Abstract
Mating induces behavioral and physiological changes in the plant bug Lygus hesperus Knight (Hemiptera: Miridae). After receiving seminal products, which include the systemic regulator juvenile hormone (JH), females enter a post-mating period lasting several days during which they enhance their oviposition rate and lose interest in remating. To elucidate the regulation of these behavioral changes in L. hesperus, biogenic amines were quantified in the heads of females at 5 min, 1 h and 24 h after copulation and compared to levels in virgins using high-performance liquid chromatography coupled with electrochemical detection. Mating significantly increased dopamine (DA) after 1 and 24 h, and decreased octopamine (OA) after 5 min and 1 h. Serotonin did not change with mating, but tyramine was significantly reduced after 5 min. While injection of amines into virgin females did not influence sexual receptivity, OA caused a decrease in oviposition during the 24 h following injection. Topical application of the JH analog methoprene to virgins caused an increase in DA, and a decline in mating propensity, but did not influence other amines or the oviposition rate. The results suggest the decline in OA observed immediately after mating may promote egg laying, and that male-derived JH may induce an increase in DA that could account for the post-mating loss of sexual receptivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many insects, mating can strongly influence sexual receptivity, gamete production, the release and detection of sex pheromones, and myriad other aspects of reproductive activity (reviewed in Ringo 1996; Gillot 2003). Similarly, in many mirids, mating causes individuals to enter a refractory period, during which sexual activity is inhibited. For a few species, this may be a permanent behavioral transition, but for most the refractory period is temporary, allowing multiple matings (reviewed in Wheeler 2001). In the western tarnished plant bug, Lygus hesperus Knight, newly mated females have a greatly diminished sexual receptivity, ignoring or avoiding courting males. This refractory period typically lasts 5 days (Strong et al. 1970; Brent 2010a). During this time, the female also increases her rate of oviposition (Brent et al. 2011). As is typical for many insects (Gillot 2003; Wolfner et al. 2005), seminal products from male accessory glands appear to drive many of the behavioral changes observed in L. hesperus females (Brent 2010b; Brent and Hull 2014). Direct injection of the male spermatophore contents into the abdomen of virgins can induce the same behavioral responses as mating, suggesting the female response if induced by one or more key components of the spermatophore, rather than the stimulus of mating (Brent 2010b). Contained within the spermatophore is a substantial quantity of juvenile hormone (JH; Brent and Hull 2014). In other insects, JH is a systemic regulator of development, gonadal activity and reproductive behaviors (Ringo 1996, Raikhel et al. 2005), including female sexual receptivity (Manning 1966; Bouletreau-Merle 1973; Ringo et al. 1991, 2005). While potentially important in regulating the reproductive behavior of L. hesperus females, the role of JH in this species is currently unknown, as is the mechanism by which it and other male-derived factors might act to produce the observed post-mating changes.
These regulatory factors must act through the brain or other parts of the central nervous system (CNS) to enhance or inhibit the likelihood of reproductive activity. Linking the CNS to the peripheral nervous system (PNS) and key regulatory organs are the biogenic monoamines. These neurotransmitters, neuromodulators and neurohormones are capable of inducing both behavioral and physiological changes in invertebrates (Blenau and Baumann 2001; Fahrbach and Mesce 2005; Roeder 2005). However, the function of each amine varies widely across the insects, necessitating investigation within individual species. Four biogenic amines have been implicated in the regulation of insect reproduction: dopamine (DA), octopamine (OA), serotonin (5-hydroxytryptamine, 5-HT) and tyramine (TA).
DA has been linked with oogenesis and oviposition (Bloch et al. 2000; Boulay et al. 2001; Dombroski et al. 2003; Sasaki et al. 2007), JH metabolism (Thompson et al. 1990; Pastor et al. 1991; Gruntenko et al. 2005a, 2005b), mate-seeking (Harano et al. 2008), and olfaction (Mercer and Erber 1983; Macmillan and Mercer 1987), a sense that is crucial to mate attraction in L. hesperus (Brent and Byers 2011; Byers et al. 2013). OA and TA are related compounds with frequently overlapping functions, but they are neuroactively independent (reviewed in Lange 2009), and can even have opposing effects (Saraswati et al. 2004). OA has been linked to oviposition (Orchard and Lange 1985; Lange and Orchard 1986; Clark and Lange 2003; Monastirioti 2003; Donini and Lange 2004; Rafaeli 2004; Hirashima et al. 2006; Yamane 2013), mate-seeking behavior (Linn and Roelofs 1986), and JH titers (Thompson et al. 1990; Rachinsky 1994; Woodring and Hoffmann 1994; Granger et al. 1996; Hirashima et al. 1999; Gruntenko et al. 2007), while TA has been implicated in the post-mating loss of sexual receptivity (Yamane and Miyatake 2010). 5-HT is implicated in the modulation of various physiological processes and complex behaviors in insects (reviewed in Blenau et al. 2013), including oviposition (Lange 2004) and sexual receptivity (Obara et al. 2011). 5-HT may influence reproductive behaviors by modifications to sensory perception (Blenau and Erber 1998; Scheiner et al. 2006), JH metabolism (Rachinsky 1994), and reproductive organ contractile activity (Lee et al. 2001; Lange 2004).
