Abstract
When crayfish have attained dominant status after agonistic bouts, their avoidance reaction to mechanical stimulation of the tailfan changes from a dart to a turn response. Ascending interneurones originating in the terminal ganglion receive sensory inputs from the tailfan and they affect spike activity of both uropod and abdominal postural motor neurones, which coordinates the uropod and abdominal postural movements. Despite the varying output effects of ascending interneurones, the synaptic responses of all interneurones to sensory stimulation were enhanced when they acquired a dominant state. The number of spikes increased as did a sustained membrane depolarizations. Regardless of social status, the output effects on the uropod motor neurones of all interneurones except VE-1 remained unchanged. VE-1 mainly inhibited the uropod opener motor neurones in naive animals, but tended to excite them in dominant animals. Synaptic enhancement of the sensory response of ascending interneurones was also observed in naive animals treated with bath-applied serotonin. However, subordinate animals or naive animals treated with octopamine had no noticeable effect on the synaptic response of their ascending interneurones to sensory stimulation. Thus, enhancement of the synaptic response is a specific neural event that occurs when crayfish attain social dominance and it is mediated by serotonin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agonistic bouts and the establishment of dominant-subordinate relationships are essential for maintaining social stability in intraspecies communication (Wilson 1975). A winner-loser hierarchy is formed through conflicts between conspecifics. Losers reduce their risks of severe injury or death by avoiding encounters with other conspecifics, while winners may increase their access to high-quality resources such as food, shelter and mating partners (Clutton-Brock and Parker 1995; Herberholz et al. 2007).
When animals become dominant, their motivation to engage in agonistic bouts increases (Hsu et al. 2006). Biogenic amines are known to regulate aggression levels in animals. In vertebrates, noradrenaline, dopamine, and androgen are known to promote aggressive behaviour (Haller et al. 1998; Annemoon and Miczek 2000; Oliveira et al. 2009; Fuxjager et al. 2010). Serotonin reduces aggression in fish (Winberg et al. 1992), lizards (Larson and Summers 2001) and rodents (Saudou et al, 1994; de Boer and Koolhaas 2005), or promotes aggression in birds (Shea et al. 1991; Buchanan et al. 1994), dogs (Badino et al. 2004) and rodents (Takahashi et al. 2010). Serotonin also increases aggression in invertebrates such as crustaceans and trap-jaw ants (Antonsen and Paul 1997; Edwards and Kravitz 1997; Huber et al. 1997, 2001; Huber 2005; Momohara et al. 2013, 2016; Aonuma 2020). In crickets, octopamine (the phenol analogue of noradrenaline) increases the motivation to engage in aggressive encounters (Stevenson et al. 2000; Rillich et al. 2011).
In addition to the increase in the aggression of winners, other behavioural and physiological changes are also known to occur after the establishment of social status (whether dominant or subordinate; Dyakonova and Schurmann 1999; Hofmann et al. 1999; von Holst et al. 1999; Miller et al. 2017). Herberholz et al. (2003) show that dominant crayfish spend substantially more time burrowing than subordinates. Dominance acquisition also decreases the rate of habituation of the lateral giant interneurone (LG)-mediated tailflips (Araki et al. 2013). Furthermore, when crayfish become dominant, their avoidance response to sensory stimulation of the tailfan changes from an escape-like dart response to a defence-like turn response (Fujimoto et al. 2011). During a dart response, the crayfish closes its uropods on both sides and walks forwards with an extended abdomen. During a turn response, the crayfish turns towards the source of the stimulus with flexion or extension of the abdomen in random order following asymmetrical uropod movements (Nagayama et al. 1986; Momohara et al. 2015). Momohara et al. (2015) show that the pattern of motor neurone spikes in response to sensory stimulation changes from dart-like to turn-like when animals became dominant, and that this change in response is also elicited by administration of serotonin. However, the neural basis that drives status-dependent behavioural changes remains unclear.
During the avoidance response, the same sensory stimulus triggers opposing motor output patterns. Thus, the avoidance response is a good model for investigating the plasticity of the neural circuit. The uropod movements during avoidance reaction are essentially controlled by local circuit within the terminal abdominal ganglion. Furthermore, a coordination between uropod and abdominal postural movement is indispensable. Approximately 30 ascending interneurones originating in the terminal abdominal ganglion can be identified by their gross morphology and physiology (Nagayama et al. 1993a, 1994; Nagayama and Sato 1993; Aonuma et al. 1994). They also receive direct excitatory inputs from sensory neurones. Some ascending interneurones are GABAergic and have inhibitory outputs to abdominal postural motor neurones (Aonuma and Nagayama 1999). Other ascending interneurones have excitatory connections to the non-spiking interneurones that are essential to control motor outputs of the uropod movement (Nagayama 1997). Thus, the ascending interneurones are coordinating neurones for the avoidance response by both receiving sensory inputs from the tailfan and having output effects on the uropod and abdominal postural motor neurones (Nagayama 1997). To clarify social-dependent neural switching mechanism, firstly, we assessed whether the synaptic response of intersegmental ascending interneurones changes according to social status. Their response to sensory inputs and their output effects on motor neurones were electrophysiologically compared between naive and dominant animals. We also investigated the effect of bath-applied serotonin on the synaptic response of ascending interneurones.
