005). In mygalomorph spiders, tested in Brachypelma, metabolic scopes during aerobic metabolism (slow running) were 8× times the resting rate, while the heart rate is 3.2×, and the ventilation rate is 11× the resting rate (Anderson and Prestwich 1985). In the same species, metabolism was aerobic in resting phases (RQ 0.7), became anaerobic during maximum activity (while running) and had their maximum O2 uptake rates during the long recovery phase in which the O2 debt is payed back. The CO2 released during recovery partly arises from buffering the anaerobically produced pH depression. A delay in CO2 release compared to the O2 uptake is caused by the circular transit time which is higher in larger animals (Anderson and Prestwich 1985; Paul 1986, 1991, 1992; Paul and Fincke 1989; Paul et al. 1989a).
CO2 release of spiders during high activities. In a, b the same spider was taken for being tested intact or with glued spiracles. a Gives the CO2 traces of a jumping spider (Marpissa muscosa). The animal ran for 6 min at a velocity of 2.5 cm s−1 on a treadmill. The intact animal showed the highest CO2 release. If the lung is sealed, the CO2 release is the smallest, while gluing of the tracheal spiracle has a smaller influence. b Gives the CO2 release of a wolf spider (Pardosa lugubris). Gluing of one lung has a larger influence as in the jumping spider. In c the CO2 release traces of the wolf and the jumping spider and in addition of a cellar spider (Pholcus phalangioides) are given by shaking the experimental vessel for 2 min (red bar). In Pholcus the CO2 release is lower than in the two other species and in addition it is reduced by gluing one lung spiracle. Data are taken from (Schmitz 2004, 2005, 2015)
Comparing species with different respiratory organs, spiders with tracheae have higher maximum metabolic rates. We tested jumping spiders (Marpissa muscosa, well-developed tracheal system), and cellar spiders (Pholcus phalangioides, only lungs) (Schmitz 2004, 2005, 2015). M. muscosa has maximum rates of CO2 release that are 30–150 % higher than in P. phalangioides (Fig. 3a, c). This is higher than the lungs could manage as the diffusing capacity for O2 of the lungs is only up to 30 % higher than in P. phalangioides (Schmitz 2015). Even if one considers the different body masses of jumping spiders (27–39 mg) and cellar spiders (11–28 mg), jumping spiders have the highest metabolic rates (Schmitz 2015). Prestwich compared a wolf spider, Lycosa lenta (four simple tube tracheae), a jumping spider, Phidippus audax (well-developed tracheal system), and in addition a filistatid spider (Filistata hibernalis), which has only rudimentary tracheae (Prestwich 1983b). Animals were stimulated to maximum activity for 120 s. The anaerobic dependence of maximum metabolism is inversely associated with respiratory surface area: in Filistata 87–95 % of the power came from anaerobic energy production, in Lycosa 65 % and in Phidippus 55 %. Aerobic scopes, maximum respiratory rates and also the increase during exercise were greatest in Phidippus and least in Filistata. In addition, recovery periods were shortest in Phidippus (Prestwich 1983a, b, 1988a, b). The jumping spider with the well-developed tracheal system is therefore also the most aerobic spider. But as already stated, the mobilization of the anaerobic partition of respiration depends on the running velocity and the arrangement of the gas exchange organs, but also depends on the aerobic capabilities of the muscle tissue. This was not yet studied in spiders. Moreover, for jumping spiders it was shown that tracheae support aerobic metabolism at high intense activity (Fig. 3a, b). This indicates that tracheae may have evolved because of higher aerobic needs in this spider group (Prestwich 1988a; Schmitz 2005).
In other spider species, factorial scopes during locomotory activity are between 2 and 18. In Lycosidae they are sometimes even up to 22 for short periods in males of Hydrolycosa rubrofasciata during sexual signaling (drumming) (Anderson 1970; Culik and McQueen 1985; Ford 1977a, b; Humphreys 1977; Kotiaho 1998; McQueen 1980; McQueen and Culik 1981; Miyashita 1969; Schmitz 2004, 2005, 2015; Seymour and Vinegar 1973; Shillington and Peterson 2002; Watson and Lighton 1994). In Pardosa prativaga (Lycosidae) the factorial scope was 5–6 between fasting and feeding (Jensen et al. 2010). In Geolycosa domifex (Lycosidae) activity rates were measured to be 3.2–10× resting rates (different in burrow and running activities) but were found to be 17.8× resting rates as a maximum value during running (Culik and McQueen 1985; McQueen 1980; McQueen and Culik 1981; McQueen et al. 1979).