Given the role that JH normally plays in regulating reproductive functions (Raikhel et al. 2005), we hypothesized that the loss of sexual receptivity and increased ovipositional behavior exhibited by mated L. hesperus females was in response to the JH delivered in the spermatophore. Further, because biogenic amines seem to be involved in modulating reproductive behaviors, we hypothesize that the behavioral responses subsequent to mating are mediated by changes to the circulating titers of biogenic amines. The influence of male-derived JH on female behavior was investigated using topical application of methoprene, a JH analog. To determine the potential intermediary role of biogenic amines, we used high-performance liquid chromatography (HPLC) coupled with electrochemical detection to measure the amine content in the heads of virgin and mated females, and virgins treated with methoprene. We also examined whether one or more biogenic amines modulate reproductive behaviors by monitoring mating propensity and rates of egg production after directly injecting the compounds into virgin females.
Materials and methods
Insects
The L. hesperus used in this study were obtained from a large laboratory colony maintained at the USDA-ARS, US Arid Land Agricultural Research Center (Maricopa, AZ, USA). The individuals in this colony are periodically outbred with locally caught conspecifics to maintain vigor. The stock insects were given unrestricted access to a supply of green bean (Phaseolus vulgaris L.) pods and an artificial diet mix (Debolt 1982) packaged in Parafilm M (Pechiney Plastic Packaging, Chicago, IL, USA) (Patana 1982). Both food sources were replenished as needed. Insects were reared at 25 °C, 20 % relative humidity, under a L14:D10 photoperiod.
Adults were produced from randomly selected groups of 5th instar nymphs of varied parentage. The nymphs were reared in 1890-mL waxed chipboard cups (Huhtamaki, De Soto, KS, USA) at a density known to have minimal effect on L. hesperus development (≤100 nymphs/container; Brent 2010c). Nymphs in each container were provided approximately 20 g of fresh green beans and 12 g of artificial diet, which was replaced every 48 h. Rearing cups were covered with a nylon mesh to ensure air circulation and light exposure. Daily monitoring allowed adults to be collected within 24 h of emergence. Cohorts of adults of the same age and sex were reared under conditions matching those for nymphs, but with population densities ranging between 50 and 120 adults/container.
Effects of methoprene application on behavior and biogenic amine levels
The JH analog methoprene was used to determine whether JH, such as that delivered in the male spermatophore, could induce effects in female Lygus that are similar to those observed after mating. Methoprene was chosen due to its ability to trigger many of the same responses as JH, and because it is more persistent in the hemolymph thereby extending any behavioral or physiological effects. In the first test, topical applications were used to determine how JH might influence biogenic amine levels. To generate samples, two females and two males, all virgins taken from the same cohort, were sorted together into a covered plastic Petri dish (60 × 15 mm) and allowed to interact while under continuous observation. Almost all females were courted by males, and a subset copulated. Females observed copulating had their insemination status confirmed by inspecting the dorsal abdomen for a spermatophore lying just underneath the cuticle (Cooper 2012). Mated females were isolated in new petri dishes containing a section of green bean. At the same time, the females that had been courted by males but were still virgins were similarly set aside. All females used were aged 7 days post-eclosion, a time when they are sexually mature and willing to mate (Brent 2010a). Three treatment groups were generated: newly mated females treated with 1 uL acetone, virgin females treated with acetone and virgin females treated with 5.0 µg methoprene (Sigma-Aldrich) in 1 µL acetone. Although this dose is five thousand-fold higher than the amount of JH found in the typical Lygus spermatophore (Brent and Hull 2014), the amount actually penetrating the cuticle would be greatly reduced but still should be sufficient to induce physiological responses. We avoided using substantially higher dosages, typically chosen because they are just sub-lethal, because they can produce a variety of consequences that would confound data interpretation. All applications were made to the posterior apex of the abdomen. Immediately after the solvent dried, females were placed individually in Petri dishes with a section of green bean and housed under standard rearing conditions. To determine the effect of methoprene on oviposition rate, egg counts were made 48 h after application, with each bean section examined under a dissecting scope to determine egg number. Methoprene is persistent so that any difference between treatment groups is amplified over time. Each treatment group consisted of 62 females, but counts were excluded for females that died prior to 48 h after treatment. Mortality rates were low and similar across treatment groups.
To determine the effect of methoprene on female mating receptivity, virgin females were topically treated with either acetone or 5 µg methoprene. Subjects were then placed in Petri dishes with a section of green bean and one 8–10-day-old male and returned to standard rearing conditions for 24 h. After this time, the females were dissected to determine whether they had mated, as indicated by the presence of a spermatophore. Data were not collected from females that died prior to sampling. Mortality rates were low and similar across treatment groups. Each treatment group contained 205 females.
The responses of biogenic amines to JH were determined by quantifying amine levels in the heads of 7-day-old adult virgin females after treatment with acetone or 5 µg methoprene. There were 21–27 samples per combination of treatment and sample time. Individuals were flash frozen in liquid nitrogen at 1 h, or 24 h after treatment. Subjects for each sample period group were collected separately at different times, each using a new cohort of individuals. This sampling approach precluded comparisons between time intervals. Collected individuals were decapitated and the antennae and proboscis were removed from the head. For each sample, 10 heads were pooled to ensure quantifiability. Because the detection limit of the HPLC was 25 pg, it would not have been possible to analyze the contents of individual heads for amines other than DA. Samples were stored at −80 °C until analysis. Heads were placed in a 1.5-mL centrifuge tube and homogenized with a pestle in 20 µL of chilled perchloric acid (0.2 M) that contained dihydroxybenzylamine (DHBA, 87 pg/µL; Sigma-Aldrich, St. Louis, MO, USA) and synephrine (50 pg/µL; Sigma-Aldrich) as internal standards. Tissue was further disrupted with 5 min of sonication in a covered ultrasonic bath (Branson 2510, Branson Ultrasonics Corp., Danbury, CT, USA) filled with an ice water slurry. After sonication, the samples were allowed to sit in the water bath for an additional 20 min to complete amine extraction from the brain tissue. Samples were then spun at 12,000 RCF for 10 min in a refrigerated (4 °C) centrifuge. Samples were kept on ice in a covered container until analyzed. Only five samples were prepared at a time to minimize the delay between removal from the freezer and amine quantification.