Materials and methods
Animals and pairing
Adult male crayfish Procambarus clarkii (Girard), 6–9 cm long from the rostrum to telson, were obtained from a commercial supplier and used for all experiments. The crayfish were kept individually in small separate opaque containers measuring 19 × 33 × 15 cm (width × length × height). The containers were filled with water to a depth of 10 cm and maintained at approximately 23 °C for 30—60 days on a 12 h light–dark cycle. The crayfish were fed equal amounts of small food pellets weekly. Animals that moulted in the week before experiments were run were excluded from the study.
Crayfish with 10–13% difference in body length were paired up to form pairs comprising a dominant and a subordinate animal. Pairings were conducted using a previously described protocol (Momohara et al. 2013). A fighting arena measuring 26 × 38 × 24 cm (width × length × height) was filled with water to a depth of 12 cm and separated into two chambers with an opaque plastic divider. One crayfish was placed on each side of the divider and allowed to acclimate for at least 10 min before the pairing. Agonistic bouts between the pairs of crayfish were observed after the divider had been removed. We considered dominance to have been established once the subordinate crayfish had retreated or tailflipped after the dominant crayfish had attacked at least three consecutive times (Sato and Nagayama 2012), since a behavioural change from approach to attack is a distinctive characteristic of dominant animals (Watanabe et al. 2016). The chasing speed of attack was considerably faster than that of approach, and individuals exhibited a raised position during the attack. After 1 h of pairing, the crayfish were re-isolated and used for physiological experiments, which were conducted on the same day.
Behavioural observation of avoidance reaction
In 18 dominant animals, a pattern of avoidance reaction elicited by a gentle mechanical stimulation to the exopodite with a fine paintbrush was examined before and after pairings with smaller opponents. Three stimulations were applied at an interval of 5–10 min, respectively.
Preparations, extracellular recording and stimulation
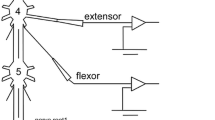
Each crayfish was decapitated quickly and its abdomen pinned in a dissecting chamber containing cooled van Harreveld’s (1936) solution. The nerve chain from the second to sixth (terminal) abdominal ganglia, with the relevant nerve roots, was isolated from the abdomen and pinned, ventral side up, in a 4 mL Sylgard-lined (Sylgard 184, Toray-Daw Corning, Tokyo, Japan) perfusion chamber containing van Harreveld’s (1936) solution (Fig. 1).
Experimental arrangement of the extracellular and intracellular recordings of neural activity and the electrical stimulation of the abdominal nervous system. The activity of the abdominal extensor motor neurones was recorded from the second nerve roots of the fourth abdominal ganglion. The activity of uropod opener motor neurones was recorded from the third nerve root motor bundles of the terminal abdominal ganglion. To stimulate the sensory afferents innervating the exopodite, a different suction electrode was placed on the second root sensory bundle. Intracellular recording was carried out from the right half of the terminal ganglion neuropils with glass microelectrodes filled with a 3% solution of Lucifer yellow CH in 1 mol l−1 lithium chloride
The spiking activity of the uropod opener motor neurones was recorded extracellularly via suction electrode from the right nerve root 3 motor bundle in the terminal abdominal ganglion at the bifurcation of the ventral rotator and abductor exopodite muscles. At least three excitatory opener motor neurones innervating the abductor exopodite ventral muscle showed tonic activity (Nagayama et al. 1984; Momohara et al. 2015). The spike activity of the slow extensor motor neurones controlling tonic movement and the posture of the abdomen was also recorded extracellularly from the right nerve root 2 motor bundle in the fourth abdominal ganglion. To stimulate the sensory neurones innervating hairs on the surface of the exopodite, another suction electrode was placed over the cut over the cut end of the right nerve root sensory bundle in the terminal abdominal ganglion. Square pulses of 0.05 ms duration at 20 Hz (= 11 shocks) were delivered to the stimulating electrode to mimic mechanical touch. Also, square pulses of 0.05 ms duration at 1 Hz (= 10 shocks) were delivered to the stimulating electrode to analyse synaptic response of interneurones quantitatively.