As the metabolism decreases under starvation (see above), starvation increases also the factorial scope which was shown for Pardosa astrigera (Tanaka and Itô 1982). Moreover, the factorial scope in Nephila (Nephilidae) was 4.2 for the spontaneous activity of males (Kasumovic and Seebacher 2013), while it was 6.6 for Euathlus truculentus (Theraphosidae) for feeding (Nespolo et al. 2011). In the two crab spiders, M. asperata and M. formosipes, the resting rate was increased by feeding to about 3–5× (Schmalhofer 2011).
Hardly investigated is the metabolic activity in response to a predator. In jumping spiders it was shown that the metabolism reacts to a visual stimulus with a first increase and is then decreasing to a lower level for a longer time period (Okuyama 2015).
Respiration under water
Finally, I want to give a very short introduction in respiration under water in spiders. Argyroneta aquatica is the only spider species that lives under water. It breathes almost exclusively with tracheae (Braun 1931; Crome 1952/53). A study reveals that the diving bell of Argyroneta works as a physical gill to meet their metabolic O2 requirements by diffusive O2 uptake from the water (Seymour and Hetz 2011). This bell is sufficient for more than 1 day when spiders are resting and frequent replenishment with air from the surface is necessary only in severely hypoxic water or in exceptionally small bells. The bell itself acts as an O2 collecting device but it only works with a certain partial pressure difference between bubble and water (Seymour and Hetz 2011). This leads to a low PO2 within the bubble which has consequences for the PO2 the spider has to cope with. In the raft spider (Dolomedes fimbriatus), which hunts underwater, the abdomen is superhydrophobic and retains a thin gas film while submerged (visible as a silvery layer). The gas film acts as a physical gill for a minimum of 20 min (Pedersen and Colmer 2012).
Conclusions
Respiration in spiders is a very interesting topic and almost each spider family has its own morphological and physiological characteristics. It is very interesting to see in one animal group that two respiratory organs (lungs and tracheae) are developed with very different characteristics in each family. There is the tendency that higher developed spiders with a well-developed tracheal system reduce the concentration and/or the function of their respiratory pigment and increase the maximum metabolic rates. But tracheae are also helpful in saving water and especially to provide O2 to special organs, such as the nervous system or some highly active leg muscles. Not only the equipment with lungs or tracheae influences the metabolism in spiders, but also other factors as life time, sex, and prey availability have a large influence on the use of the respiratory system. Metabolic rates can be analyzed from the physiological limits point of view and from the ecological constraints point of view. Forthcoming studies have to deal more with these conflicting approaches.
References
Anderson JF (1970) Metabolic rates in spiders. Comp Biochem Physiol 33:51–72
Anderson JF (1974) Responses to starvation in the spiders Lycosa lenta (Hentz) und Filistata hibernalis (Hentz). Ecology 55:576–585
Anderson JF (1994) Comparative energetics of comb-footed spiders (Araneae: Theridiidae). Comp Biochem Physiol 109A(1):181–189
Anderson JF (1996) Metabolic rates of resting salticid and thomisid spiders. J Arachnol 24:129–134
Anderson JF, Prestwich KN (1975) The fluid pressure pumps of spiders (Chelicerata, Araneae). Z Morph Tiere 81:257–277
Anderson JF, Prestwich KN (1982) Respiratory gas exchange in spiders. Physiol Zool 55(1):72–90
Anderson JF, Prestwich KN (1985) The physiology of exercise at and above maximal aerobic capacity in a theraphosid (tarantula) spider Brachypelma smithi. J Comp Physiol B 155:529–539
Angersbach D (1978) Oxygen transport in the blood of the tarantula Eurypelma californicum: pO2 and pH during rest, activity and recovery. J comp Physiol 123:113–125