The biogenic amine content of 10 µL of the supernatant was determined by high-performance liquid chromatography (HPLC). The assessed quantities reflect both the stored and circulating biogenic amines from multiple organs associated with the head. The HPLC system (ESA, Chelmsford, MA, USA) consisted of a Coularray model 5600A with a four-channel electrochemical detector, a model 582 pump, and a reverse-phase catecholamine HR-80 column. Samples were delivered via a manual injector (Rheodyne 9125, Rohnert Park, CA, USA) with a 20-μL loop. Channel 1 was set at 650 mV for octopamine and tyramine. Channel 2 was set at 425 mV for dopamine and serotonin. Amine identity was confirmed by peak responses on a third channel set at 175 mV. The mobile phase was composed of polished water, 15 % methanol, 15 % acetonitrile, 1.5 mmol L−1 sodium dodecyl sulfate, 85 mmol L−1 sodium phosphate monobasic, and 5 mmol L−1 sodium citrate. The pH was adjusted to 5.6 using phosphoric acid. The flow rate of the mobile phase was 0.5 mL min−1. Results are expressed on a per-head basis. The size of resultant peaks was compared to a serial set of external standards (hydrochloride forms of DA, OA, 5-HT, TA; Sigma-Aldrich) run before and after each set of five samples to determine the equivalent quantity in picograms.
Effects of mating on biogenic amine levels
To identify changes to female amine levels after mating, mated and virgin females were generated as above and were then flash frozen in liquid nitrogen at 5 min, 1 h, or 24 h after their courtship or mating interactions. For each time period, twenty samples were collected and stored at −80 °C until analysis. Biogenic amine content was determined for each time period using HPLC as described above.
Effects of injected biogenic amines on behavior
To test for effects of amines on female ovipositional behavior, 8–10-day-old virgins were injected with 0.5 µL of either insect saline (solvent control; cockroach Ringer’s (Seshan 1976)) or a 1.0 %m/v solution of one of the four amines (total injected = 5.0 µg) in saline. Amine concentrations were chosen based on previous studies where significant behavioral and physiological effects were induced with similar concentrations in adult insects (Linn and Roelofs 1986; Linn et al. 1994; Scheiner et al. 2002; Yamane 2013). Injected females were then placed in Petri dishes with a section of green bean. For each treatment, ninety females were injected using a graduated borosilicate glass syringe, made from a fused glass capillary tube. The needle insertion point was between the 6th and 7th sternite, to the left of the ovipositor. Injections were into the abdominal hemocoel where there is an estimated interior hemolymph content of 2.5 µL. The head of Lygus is quite small relative to the abdomen such that it could not accommodate the volume of injected fluid and would likely be severely traumatized by the needle. Similarly, injections into the thorax failed to contain the injection volume. Abdominally injected amines are able to passively diffuse in the hemolymph to the brain and other key organs involved in reproductive functions. Oviposition data were collected as described above. A 24-h sample period was used because the induction effect of mating on ovipositional activity is rapid and causes an observable increase within a short span (Brent et al. 2011). Further, unbound biogenic amines degrade quickly (Linn et al. 1994) and by 24 h would have been cleared out of the hemolymph. Data were not collected from females that died prior to sampling. Based on the results from this initial test, a second group was tested to determine whether changing OA concentration could produce a graded effect. Identical conditions were used, except females were injected with IS, 0.75 % (3.75 µg) or 1.5 % (7.5 µg) OA.
To test for effects of amines on female mating receptivity, mature virgins were injected with 0.5 µL of either insect saline (control) or a 1.0 % solution of one of the four amines in saline. Subjects were then placed in Petri dishes with a section of green bean and one 8–10-day-old male. Sixty females were assigned to each treatment. They were returned to standard rearing conditions for 24 h, after which the females were dissected to determine whether they had mated. Data were not collected from females that died prior to sampling. Mortality rates were low and similar across treatment groups.
Statistical analysis
For most experiments, the data were non-normally distributed, necessitating the use of non-parametric tests. A Mann–Whitney rank sum test was used to compare between virgin and mated individuals for each of the biogenic amines. Kruskal–Wallis ANOVA on ranks was used to compare oviposition rates among females injected with amines or treated with methoprene, and for comparison of amine responses after methoprene treatment. Dunn’s test for multiple comparisons was used post hoc with α = 0.05. Chi-square analysis was used to determine whether mating receptivity was associated with amine or JH treatment. All analyses were conducted using Sigmaplot 11.0 (Systat Software Inc. 2008).