Intracellular recordings and staining
For intracellular recordings, the terminal abdominal ganglion was stabilised on a silver platform and treated with protease (Sigma type XIV, Sigma-Aldrich, St Louis, MO, USA) for 30 s to soften the ganglionic sheath and thereby aid penetration with an intracellular electrode. Intracellular recording was carried out from the right half of the terminal ganglion neuropils with glass microelectrodes filled with a 3% solution of Lucifer yellow CH (Sigma-Aldrich, St Louis, MO, USA) in 1 mol l−1 lithium chloride (electrode resistance range 30–80 MΩ). All recordings were stored on computer using a Power Lab (AD Instruments, USA) for subsequent offline analysis. Following the physiological investigation, Lucifer yellow was iontophoretically injected using 1–10 nA hyperpolarising current pulses of 500 ms at 1 Hz for 10–15 min for staining. The terminal abdominal ganglion was dissected, fixed in 10% formalin, dehydrated in an ascending alcohol series and cleared with methyl salicylate before being imaged on a BX51 (Olympus, Japan) fluorescence microscope fitted with a CCD camera AR-MC200HD (Arms System, Japan). Interneurones were identified according to published morphological and physiological criteria (Nagayama et al. 1993a; Aonuma et al. 1994). Interneurones recorded on multiple occasions were analysed. Data were based on recording from 34 naive, 31 dominant and 4 subordinate animals. Our previous behavioural and electrophysiological studies (Fujimoto et al. 2011; Momohara et al. 2015) showed that motor patterns of both the uropod and abdominal postural motor neurones were not changed drastically when the animals became subordinate, we focused on this study to compare the response pattern of ascending interneurones to the sensory stimulation between naive and dominant animals.
Drug applications
Serotonin creatinine sulphate complex (5HT) and ( ±)-octopamine hydrochloride (OA) were obtained from Sigma-Aldrich (St Louis, MO, USA) and dissolved in physiological saline to make a 50 μM concentration prior to each experiment. Drug concentration was based on Momohara et al. (2013, 2015). After physiological identification of interneurones in normal saline, the recording chamber was perfused for 20 min with serotonin or octopamine and the response of the interneurones to a sensory stimulation of the same intensity was tested again. The bath solution was then washed out with normal saline for 60 min to check for response recovery. Data were collected from 30 naive animals treated with serotonin and from five naive animals treated with octopamine.
Statistical analysis
Statistical analyses were carried out using SigmaPlot v13. Differences in the response patterns of ascending interneurones and motor neurones were analysed using the binomial test, Mann–Whitney rank-sum test, Fisher’s exact test, and Wilcoxon signed-rank test. In this study, we characterized the responses of 34 ascending interneurones (18 VE-1, 3 CI-1, 3 RO-2, 3 RC-3, 4 CA-1 and 3 NE-4) in the naive animals and 31 interneurones (15 VE-1, 4 CI-1, 4 RO-2, 3 RC-3 4 CA-1 and 1 NE-4) in the dominant animals. Except for VE-1, we did not perform statistic analyses for each identified neurone since the sample number was rather small.
Results
Neuronal activity patterns during turn responses
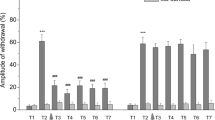
Behavioural observations showed that naive crayfish exhibited a dart response to 90% of the stimulations applied to the tailfan. The same mechanical stimulation predominantly elicited a turn response in crayfish that had acquired dominant status while pairing with another crayfish. The uropod on the stimulated side opened and the crayfish turned towards the stimulus source with its abdomen either extended or flexed. Of 18 dominant crayfish, eight extended their abdomens (44.4%) and 10 (55.6%) flexed them. There was no significant difference between these two groups (P = 0.8145; binomial test). The turn-like pattern of both the opener and the extensor motor neurone activity was elicited in the isolated nerve chain preparations (Fig. 2). Electrical stimulation of sensory afferents mainly (in 27 of 32 preparations) elicited an inhibitory response in the opener motor neurone in naive animals, whereas an excitatory response was elicited in 24 out of 29 preparations from the dominant animals. In both cases, the occurrence rate of the two types of response differed significantly (P < 0.0001; binomial test). The response pattern of the opener motor neurones differed significantly between naive and dominant animals (P < 0.0001; Fisher’s exact test). In naive animals, most (26 of 28 preparations) of the extensor motor neurones (Fig. 2b) produced an excitatory response to sensory stimulation. While 57% of the dominant animals (16 of 28 preparations) exhibited an excitatory response, the remaining 43% (12 of 28 preparations) displayed an inhibitory response. The rates of excitatory and inhibitory responses differed significantly in naive animals (P < 0.0001; binomial test) but not in dominant animals (P = 0.5716; binomial test). The response pattern of the extensor motor neurones also differed significantly between naive and dominant animals (P = 0.0043; Fisher’s exact test).