Ballweber P, Markl J, Burmester T (2002) Complete hemocyanin subunit sequences of the hunting spider Cupiennius salei—recent hemocyanin remodeling in entelegyne spiders. J Biol Chem 277:14451–14457
Berner RA, Vandenbrooks JM, Ward P (2007) Oyxgen and evolution. Science 316:557–558
Bertkau P (1876) Über die Respiationsorgane der Araneen. Archiv für Naturgeschichte 38:208–233
Blest AD (1976) The tracheal arrangement and the classification of linyphiid spiders. J Zool Lond 180:185–194
Braun F (1931) Beiträge zur Biologie und Atmungsphysiologie der Argyroneta aquatica Cl. Zoolog Jahrb Syst 62:175–262
Bromhall C (1987a) Spider heart-rates and locomotion. J Comp Physiol B 157:451–460
Bromhall C (1987b) Spider tracheal systems. Tissue Cell 19(6):793–807
Burmester T (2013) Evolution and adaptation of hemocyanin within spiders. In: Nentwig W (ed) Spider ecophysiology. Springer, Berlin, pp 3–14
Cady AB, Delaney KJ, Uetz GW (2011) Contrasting energetic costs of courtship signaling in two wolf spiders having divergent courtship behaviors. J Arachnol 39:161–165
Canals M, Salazar MJ, Duran C, Figueroa D, Veloso C (2007) Respiratory refinements in the mygalomorph spider Grammostola rosea walckenaer 1837 (Araneae, Theraphosidae). J Arachnol 35:481–486
Canals M, Figueroa D, Alfaro C, Kawamoto T, Torres-Contreras H, Sabat P, Veloso C (2011) Effects of diet and water supply on energy intake and water loss in a mygalomorph spider in a fluctuating environment of the central Andes. J Insect Physiol 57:1489–1494. doi:10.1016/j.jinsphys.2011.07.016
Canals M, Veloso C, Moreno L, Solis R (2015a) Low metabolic rates in primitive hunters and weaver spiders. Physiol Entomol 40:232–238. doi:10.1111/phen.12108
Canals M, Veloso C, Solis R (2015b) Adaptation of the spiders to the environment: the case of some Chilean species. Front Physiol. doi:10.3389/fphys.2015.00220
Carrel JE (1987) Heart rate and physiological ecology. In: Nentwig W (ed) Ecophysiology of spiders. Springer, Berlin, pp 95–110
Carrel JE, Heathcote RD (1976) Heart rate in spiders: influence of body size and foraging strategies. Science 193:148–150
Crome W (1952/53) Die Respirations- und Circulationsorgane der Argyroneta aquatica Cl. (Araneaea). Wiss Zeitschr Humboldt Universit”t Berlin 3/4:53–83
Culik BM, McQueen DJ (1985) Monitoring respiration and activity in the spider Geolycosa domifex (Hancock) using time-lapse televison and CO2-analysis. Can J Zool 63:843–846
Edwards GA (1946) The influence of temperature upon the oxygen consumption of several arthropods. J Cell Comp Physiol 27:53–64
Ellis CH (1944) The mechanism of extension in the legs of spiders. Biol Bull 86:41–50
Figueroa DP, Sabat P, Torres-Contreras H, Veloso C, Canals M (2010) Participation of book lungs in evaporative water loss in Paraphysa parvula, a migalomorph spider from Chilean Andes. J Insect Physiol 56:731–735. doi:10.1016/j.jinsphys.2010.01.001
Fincke T, Paul R (1989) Book lung function in arachnids III. The function and control of the spiracles. J Comp Physiol B 159:433–441
Foelix RF (1992) Biologie der Spinnen, Second edn. Georg Thieme Verlag, New York
Ford MJ (1977a) Energy costs of the predation strategy of the web-spinning spider Lethyphantes zimmermanni Bertkau (Linyphiidae). Oecologica 28:341–349
Ford MJ (1977b) Metabolic costs of the predation strategy of the spider Pardosa amentata (Clerck) (Lycosidae). Oecologica 28:333–340
Forster RR (1980) Evolution of the tarsal organ, the respiratory system and the female genitalia in spiders. Int Congr Arachnol 8:269–284
Greenstone MH, Bennett AF (1980) Foraging strategy and metabolic rates in spiders. Ecology 61(5):1255–1259
Haller B (1912) Über die Atmungsorgane der Arachnoiden. Ein Beitrag zur Stammesgeschichte dieser Tiere Arch f Mikrosk Anat 79:1–58
Hemmingsen AM (1960) Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep Steno Mem Hosp 9:1–110