Results
Topical application of methoprene, to simulate the transmission of male-derived JH during mating, failed to influence oviposition rates. Although there was an overall effect of treatment (Fig. 1a; KW-ANOVA, H = 13.71, df = 2, P < 0.01), this was driven wholly by an increase in egg deposition by mated females relative to virgins treated with acetone (Dunn’s, Q = 3.36, P < 0.05) or methoprene (Dunn’s, Q = 3.02, P < 0.05). There was no difference between virgin groups (Dunn’s, Q = 0.35, P > 0.05). In contrast to these results, methoprene caused a significant decrease in mating behavior in virgins relative to the acetone control (Fig. 1b; χ 2 = 6.327, df = 1, P = 0.01).
a Eggs produced during 48 h in newly mated L. hesperus females topically treated with acetone, and virgins treated with acetone or methoprene, and b percent of virgin females that mated within 24 h after treatment with acetone or methoprene. Shown are the medians, interquartile ranges, 90th and 10th percentiles (whiskers), and any outlier data points exceeding these outer bounds (Ο). Significant differences (Kruskal–Wallis ANOVA on ranks followed by Dunn’s post hoc analysis, α = 0.05) between groups are indicated by lines over the boxes. Sample sizes are given
Methoprene application did selectively impact the biogenic amine levels of virgin females at 1 h but not at 24 h post-treatment (Fig. 2). DA differed between acetone- and methoprene-treated virgins at 1 h (KW-ANOVA, H = 11.31; df = 2; P < 0.01). Acetone-treated virgins had significantly less DA than those given methoprene (Dunn’s, Q = 15.69; P < 0.05) or mated females (Dunn’s, Q = 19.70; P < 0.05), while the latter groups were comparable (Dunn’s, Q = 0.63; P > 0.05). The methoprene-induced increase was not as persistent as the mating effect; by 24 h after treatment, DA did not differ between acetone- or methoprene-treated virgins (Dunn’s; Q = 0.34; P > 0.05), while mated females still had significantly more DA than acetone (Dunn’s, Q = 4.05; P < 0.05) or methoprene-treated virgins (Dunn’s, Q = 4.03; P < 0.05). DA was much higher in mated females at 24 h than the elevated levels observed in methoprene-treated virgins at 1 h (Mann–Whitney test, T 23,23 = 683.0, P < 0.01). OA levels differed at 1 h (KW-ANOVA, H = 14.50, df = 2, P < 0.01), being significantly reduced in mated females relative to virgins receiving either acetone (Dunn’s, Q = 3.69, P < 0.05) or methoprene (Dunn’s, Q = 2.62, P < 0.05). Again, there was no difference between the two virgin groups (Dunn’s, Q = 0.96, P > 0.05). By 24 h, OA no longer differed between groups (KW-ANOVA, H = 2.32, df = 2, P = 0.31). Concentrations of the remaining amines were similar across treatments at both 1 h (5-HT: KW-ANOVA, H = 0.70, df = 2, P = 0.71; TA: KW-ANOVA, H = 4.82, df = 2, P = 0.09) and 24 h (5-HT: KW-ANOVA, H = 0.93, df = 2, P = 0.63; TA: KW-ANOVA, H = 1.39, df = 2, P = 0.50).
Biogenic amine concentrations of adult L. hesperus females that were newly mated and topically treated with acetone, virgin and treated with acetone, or virgin and treated with methoprene in acetone. Concentrations were measured 1 h or 24 h after treatment. Each sample consisted of 10 heads. Shown are the medians, interquartile ranges, 90th and 10th percentiles (whiskers), and any outlier data points exceeding these outer bounds (Ο). Significant differences (Mann–Whitney rank sum test, α = 0.05) between virgin and mated individuals for each time period are indicated by lines over the boxes. Sample sizes are given
Mating was found to influence biogenic amine levels, although the effects varied by amine and over time (Fig. 3). DA content did not differ at 5 min (Mann–Whitney test, T 19,20 = 445.0, P = 0.07); however, there was a significant increase in DA at 1 h (Mann–Whitney test, T 19,20 = 457.0, P = 0.03) and 24 h (Mann–Whitney test, T 16,16 = 190.0, P < 0.01). There were also significant changes in OA after mating, but unlike DA these occurred earlier and were short lived. OA content decreased 5 min after mating (Mann–Whitney test, T 19,20 = 461.5, P = 0.02), stayed low through the first hour (Mann–Whitney test, T 18,19 = 273.5, P = 0.04), but rose back to the levels observed in virgin females by 24 h (Mann–Whitney test, T 14,16 = 203.5, P = 0.59). Serotonin content did not differ between mated and virgin females during any of the periods sampled. There was a similar lack of response with tyramine, except that at 5 min there was a small but significant drop in mated females (Mann–Whitney test, T 18,20 = 424.5, P = 0.03).
Biogenic amine concentrations of virgin or newly mated adult L. hesperus females sampled at three different intervals corresponding to the amount of time passed since they mated or otherwise interacted with males. Each sample consisted of 10 heads. Shown are the medians, interquartile ranges, 90th and 10th percentiles (whiskers), and any outlier data points exceeding these outer bounds (Ο). Significant differences (Mann–Whitney rank sum test, α = 0.05) between virgin and mated individuals for each time period are indicated by lines over the boxes. Sample sizes are given
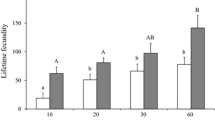
Female oviposition rate was influenced by injection of 1.0 % biogenic amine solutions (Fig. 4a; KW-ANOVA, H = 18.4, df = 4, P < 0.01), but only the OA solution caused a significant reduction in egg laying over 24 h relative to females injected with insect saline (Dunn’s, Q = 3.80, P < 0.05) or DA (Dunn’s, Q = 3.21, P < 0.05). The effect appeared to be dosage dependent (Fig. 4b; KW-ANOVA, H = 6.86, df = 2, P = 0.03), with a significant reduction relative to the saline injection in the second test in those receiving the 1.5 % (Dunn’s, Q = 3.36, P < 0.05), but not the 0.75 % (Dunn’s, Q = 0.42, P > 0.05) solution.