Response of motor neurones to sensory stimulation in naive and dominant (dom) crayfish. a Uropod opener motor neurones (op mns). b Abdominal extensor motor neurones (ext mns). The response patterns were classified into excitatory (black) and inhibitory (grey). Asterisks indicate significant differences in excitatory and inhibitory response patterns (*** P < 0.0001; binomial test). Hash symbols indicate significant differences in the response patterns of naive and dominant animals (## P < 0.01, ### P < 0.001; Fisher’s exact test)
Status-dependent modulation of the response of ascending interneurones to sensory stimulation
The ascending interneurone RC-3 was identified by its cell body position and the projection pattern of its main branches (Fig. 3d; also see Nagayama et al. 1993a). RC-3 had reciprocal output effects on opener and closer uropod motor neurones and similar excitatory output effects on extensor and flexor motor neurones (Aonuma et al. 1994). When the sensory stimulation (11 shocks at 20 Hz) was applied to preparations from naive crayfish, the number of RC-3 tonic spikes increased, with inhibition of opener motor neurones or excitation of extensor motor neurones (Fig. 3a). In preparations from dominant animals, the sensory stimulation increased the spike discharge of both the extensor and opener motor neurones. In comparison to the RC-3 in naive animals, the number of RC-3 tonic spikes in dominant animals was further increased, with a sustained membrane depolarization (Fig. 3b). This enhancement of the synaptic response of RC-3 in dominant animals was also evident when a single stimulus was repeated at 1 Hz (Fig. 3c). RC-3 in naive animals responded with 2.4 spikes and a membrane depolarization of approximately 7 mV (7.0 ± 0.8 mV [mean ± standard error of the mean, SEM]; n = 3 naive animal preparations), while RC-3 in dominant animals responded with 3.9 spikes per stimulus and a depolarization of more than 10 mV (11.6 ± 1.3 mV; n = 3 dominant animal preparations). Furthermore, the membrane depolarization in the RC-3 from dominant animals was substantially prolonged (n = 3).
a, b Responses of RC-3 in naive (a) and dominant (dom) (b) crayfish to sensory stimulation at 20 Hz (11 shocks) was recorded intracellularly. Spiking activity of the extensor (ext; top) and opener (op; middle) motor neurones was monitored extracellularly. Arrowheads indicate start and end of stimuli. c Superimposed sweeps of RC-3 responses in naive (top) and dominant (bottom) crayfish to sensory stimulation at 1 Hz (10 shocks). Arrows indicated measuring points of maximum depolarization. d Ventral view of Lucifer yellow-stained RC-3 (anterior side uppermost)
RO-2 was identified from three naive animals and four dominant animals (Fig. 4c). RO-2 increases the spiking activity of opener and flexor motor neurones and decreases that of closer and extensor motor neurones (Aonuma et al. 1994). One of the characteristic sensory responses of RO-2 is anti-facilitation in response to repetitive stimulation (Nagayama et al. 1993a). In naive animals, RO-2 only responded to the first stimulus with a spike, and no further spikes could be elicited, although small-amplitude excitatory postsynaptic potentials with sustained membrane hyperpolarization were detected in response to subsequent stimuli (Fig. 4a). In dominant animals, spikes were frequently elicited by the later stimuli (Fig. 4b) suggesting that the synaptic response of RO-2 is enhanced when a dominant status is attained.