Hsia CCW, Schmitz A, Lambertz M, Perry SF, Maina JN (2013) Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol 3:849–915
Humphreys WF (1977) Respiration studies on Geolycosa godeffroyi (Aranea: Lycosidae) and their relationship to field estimates of metabolic heat loss. Comp Biochem Physiol 57A:255–263
Jensen K, Mayntz D, Wang T, Simpson SJ, Overgaard J (2010) Metabolic consequences of feeding and fasting on nutritionally different diets in the wolf spider Pardosa prativaga. J Insect Physiol 56:1095–1100. doi:10.1016/j.jinsphys.2010.03.001
Kästner A (1929) Bau und Funktion der Fächertracheen einiger Spinnen. Z f Morphol d Tiere 13:463–558
Kasumovic MM, Seebacher F (2013) The active metabolic rate predicts a male spider’s proximity to females and expected fitness. Biol Lett 9:1–4. doi:10.1098/rsbl.2012.1164
Kawamoto TH, Machado FDA, Kaneto GE, Japyassu HF (2011) Resting metabolic rates of two orbweb spiders: a first approach to evolutionary success of ecribellate spiders. J Insect Physiol 57:427–432. doi:10.1016/j.jinsphys.2011.01.001
Kotiaho J (1998) Sexual differences in metabolic rates of spiders. J Arachnol 26:401–404
Lamy E (1902) Les trachées des araignées. Ann Sci Natur Zool 15(8):149–280
Levi HW (1967) Adaptations of respiratory systems of spiders. Evolution 21:571–583
Levi HW (1976) On the evolution of tracheae in Arachnids. Bull Br Arachnol Soc 3(7):187–188
Linzen B, Gallowitz P (1975) Enzyme activity patterns in muscles of the lycosid spider Cupiennius salei. J Comp Physiol 96:101–109
Mangum CP (1985) Oxygen transport in invertebrates. Am J Physiol 248:505–514
Markl J (1986) Evolution and function of structurally diverse subunits in the respiratory protein hemocyanin from Arthropods. Biol Bull 171:90–115
Markl J, Decker H (1992) Molecular structure of the arthropod hemocyanins. Adv Comp Environ Physiol 13:325–376
Markl J, Stöcker W, Runzler R, Precht E (1986) Immunological correspondence between the hemocyanin subunits of 86 arthropods: evolution of a multigene protein family. In: Linzen B (ed) Invertebrate oxygen carriers. Springer, Berlin, pp 281–299
McQueen DJ (1980) Active respiration rates for the burrowing wolf spider Geolycosa domifex (Hancock). Can J Zool 58:1066–1074
McQueen DJ, Culik B (1981) Field and laboratory activity patterns in the burrowing wolf spider Geolycosa domifex (Hancock). Can J Zool 59:1263–1271
McQueen DJ, Jensen IM, Dyer BS (1979) Resting and diel respiration rates for burrowing wolf spider Geolycosa domifex (Hancock). Can J Zool 57:1922–1933
Millidge AF (1986) A revision of the tracheal structures of the Linyphiidae (Araneae). Bull Br Arachnol Soc 7(2):57–61
Miyashita K (1969) Effects of locomotory activity, temperature and hunger on the respiratory rate of Lycosa t-insignita Boes et. Str. (Araneae: Lycosidae). Appl Ent Zool 4:105–113
Moore SJ (1976) Some spider organs as seen by the scanning electron microscope, with special reference to the book-lung. Bull Br Arachnol Soc 3(7):177–187
Nakamura K (1987) Hunger and starvation. In: Nentwig W (ed) Ecophysiology of spiders. Springer, Berlin, pp 287–295
Nespolo RF, Correa L, Perez-Apablaza CX, Cortes P, Bartheld JL (2011) Energy metabolism and the postprandial response of the Chilean tarantulas, Euathlus truculentus (Araneae: Theraphosidae). Comp Biochem Physiol A-Mol Integr Physiol 159:379–382. doi:10.1016/j.cbpa.2011.04.003
Okuyama T (2015) Metabolic responses to predation risk in a jumping spider. J Zool 297:9–14. doi:10.1111/jzo.12251
Opell BD (1979) Revision of the genera and tropical American species of the spider family Uloboridae. Bull Mus Comp Zool 148(10):443–549
Opell BD (1987) The influence of web monitoring tactics on the tracheal systems of spiders in the family Uloboridae (Arachnida, Araneida). Zoomorphology 107:255–259
Opell BD (1989) Centers of mass and weight distribution in spiders of the family Uloboridae. J Morphol 202:351–359
Opell BD (1990) The relationships of book lung and tracheal systems in the spider family Uloboridae. J Morphol 206:211–216