Eggs produced 24 h after virgin L. hesperus females were injected with a insect saline (IS), 1.0 % m/v solutions of dopamine (DA), octopamine (OA), serotonin (5-HT) or tyramine (TA); b insect saline, or 0.75 or 1.5 % OA. Shown are the medians, interquartile ranges, 90th and 10th percentiles (whiskers), and any outlier data points exceeding these outer bounds (Ο). Treatment groups with different letters were significantly different from other groups, while those with the same letter did not significantly differ (Kruskal–Wallis ANOVA on ranks followed by Dunn’s post hoc analysis, α = 0.05). Sample sizes are given
None of the biogenic amines appeared to influence mating behavior when virgin females were examined 24 h after injection. Mating probability after treatment with each of the amine solutions was comparable to that of the insect saline control (Fig. 5; χ 2 = 1.67, df = 4, P = 0.80).
Discussion
The results of this study at least partially support the hypothesis that male-derived JH drives post-mating changes to reproductive behaviors observed in L. hesperus, and that biogenic amines play an intermediary role in regulating this shift. Juvenile hormone may act as both a mediator and driver for the post-mating changes to Lygus females. For most insects, JH plays a central role in regulating reproductive traits and behaviors (Raikhel et al. 2005). When male-derived JH is transferred to females in other insects, it can cause organizational shifts that influence oogenesis, sexual receptivity and oviposition rate (Shirk et al. 1980; Bali et al. 1996; Ramaswamy et al. 1997; Park and Ramaswamy 1998; Park et al. 1998; Pszczolkowski et al. 2006). JH also has been shown to modulate levels of both DA (Gruntenko and Rauschenbach 2008) and OA (Thompson et al. 1990; Rachinsky 1994; Woodring and Hoffmann 1994; Hirashima et al. 1999; Gruntenko et al. 2007; Gruntenko and Rauschenbach 2008). Our topical application of methoprene to virgin Lygus to imitate the JH transferred in the male spermatophore (Brent and Hull 2014) did reduce the incidence of mating but failed to influence oviposition rate.
Both dopamine and octopamine are implicated in the post-mating behavioral responses. While serotonin and tyramine had no evident direct effect on egg laying and sexual receptivity, they have been shown to influence behavior by physiological preparation rather than direct induction (Blenau and Thamm 2011). Such indirect contributions would not be evident with our approach. DA rose after mating and stayed elevated for at least 24 h. Whether this is indicative of a general rise in the circulating concentration of DA or was a response localized to the source of the amines is unknown. The change in DA was not evident until an hour after mating, a delay that may indicate that the response is not a direct result of nervous stimulation caused by copulation. Instead, the shift may be the product of a cascade of post-mating organizational events, such as changing receptor expression or the activation of reproductive organs. However, methoprene did produce an increase in DA that mimicked the change observed in mated females, suggesting a possible direct link between DA and JH in this species. Although the increase in DA lasted less than 24 h with our approach, a sustained signal, such as the steady transfer of JH from the spermatophore into the female’s hemolymph, may be sufficient to promote a continuous elevation of DA. Although injection of DA had no direct effect on oviposition rate or mating behavior, the persistent increase following mating suggests DA may play a role in mediating the prolonged refractory period of mated females. For instance, the change in DA might reduce locomotory behavior, as it does in newly mated honey bee queens (Harano et al. 2008). This would diminish the likelihood of nuisance encounters with additional courters and could promote greater egg deposition. Despite the apparent lack of an effect of the DA injections, it is possible that a sustained elevation in DA might also directly suppress receptivity. Once introduced into the hemolymph, the injected amines would rapidly degrade (Linn and Roelofs 1986), so that any response requiring a continuous stimulus above a set activational threshold would be short lived. The refractory period of females lasts an average of 5 days, with some females taking much longer to remate (Brent 2010b). A short burst of DA would be insufficient to maintain this prolonged inhibition unless it was coupled with lasting organizational effects.
Despite the relatively short duration of the induced DA increase, topical methoprene was still able to cause a decline in female mating propensity. Whether this was through its influence over DA levels or by a less direct pathway remains to be determined. DA has been shown to induce changes to the production or degradation of juvenile hormone in Blattella germanica (Pastor et al. 1991), Drosophila virilis (Gruntenko et al. 2005a, b, 2007; Gruntenko and Rauschenbach 2008), Gryllus bimaculatus (Woodring and Hoffmann 1994), and Manduca sexta (Granger et al. 1996). Because JH influences vitellogenin biosynthesis and uptake in most insects, changes to the levels of the hormone could influence the rate at which eggs are produced (Raikhel et al. 2005). If L. hesperus females respond to male-derived JH with an increase in DA that causes a decline in their own JH production, the net effect could be a delay in the production of eggs to replace those being oviposited. Such a reduction in oogenesis could produce a longer term reduction in the female’s motivation to remate (Brent 2010b). JH might also induce changes in the expression of amine receptors so that even if DA levels fall after an initial surge, target organs such as the ovaries or central nervous system might still be primed to respond to a lower threshold concentration.