Response of RO-2 and CI-1 to sensory stimulation. a, b Response of RO-2 in naive (a) and dominant (dom) (b) crayfish to sensory stimulation at 20 Hz (11 shocks). c, d Ventral view of Lucifer yellow-stained RO-2 (c) and CI-1 (d) (anterior side uppermost). e, f Superimposed sweeps of CI-1 responses in naive (e) and dominant (f) crayfish to sensory stimulation at 1 Hz (10 shocks). g, h Depolarising current (2 nA) injected into CI-1 in naive (g) and dominant (h) crayfish. Arrowheads indicate the start and end of stimuli; ext, extensor motor neurones; op, opener motor neurones
The sensory response of CI-1 was also enhanced in dominant animals (Fig. 4d). CI-1 was identified in three naive and four dominant animals and analysed. Although one or two spikes were elicited by individual stimuli in CI-1 taken from naive animals (Fig. 4e), 6–8 spikes were elicited in those from dominant animals (Fig. 4f). CI-1 is known to have inhibitory output effects on opener and closer motor neurones and excitatory output effects on extensor and flexor motor neurones (Aonuma et al. 1994). These output effects are consistent with those of the dominant crayfish. In both naive (Fig. 4g) and dominant (Fig. 4h) animals, a depolarising current injected into CI-1 caused an increase in the spike discharge of the extensor motor neurones and a decrease in the spike discharge of the opener motor neurones. Regardless of whether the animals had attained social dominance or not, the output effects of all ascending interneurones examined in this study were consistent, with the exception of VE-1.
VE-1 (Fig. 5a) was the most accessible interneurone in this study and we identified 18 in the naive animal preparations and 15 in the dominant animal preparations. The output effects of VE-1 on the abdominal postural motor neurones consistently excited extensor and inhibited flexor motor neurones, while its output effects on the uropod motor neurones were variable (Nagayama et al. 1993a; Aonuma et al. 1994). Since VE-1 simultaneously received a weak inhibitory input with an excitatory sensory input (Nagayama et al. 1993a), in naive animals, multiple spikes were usually elicited at the first electrical pulse, but only one spike was elicited by subsequent stimuli at 20 Hz (Fig. 5b). In contrast, in dominant animals, each stimulus consistently elicited multiple spikes, accompanied by a sustained membrane depolarization (Fig. 5c). The mean number of spikes elicited by 10 repeated stimuli at 1 Hz differed significantly (P = 0.028; Mann–Whitney rank-sum test) between preparations from naive (1.5 spikes) and dominant animals (2.0 spikes; Fig. 5d). Distinct inhibitory postsynaptic potentials were identified in VE-1 s from 78% of the naive animals (14 of 18 animals) but only 40% of the dominant animals (6 of 15 animals). In this study, 11 VE-1 s (61.1%) from naive animals had inhibitory output effects on opener motor neurones, while four (22.2%) had excitatory output effects (Fig. 5e). When animals had attained dominance, the excitatory output effects on opener motor neurones increased (Fig. 5e). Approximately half the VE-1 s (n = 7) had excitatory output effects, while four (26.7%) had inhibitory output effects.
Response of VE-1 to sensory stimulation. a Ventral view of Lucifer yellow-stained VE-1 (anterior side uppermost). b, c Response of VE-1 in naive (b) and dominant (dom) (c) crayfish to sensory stimulation at 20 Hz (11 shocks). Arrowheads indicate the start and end of stimuli; op, opener motor neurones. d Number of spikes elicited by a single electrical shock. Solid black lines in the boxes represent the medians, box lengths indicate interquartile ranges, whiskers indicate the tenth and ninetieth percentiles, and the dot is an outlier. The asterisk indicates the significance of the difference in number of spikes (*P < 0.05; Mann–Whitney rank-sum test). e Output effects of VE-1 on opener motor neurones in naive (black) and dominant (grey) crayfish. The response patterns were classified as excitatory, inhibitory, or no effect
In total ascending interneurones, the mean number of spikes elicited by 1 Hz sensory stimulation of 34 naive animals was 1.0 ± 0.06 while that of 31 dominant animals was 1.4 ± 0.09 that was statistically significantly different (P = 0.002; Mann–Whitney rank-sum test). In contrast to dominant animals, we did not observe significant changes in the responses of ascending interneurones to sensory stimulation in subordinate animals. For example, the response of VE-1 to sensory stimulation in subordinate animals at 20 Hz was similar to naive animals, and no strong depression or enlargement of VE-1 activity was observed (not shown). The mean number of spikes of VE-1 per stimulus elicited by 10 repeated stimulations at 1 Hz was similar in subordinate (1.47 ± 0.2, n = 4) and naive animals (1.5 ± 0.1, n = 15).
Effects of serotonin on the sensory responses of ascending interneurones
The pattern of the responses of opener motor neurones to sensory stimulation was also modulated by bath-applied serotonin in the naive animals (Fig. 6). The spike discharge of the opener motor neurones in response to sensory stimulation decreased prior to the serotonin bath treatment but increased 15 min after the treatment. This reversal of the activity pattern of the motor neurones partly returned to control levels approximately 60 min after washing with normal saline. In total, the opener motor neurones in 23 of 32 naive animals induced an inhibitory response before serotonin treatment and 20 of them changed to an excitatory response following serotonin treatment. The responses before and after serotonin treatment differed significantly (P < 0.001; binomial test).