Opell BD (1998) The respiratory complementary of spider book lung and tracheal systems. J Morphol 236:57–64
Opell BD, Konur DC (1992) Influence of web-monitoring tactics on the density of mitochondria in leg muscles of the spider family Uloboridae. J Morphol 213:341–347
Paul R (1986) Gas exchange and gas transport in the tarantula Eurypelma californicum—an overview. In: Linzen B (ed) Invertebrate oxygen carriers. Springer, Berlin, pp 321–326
Paul RJ (1991) Oxygen transport from book lungs to tissues—environmental physiology and metabolism in arachnids. Verh Dt Zool Ges 84:9–14
Paul RJ (1992) Gas exchange, circulation, and energy metabolism in arachnids. In: Wood SC, Weber RE, Hargens AR, Millard RW (eds) Physiological adaptations in vertebrates. Marcel Dekker, New York, pp 169–197
Paul R, Fincke T (1989) Book lung function in arachnids II. Carbon dioxide and its relations to respiratory surface, water loss and heart frequency. J Comp Physiol 159:419–432
Paul R, Fincke T, Linzen B (1987) Respiration in the tarantula Eurypelma californicum: evidence for diffusion lungs. J Comp Physiol B 157:209–217
Paul R, Fincke T, Linzen B (1989a) Book lung function in arachnids. I. Oxygen uptake and respiratory quotient during rest, activity and recovery—relations to gas transport in the haemolymph. J Comp Physiol B 159:409–418
Paul R, Tiling K, Focke P, Linzen B (1989b) Heart and circulatory functions in a spider (Eurypelma californicum): the effects of hydraulic force generation. J Comp Physiol B 158:673–687
Paul RJ, Bergner B, Pfeffer-Seidl A, Decker H, Efinger R, Storz H (1994a) Gas transport in the haemolymph of Arachnids I. Oxygen transport and the physiological role of haemocyanins. J exp Biol 188:25–46
Paul RJ, Bihlmayer S, Colmorgen M, Zahler S (1994b) The open circulatory system of spiders (Eurypelma californicum, Pholcus phalangioides): a survey of functional morphology and physiology. Physiol Zool 67(6):1360–1382
Pedersen O, Colmer TD (2012) Physical gills prevent drowning of many wetland insects, spiders and plants. J Exp Biol 215:705–709. doi:10.1242/jeb.065128
Peters HM (1987) Fine structure and function of capture threads. In: Nentwig W (ed) Ecophysiology of spiders. Springer, Berlin, pp 187–202
Prestwich KN (1983a) Anaerobic metabolism in spiders. Physiol Zool 56(1):112–121
Prestwich KN (1983b) The roles of aerobic and anaerobic metabolism in active spiders. Physiol Zool 56(1):122–132
Prestwich KN (1988a) The constraints on maximal activity in spiders I. Evidence against the fluid insufficiency hypothesis. J Comp Physiol 158:437–447
Prestwich KN (1988b) The constraints on maximal activity in spiders. II. Limitations imposed by phosphagen depletion and anaerobic metabolism. J Comp Physiol B 158:449–456
Purcell F (1895) Note on the development of the lungs, entapophyses, tracheae and genital ducts in spiders. Zool Anz 486:1–5
Purcell WF (1909) Development and origin of the respiratory organs in Araneae. Quart J Microsc Sci 54(1):1–110
Purcell WF (1910) The phylogeny of tracheae in Araneae. Quart J Microsc Sci 54(4):519–563
Ramirez MJ (2000) Respiratory system morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae). J Arachnol 28:149–157
Rehm P, Pick C, Borner J, Markl J, Burmester T (2012) The diversity and evolution of chelicerate hemocyanins. BMC Evolut Biol 12:19. doi:10.1186/1471-2148/12/19
Reisinger PWM, Focke P, Linzen B (1990) Lung morphology of the tarantula, Eurypelma californicum, Ausserer, 1871 (Araneae: Theraphosidae). Bull Br Arachnol Soc 8:165–170
Reisinger PWM, Tutter I, Welsch U (1991) Fine structure of the gills of the horseshoe crabs Limulus polyphemus and tachypleus tridentatus and of the book lungs of the spider Eurypelma californicum. Zool Jb Anat 121:331–357
Schmalhofer VR (2011) Impacts of temperature, hunger and reproductive condition on metabolic rates of flower-dwelling crab spiders (Araneae: Thomisidae). J Arachnol 39:41–52