The response of octopamine to mating was less pronounced than that of DA, but more rapid. There was a significant decrease in the OA content of the heads within 5 min of mating, but OA reverted to pre-mating levels before 24 h had passed. This may have resulted from a decrease in production/release or an increase in degradation of OA. The concurrent decline at 5 min in TA, a precursor to OA (Lange 2009), may support a reduced production rate, but given the inconsistency of TA levels with the other changes to OA, these results are inconclusive. The relative rapidity of the changes in OA suggests they may have been directly induced by mating stimuli, possibly through the activation of stretch receptors in the female’s filled seminal depository. Because the decline in OA is short lived, the observed response is unlikely to directly regulate long-term changes to mating propensity and egg deposition behaviors. However, the decline may induce or be associated with more persistent organizational changes that influence these behaviors (reviewed in Farooqui 2012). But these changes do not appear to be directly related to JH titer; methoprene application failed to influence OA levels, and perhaps not coincidentally had no effect on oviposition rate. This may reflect a lack of effect of JH on OA expression or it may be in part due to activational differences between JH and methoprene. If the former scenario is true, then another component of the spermatophore is likely responsible for inducing post-mating changes to OA.
OA does appear to have some direct control over oviposition; virgin females injected with OA decreased egg production over a 24-h period in a dose-dependent manner. The response is similar to that seen in L. migratoria, in which OA inhibits oviduct muscle contraction (Lange and Orchard 1986; Donini and Lange 2004). However, this contrasts with the response of another mirid, Trigonotylus caelestialium, in which injected OA increased the number of eggs laid (Yamane 2013). It is possible that the high concentration of OA used in this experiment may have been sufficient to hamper oviposition by negatively impacting physiological processes other than those of interest, but this seems unlikely given that high concentrations of the other amines were unable to produce a similar behavioral effect. Differences between T. caelestialium and L. hesperus in the tissue-specific expression patterns for the OA receptor could also explain the divergent responses. Such variation in receptor expression and the role of OA in regulating behavior has been found between species (Blenau and Baumann 2001) and even within individuals of the same species at different ages or when developing under different conditions (Verlinden et al. 2010; Cunningham et al. 2014; Reim and Scheiner 2014). Collectively, the data for L. hesperus suggest that OA may suppress egg laying at higher circulating concentrations and that mating releases a female from this inhibition by lowering OA levels. Whether the OA acts directly on the ovaries or on the central nervous system remains to be determined.
References
Bali G, Raina AK, Kingan TG, Lopez JD (1996) Ovipositional behavior of newly colonized corn earworm (Lepidoptera: Noctuidae) females and evidence for an oviposition stimulating factor of male origin. Ann Entomol Soc Am 89:475–480
Blenau W, Baumann A (2001) Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48:13–38
Blenau W, Erber J (1998) Behavioural pharmacology of dopamine, serotonin and putative aminergic ligands in the mushroom bodies of the honeybee (Apis mellifera). Behav Brain Res 96:115–124
Blenau W, Thamm M (2011) Distribution of serotonin (5-HT) and its receptors in the insect brain with focus on the mushroom bodies. Lessons from Drosophila melanogaster and Apis mellifera. Arthro Struct Develop 40:381–394
Blenau W, Thamm M, Baumann A (2013) Serotonin in insects: distribution, biosynthesis, uptake, inactivation, receptors, functions, and implications for human health. In: Hall FS (ed) Serotonin: biosynthesis, regulation and health implications. NOVA Publishers, New York, pp 1–26
Bloch G, Simon T, Robinson GE, Hefetz A (2000) Brain biogenic amines and reproductive dominance in bumble bees (Bombus terrestris). J Comp Physiol A 186:261–268
Boulay R, Hooper-Bui LM, Woodring J (2001) Oviposition and oogenesis in virgin fire ant females Solenopsis invicta are associated with a high level of dopamine in the brain. Physiol Entomol 26:294–299
Bouletreau-Merle J (1973) Receptivite sexuelle et vitellogenese chez les femelles de Drosophila melanogaster: effets d’une application d’hormone juvenile et de deux analogues hormonaux. CR Acad Sci Ser D 277:2045–2048
Brent CS (2010a) Reproduction of the western tarnished plant bug, Lygus hesperus, in relation to age, gonadal activity and mating status. J Insect Physiol 56:28–34
Brent CS (2010b) Reproductive refractoriness in the western tarnished plant bug (Hemiptera: Miridae). Ann Entomol Soc Am 103:300–306
Brent CS (2010c) Stage-specific effects of population density on the development and fertility of the western tarnished plant bug. Lygus hesperus. J Insect Sci 10:49
Brent CS, Byers JA (2011) Female attractiveness modulated by a male-derived antiaphrodisiac pheromone in a plant bug. Animal Behav 82:937–943