Effect of bath-applied serotonin on the response of opener motor neurones to sensory stimulation in the naive animal. Initially, the sensory response of the opener motor neurones was characterised in normal saline, then the recording chamber was perfused for 20 min with a serotonin-containing saline (5HT), and finally, the bath solution was washed out with normal saline for 60 min. Arrowheads indicate the start and end of stimuli; op opener motor neurones
In total, 30 ascending interneurones were identified before and after serotonin treatment in the naive animals. Their resting potentials before and after serotonin treatment were − 60.2 ± 2.1 mV and − 63.4 ± 2.4 mV, respectively. This small change of resting potential was similar to that reported by Nagayama (2002) and was not statistically significantly different (P = 0.232; Mann–Whitney rank-sum test). Serotonin also enhanced the synaptic response of the ascending interneurones to sensory stimulation. For example, the spike discharge of the RC-3 that was elicited by sensory stimulation at 20 Hz increased, accompanied by a prolonged membrane depolarization (Fig. 7a) as shown for the RC-3 of dominant animals (Fig. 3). During serotonin treatment, the number of spikes elicited by a single electrical shock increased 2.2-fold and the period of the falling phase was 5.6 times longer than before serotonin treatment (Fig. 7b). Both of these differences were significant (P < 0.001; Wilcoxon signed-rank test). CI-1, CI-2, RO-2 and NE-1 also showed an enhanced response to sensory stimulation as a result of the serotonin treatment (not shown). Furthermore, serotonin-mediated synaptic enhancement in response to sensory stimulation was also observed in the spiking local interneurone sp-pos2 (Nagayama et al. 1993b). Spikes were consistently followed by individual pulses of sensory stimulation at 20 Hz as a result of serotonin treatment, although sp-pos2 failed to elicit a spike from second pulses of the stimulation before serotonin treatment (Fig. 7c).
The effect of serotonin (5HT, a serotonin–creatinine sulphate complex) on the response of ascending RC-3 and spiking local interneurone sp-pos2 to sensory stimulation in the naive animals. a Response of RC-3 to sensory stimulation at 20 Hz (11 shocks) before and after serotonin treatment. b Superimposed sweeps of the RC-3 responses to sensory stimulation at 1 Hz (10 shocks) before and after serotonin treatment. c Response of sp-pos2 to sensory stimulation at 20 Hz (11 shocks) before and after serotonin treatment. Arrowheads indicate the start and end of stimuli
The effect of serotonin on VE-1 neurone was characterised in six animals (Fig. 8). The synaptic response of VE-1 to sensory stimulation was also enhanced by serotonin treatment. After serotonin treatment, the number of spikes elicited by each electrical shock (10 shocks at 1 Hz) increased and the subsequent membrane hyperpolarizations frequently became indistinguishable from each other (Fig. 8a). Although a depolarising current injected into a VE-1 before serotonin treatment inhibited the spike activity of the opener motor neurones, the same depolarising current administered 15 min after the bath-applied serotonin increased it (Fig. 8b). In five out of six animals, VE-1 had an inhibitory output effect on the opener motor neurones before serotonin treatment, and four of them had the reverse output effect following the serotonin treatment (Fig. 8c).
The effect of serotonin on the input and output of VE-1 in the naive animal. a Superimposed sweeps of VE-1 responses to sensory stimulation at 1 Hz (10 shocks) before and after serotonin treatment. b Depolarising current of 12 nA injected into VE-1 before and after serotonin treatment. c Output effects of VE-1 on opener motor neurones before and after serotonin treatment. The response patterns were classified into inhibitory (black) and excitatory (grey). Arrowheads indicate the start of stimuli
Octopamine treatment
Octopamine is known to have the opposite effect to serotonin (Momohara et al. 2013). Treatment with 50 μM octopamine, however, did not significantly affect the synaptic response of VE-1 to sensory stimulation (Fig. 9). Two electrical shocks failed to elicit spikes in VE-1 under octopamine treatment, although this might be due to the membrane potential shifting to hyperpolarization. In the four other ascending interneurones, the mean number of spikes per stimulus at 1 Hz was similar before and after octopamine treatment: it was only 1.02 ± 0.5 times greater after treatment.