Schmitz A (2004) Metabolic rates during rest and activity in differently tracheated spiders (Arachnida, Araneae): Pardosa lugubris (Lycosidae) and Marpissa muscosa (Salticidae). J Comp Physiol B 174:519–526
Schmitz A (2005) Spiders on a treadmill: influence of running activity on metabolic rates in Pardosa lugubris (Araneae, Lycosidae) and Marpissa muscosa (Araneae, Salticidae). J Exp Biol 208:1401–1411
Schmitz A (2013) Tracheae in spiders: respiratory organs for special functions. In: Nentwig W (ed) Spider ecophysiology. Springer, New York, pp 29–39
Schmitz A (2015) Functional morphology of the respiratory organs in the cellar spider Pholcus phalangioides (Arachnida, Araneae, Pholcidae). J Comp Physiol B 185:637–646
Schmitz A, Paul RJ (2003) Probing of hemocyanin function in araneomorph spiders. XIIIth international conference Inv Diox Bind Prot Mainz, vol 96
Schmitz A, Perry SF (2000) Respiratory system of arachnids I: Morphology of the respiratory system of Salticus scenicus and Euophrys lanigera (Arachnida, Araneae, Salticidae). Arthropod Struct Dev 29:3–12
Schmitz A, Perry SF (2001) Bimodal breathing in jumping spiders: morphometric partitioning of lungs and tracheae in Salticus scenicus (Arachnida, Araneae, Salticidae). J Exp Biol 204:4321–4334
Schmitz A, Perry SF (2002) Respiratory organs in wolf spiders: morphometric analysis of lungs and tracheae in Pardosa lugubris (L.) (Arachnida, Araneae, Lycosidae). Arthropod Struct Dev 31:217–230
Seymour RS, Hetz SK (2011) The diving bell and the spider: the physical gill of Argyroneta aquatica. J Exp Biol 214:2175–2181. doi:10.1242/jeb.056093
Seymour RS, Vinegar A (1973) Thermal relations, water loss and oxygen consumption of a North American tarantula. Comp Biochem Physiol 44A:83–96
Shillington C (2005) Inter-sexual differences in resting metabolic rates in the Texas tarantula, Aphonopelma anax. Comp Biochem Physiol A-Mol Integr Physiol 142:439–445
Shillington C, Peterson CC (2002) Energy metabolism of male and female tarantulas (Aphonopelma anax) during locomotion. J Exp Biol 205:2909–2914
Simmons OL (1894) Development of the lungs of spiders. Am J Sci Art 48:119–129
Stoltz JA, Andrade MCB, Kasumovic MM (2012) Developmental plasticity in metabolic rates reinforces morphological plasticity in response to social cues of sexual selection. J Insect Physiol 58:985–990. doi:10.1016/j.jinsphys.2012.05.002
Strazny F, Perry SF (1984) Morphometric diffusing capacity and functional anatomy of the book lungs in the spider Tegenaria spp. (Agelenidae). J Morphol 182:339–354
Strazny F, Perry SF (1987) Respiratory system: structure and function. In: Nentwig W (ed) Ecophysiology of spiders. Springer, Berlin, pp 78–94
Tanaka K, Itô Y (1982) Decrease in respiratory rate in a wolf spider, Pardosa astrigera (L. Koch), under starvation Res. Popul Ecol 24:360–374
Tanaka K, Ito Y, Saito T (1985) Reduced respiratory quotient by starvation in a wolf spider, Pardosa astrigera (L. Koch). Comp Biochem Physiol A-Mol Integr Physiol 80:415–418
van Holde KE, Miller KI (1995) Hemocyanins. Adv Protein Chem 47:1–81
Venner S, Bel-Venner M-C, Pasquet A, Leborgne R (2003) Body-mass-dependent cost of web-building behavior in an orb weaving spider, Zygiella x-notata. Naturwissenschaften 90:269–272
Walker SE, Irwin JT (2006) Sexual dimorphism in the metabolic rate of two species of wolf spider (Araneae, Lycosidae). J Arachnol 34:368–373
Watson PJ, Lighton JRB (1994) Sexual selection and the energetics of copulatory courtship in the Sierra dome spider, Linyphia litigiosa. Anim Behav 48:615–626
Wirkner CS, Huckstorf K (2013) The circulatory system of spiders. In: Nentwig W (ed) Spider ecophysiology. Springer, Berlin, pp 15–27
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Schmitz, A. Respiration in spiders (Araneae). J Comp Physiol B 186, 403–415 (2016). https://doi.org/10.1007/s00360-016-0962-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-0962-8