Brent CS, Hull JJ (2014) Characterization of male-derived factors inhibiting female sexual receptivity in Lygus hesperus. J Insect Physiol 60:104–110
Brent CS, Fasnacht MP, Judd TM (2011) Post-mating enhancement of fecundity in female Lygus hesperus. Physiol Entomol 36:141–148
Byers JA, Fefer D, Levi-Zada A (2013) Sex pheromone component ratios and mating isolation among three Lygus plant bug species of North America. Naturwissenschaften 100:1115–1123
Clark J, Lange AB (2003) Octopamine modulates spermathecal muscle contractions in Locusta migratoria. J Comp Physiol A 189:105–114
Cooper WR (2012) External visibility of spermatophores as an indicator of mating status of Lygus hesperus (Hemiptera: Miridae) females. J Entomol Sci 47:285–290
Cunningham CB, Douthit MK, Moore AJ (2014) Octopaminergic gene expression and flexible social behavior in the subsocial burying beetle Nicrophorus vespilloides. Insect Mol Biol 23:391–404
Debolt JW (1982) Meridic diet for rearing successive generations of Lygus hesperus. Ann Entomol Soc Am 75:119–122
Dombroski TCD, Simoes ZLP, Bitondi MMG (2003) Dietary dopamine causes ovary activation in queenless Apis mellifera workers. Apidologie 34:281–289
Donini A, Lange AB (2004) Evidence for a possible neurotransmitter/neuromodulator role of tyramine on the locust oviducts. J Insect Physiol 50:351–361
Fahrbach SE, Mesce KA (2005) “Neuroethoendocrinology”: integration of field and laboratory studies in insect neuroendocrinology. Horm Behav 48:352–359
Farooqui T (2012) Review of octopamine in insect nervous systems. Open Access Insect Physiol. 4:1–17
Gillot C (2003) Male accessory gland secretions: modulators of female reproductive physiology and behavior. Ann Rev Entomol 48:163–184
Granger NA, Sturgis SL, Ebersohl R, Geng C, Sparks TC (1996) Dopaminergic control of corpora allata activity in the larval tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol 32:449–466
Gruntenko NE, Rauschenbach IY (2008) Interplay of JH, 20E and biogenic amines under normal and stress conditions and its effect on reproduction. J Insect Physiol 54:902–908
Gruntenko NE, Karpova EK, Adonyeva NV, Chentsova NA, Faddeeva NV, Alekseev AA, Rauschenbach IY (2005a) Juvenile hormone, 20-hydroxyecdysone and dopamine interaction in Drosophila virilis reproduction under normal and nutritional stress conditions. J Insect Physiol 51:417–425
Gruntenko NE, Karpova EK, Alekseev AA, Chentsova NA, Saprykina ZV, Bownes M, Rauschenbach IY (2005b) Effects of dopamine on juvenile hormone metabolism and fitness in Drosophila virilis. J Insect Physiol 51:959–968
Gruntenko NE, Karpova EK, Alekseev AA, Chentsova NA, Bogomolova EV, Bownes M, Rauschenbach IY (2007) Effects of octopamine on reproduction, juvenile hormone metabolism, dopamine and 20-hydroxyecdysone contents in Drosophila. Arch Insect Biochem Physiol 65:85–94
Harano K, Sasaki M, Nagao T, Sasaki K (2008) Dopamine influences locomotor activity in honeybee queens: implications for a behavioural change after mating. Physiol Entomol 33:395–399
Hirashima A, Suetsugu E, Hirokado S, Kuwano E, Taniguchi E, Eto M (1999) Effect of octopamine on the activity of juvenile-hormone esterase in the silkworm Bombyx mori and the red flour beetle Tribolium freemani. Gen Comp Endocrinol 116:373–381
Hirashima A, Matsushita M, Ohta H, Nakazono K, Kuwano E, Eto M (2006) Prevention of progeny formation in Drosophila melanogaster by 1-arylimidazole-2(3H)-thiones. Pest Biochem Physiol 85:15–20
Lange AB (2004) A neurohormonal role for serotonin in the control of locust oviducts. Arch Insect Biochem Physiol 56:179–190
Lange AB (2009) Tyramine: from octopamine precursor to neuroactive chemical in insects. Gen Comp Endocrinol 162:18–26
Lange AB, da Silva R (2006) Neural and hormonal control of muscular activity of the spermatheca in the locust, Locusta migratoria. Peptides 28:174–184
Lange AB, Orchard I (1986) Identified octopaminergic neurons modulate contractions of locust visceral muscle via adenosine 30,50-monophosphate (Cyclic AMP). Brain Res 363:340–349
Lee G, Villella A, Taylor BJ, Hall JC (2001) New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J Neurobiol 47:121–149
Linn CE, Roelofs WL (1986) Modulatory effects of octopamine and serotonin on male sensitivity and periodicity of response to sex pheromone in the cabbage looper moth, Trichoplusia ni. Arch Insect Biochem Physiol 3:161–171
Linn CE, Poole KR, Roelofs WL (1994) Stimulatory effect of octopamine on juvenile hormone biosynthesis in honey bees (Apis mellifera): physiological and immunocytochemical evidence. Comp Biochem Physiol C 108:99–106
Macmillan CS, Mercer AR (1987) An investigation of the role of dopamine in the antennal lobes of the honeybee Apis mellifera. J Comp Physiol A 160:359–366
Manning A (1966) Corpus allatum and sexual receptivity in female Drosophila melanogaster. Nature 211:1321–1322
Mercer A, Erber JJ (1983) The effects of amines on evoked potentials recorded in the mushroom bodies of the bee brain. J Comp Physiol A 151:469–476
Monastirioti M (2003) Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Develop Biol 264:38–49
Obara Y, Fukano Y, Watanabe K, Ozawa G, Sasaki K (2011) Serotonin-induced mate rejection in the female cabbage butterfly, Pieris rapae crucivora. Naturwissenschaften 98:989–993
Orchard I, Lange AB (1985) Evidence for octopaminergic modulation of an insect visceral muscle. J Neurobiol 16:171–181