Discussion
Synaptic enhancement of ascending interneurones
The synaptic response to sensory stimulation in specific ascending interneurones was enhanced once the crayfish had attained social dominance. Furthermore, we show that serotonin is responsible for this synaptic enhancement. On the other hand, present results that subordinate status and octopamine had no observable effect on the sensory response of ascending interneurones are consistent with our previous finding (Momohara et al. 2015). Thus, synaptic enhancement may be specific to dominant crayfish. Social-dependent switch of behavioural response is also known in cricket (Abe et al. 2018) and crayfish (Song et al. 2006). When crayfish become subordinate following agonistic bouts, their response to mechanical stimulation of the abdominal segment changes from orienting to avoidance reaction (Song et al. 2006). Bacque-Cazenave et al. (2019) have revealed the role of identified motor neurones that regulate postural changes and leg movements and their modulation by social status. Serotonin is thought to be responsible for this neural circuit reconfiguration (Cattaert et al. 2010; Issa et al. 2012). In trap-jaw ant, serotonin is known to change behavioural responses to a tactile stimulus onto the abdomen from dart escape to aggressive turn (Aonuma 2020).
When crayfish attained dominant status, the sensory inputs of ascending interneurones were enhanced, but the output effects on the uropod and abdominal postural motor neurones were unchanged for all ascending interneurones with the exception of VE-1. Furthermore, not only the interneurones that exhibited increased motor activity that opened the exopodite but also those with the opposite effect of closing the exopodite enhanced their synaptic responses. These observations suggested that certain changes in membrane properties did not occur in specific ascending interneurones, but that some changes in transmitter release occurred at the level of the sensory neurones when animals attained dominance.
Role of serotonin
Injection of serotonin elicits a dominant-like posture in both lobsters and crayfish (Livingstone et al. 1980; Kravitz 1988) and an aggressive display in squat lobsters (Antonsen and Paul 1997). Bath-applied serotonin reversed the activity pattern of the uropod motor neurones from closing to opening the exopodite (Momohara et al. 2015) and induced synaptic enhancement of ascending interneurones. Furthermore, the level of serotonin in the central nervous system is known to increase in dominant crayfish (Bacque-Cazenave et al. 2017; Momohara et al. 2018). Thus, when a crayfish attains dominant status, an increase in serotonin level might act to maintain a winner's motivation. At the same time, this serotonin would enhance synaptic input of the ascending interneurones from sensory neurones. Serotonin itself, however, has no significant direct effect on the resting potential or tonic spike activity of the ascending interneurones (Nagayama 2002). These results suggest that the enhancement of synaptic response of the ascending interneurones to sensory stimulation must be the result of changes at the level of the sensory neurones. Serotonin is known to increase the number of spikes in the sensory neurones of leech mechanoreceptors (Gascoigne and McVean 1991) and the crayfish leg chordotonal organ (El Manira et al. 1991). Serotonin also modulates the synaptic efficacy of sensory neurones in Aplysia and vertebrates. Activity-dependent facilitation is known to occur in Aplysia sensory neurones. Serotonin reduces the serotonin-sensitive K+ current of the sensory neurones to increase the amount of transmitter (Montarolo et al. 1986; Scholz and Byrne 1987). The facilitatory effects of serotonin are mediated by at least two second-messenger-activated protein kinases; A (PKA) and C (PKC) systems (Byrne and Kandel 1996). In the rabbit spinal cord, serotonin induces activation of the sensory synapses via PKC (Ping et al. 1999).
Furthermore, in the crayfish neuromuscular junction, serotonin is also known to facilitate the enhancement of transmitter release. Serotonin-induced facilitation is mediated by an IP3/PKC pathway and a cAMP/PKA pathway (Dixon and Atwood 1989a, b, c), and serotonin increases the total quantity of transmitter released without increasing the presynaptic calcium influx (Delaney et al. 1991; Vyshedskiy et al. 1998). Currently there is no evidence that serotonin affects sensory neurones directly. Further pharmacological and physiological investigations, particularly for identifying the role of second-messenger pathways in sensory neurones, are necessary.
Role of ascending interneurones
Sensory stimulation evokes primarily extension patterns of abdominal posture to walk forward in a dart response, while both flexing and extending patterns of abdominal posture are elicited equally in a turn response (Momohara et al. 2015). Abdominal posture is controlled and coordinated by ascending and descending projecting interneurones (Evoy and Kennedy 1967; Kennedy et al. 1967; Larimer and Jellies 1983; Larimer and Moore 1984; Jellies and Larimer 1986). Administration of serotonin causes excitation of the flexor motor neurones and inhibition of the antagonistic extensor motor neurones by acting on flexion-evoking command fibres (Harris-Warrick and Kravitz 1984; Harris-Warrick 1985; Ma et al. 1992). In this study, all ascending interneurones showed an enhanced response to sensory stimulation, and their trains of spikes increased. Some ascending interneurones had similar excitatory or inhibitory output effects on both the extensor and flexor motor neurones, while others had reciprocal output effects that resulted in the extending or flexing of the abdomen (Aonuma et al. 1994). During a turn response, the dominant crayfish tended to flex their abdomens when placed in the corner of the tank but extend them when in the centre of the tank (Nagayama personal observations). Thus, abdominal movement may be influenced by the combined of activity of many ascending and descending interneurones (Wiersma and Hughes 1961; Ma et al. 1992; Aonuma et al. 1994; Namba et al. 1994, 1997), and certain visual and tactile cues may be central to the choice of abdomen posture.