Park YI, Ramaswamy SB (1998) Role of brain, ventral nerve cord, and corpora cardiaca-corpora allata complex in the reproductive behavior of female tobacco budworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am 91:329–334
Park YI, Shy S, Ramaswamy SB, Srinivasan A (1998) Mating in Heliothis virescens: transfer of juvenile hormone during copulation by male to female and stimulation of biosynthesis of endogenous juvenile hormone. Arch Insect Biochem Physiol 38:100–107
Pastor D, Piulachs MD, Cassier P, Andre M, Belles X (1991) In vivo and in vitro study of the action of dopamine on oocyte growth and juvenile hormone production in Blattella germanica. CR Acad Sci III 313:207–212
Patana R (1982) Disposable diet packet for feeding and oviposition of Lygus hesperus (Hemiptera: Miridae). J Econ Entomol 75:668–669
Pszczolkowski MA, Tuckera A, Srinivasan A, Ramaswamy SB (2006) On the functional significance of juvenile hormone in the accessory sex glands of male Heliothis virescens. J Insect Physiol 52:786–794
Rachinsky A (1994) Octopamine and serotonin influence on corpora allata activity in honey bee (Apis mellifera) larvae. J Insect Physiol 40:549–554
Rafaeli A (2004) Enhanced oviposition in the moth, Plodia interpunctella, after treatment with aminergic analogues. J Stored Prod Res 40:331339
Raikhel AS, Brown MR, Belles X (2005) Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive Molecular Insect Science, vol 3., ElsevierBoston, Massachusetts, pp 433–491
Ramaswamy SB, Shu S, Park YI, Zeng F (1997) Dynamics of juvenile hormone-mediated gonadotropism in the Lepidoptera. Arch Insect Biochem Physiol 35:539–558
Reim T, Scheiner R (2014) Division of labour in honey bees: Age- and task-related changes in the expression of octopamine receptor genes. Insect Mol Biol 23:833–841
Ringo J (1996) Sexual receptivity in insects. Annu Rev Entomol 41:473–494
Ringo J, Werczberger R, Altaratz M, Segal D (1991) Female sexual receptivity is defective in juvenile hormone-deficient mutants of the apterous gene in Drosophila melanogaster. Behav Genet 21:453–468
Ringo J, Talyn B, Brannan M (2005) Effects of precocene and low protein diet on reproductive behavior in Drosophila melanogaster (Diptera: Drosophilidae). Ann Entomol Soc Am 98:601–607
Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Ann Rev Entomol 50:447–477
Saraswati S, Fox LE, Soll DR, Wu CF (2004) Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol 58:425–441
Sasaki K, Yamasaki K, Nagao T (2007) Neuroendocrine correlates of ovarian development and egg-laying behaviors in the primitively eusocial wasp (Polistes chinensis). J Insect Physiol 53:940–949
Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J (2002) Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav Brain Res 136:545–553
Scheiner R, Baumann A, Blenau W (2006) Aminergic control and modulation of honeybee behaviour. Curr Neuropharmacol 4:259–276
Seshan KR (1976) Tissue culture medium and cockroach ringer for the cockroach Periplaneta americana. Method Cell Sci 2:319–322
Shirk PD, Bhaskaran G, Roller H (1980) The transfer of juvenile hormone from male to female during mating in the Hyalophora cecropia silkmoths. Experientia 36:682–683
Strong FE, Sheldahl JA, Hughes PR, Hussein EMK (1970) Reproductive biology of Lygus hesperus Knight. Hilgardia 40:105–147
Thompson CS, Yagi K, Chen ZF, Tobe SS (1990) The effects of octopamine on juvenile hormone biosynthesis, electrophysiology, and cAMP content of the corpora allata of the cockroach Diploptera punctata. J Comp Physiol B 160:241–249
Verlinden H, Vleugels R, Marchal E, Badisco L, Tobback J, Pflüger H-J, Blenau W, Vanden Broeck J (2010) The cloning, phylogenetic relationship and distribution pattern of two new putative GPCR-type octopamine receptors in the desert locust (Schistocerca gregaria). J Insect Physiol 56:868–875
Wheeler AG (2001) Biology of the Plant Bugs (Hemiptera: Miridae). Cornell University Press, Ithaca
Wolfner MF, Heifetz Y, Appelbaum SW (2005) Gonadal glands and their gene products. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive Molecular Insect Science, vol 1., ElsevierBoston, Massachusetts, pp 179–212
Woodring J, Hoffmann KH (1994) The effects of octopamine, dopamine and serotonin on juvenile hormone synthesis, in vitro, in the cricket, Gryllus bimaculatus. J Insect Physiol 40:797–802
Yamane T (2013) Effects of the biogenic amines on female oviposition behavior in the rice leaf bug Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Entomol News 123:161–167
Yamane T, Miyatake T (2010) Reduced female mating receptivity and activation of oviposition in two Callosobruchus species due to injection of biogenic amines. J Insect Physiol 56:271–276
Acknowledgments
We thank Dan Langhorst for technical assistance. This project was supported by Agriculture and Food Research Initiative Competitive Grant No. 2011-38422-30955 from the United States Department of Agriculture (USDA), National Institute of Food and Agriculture. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Brent, C.S., Miyasaki, K., Vuong, C. et al. Regulatory roles of biogenic amines and juvenile hormone in the reproductive behavior of the western tarnished plant bug (Lygus hesperus). J Comp Physiol B 186, 169–179 (2016). https://doi.org/10.1007/s00360-015-0953-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0953-1