Both the ascending interneurones that excited the spiking activity of the opener motor neurones and those that inhibited the opener motor neurones showed synaptic enhancement. These observations suggest that the reversal of the uropod motor activity pattern from closing to opening did not usually result in a spike enlargement in the ascending interneurones when animals attained dominant status. Ascending interneurones have no direct output connections to the uropod motor neurones, but some interneurones have excitatory connections to the non-spiking interneurones (Nagayama 1997). Opposing and parallel pathways through two groups of non-spiking interneurones are essential for controlling uropod movement (Nagayama and Hisada 1987; Nagayama et al. 1994; Namba et al. 1994, 1997). Synaptic enhancement of ascending interneurones would influence the balance of activity between the two groups of non-spiking interneurones to switch motor output from dart to turn response. Further identification of the responses of non-spiking interneurones to sensory stimulation after dominant status is attained could provide deeper insights into the status-dependent neural switching mechanism.
Abbreviations
- 5HT:
-
Serotonin
- dom:
-
Dominant
- ext:
-
Extensor
- MN:
-
Motor neurone
- OA:
-
Octopamine
- op:
-
Opener
- sub:
-
Subordinate
References
Abe T, Fujiyama N, Tomimatsu H, Nagayama T (2018) Age-dependent and social status-dependent behavioural plasticity of the cricket Gryllus bimaculatus. Anim Behav 14:1–7
Antonsen BL, Paul DH (1997) Serotonin and octopamine elicit stereotypical agonistic behaviors in the squat lobster Munida quadrispina (Anomura, Galatheidae). J Comp Physiol A 181:501–510
Aonuma H (2020) Serotonergic control in initiating defensive responses to unexpected tactile stimuli in the trap-jaw ant Odontomachus kuroiwae. J Exp Biol 223:jeb228874
Aonuma H, Nagayama T (1999) GABAergic and non-GABAergic spiking interneurons of local and intersegmental group in the crayfish terminal abdominal hanglion. J Comp Neurol 410:677–688
Aonuma H, Nagayama T, Hisada M (1994) Output effect of identified ascending interneurones upon the abdominal postural system in the crayfish Procambarus clarkii (Girard). Zool Sci 11:191–202
Araki M, Hasegawa T, Komatsuda S, Nagayama T (2013) Social status-dependent modulation of LG-flip habituation in the crayfish. J Exp Biol 216:681–686
Bacque-Cazenave J, Cattaert D, Delbecque J-P, Fossat P (2017) Social harassment induces anxiety-like behaviour in crayfish. Sci Rep 7:39935
Bacque-Cazenave J, Fossat P, Issa FA, Edwards DH, Delbecque JP, Cattaert D (2019) Duality of 5-HT effects on crayfish motoneurons. Front Physiol 10:1280
Badino P, Odore R, Osella MC, Bergamasco L, Francone P, Girardi C, Re G (2004) Modifications of serotonergic and adrenergic receptor concentrations in the brain of aggressive Canis familiaris. Comp Biochem Physiol Part A 139:343–350
de Boer SF, Koolhaas JM (2005) 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526:125–139
Buchanan CP, Shrier EM, Hill WL (1994) Time-dependent effects of PCPA on social aggression in chicks. Pharm Biochem Behav 49:483–488
Byrne JH, Kandel ER (1996) Presynaptic facilitation revisited: state and time dependence. J Neurosci 16:425–435
Cattaert D, Delbecque JP, Edwards DH, Issa FA (2010) Social interactions determine postural network sensitivity of 5-HT. J Neurosci 30:5603–5616
Clutton-Brock TH, Parker GA (1995) Punishment in animal societies. Nature 373:209–216
Delaney K, Tank DW, Zucker RS (1991) Presynaptic calcium and serotonin-mediated enhancement of transmitter release at crayfish neuromuscular junction. J Neurosci 11:2631–2643
Dixon D, Atwood HL (1989a) Phosphatidylinositol system’s role in serotonin-induced facilitation at the crayfish neuromuscular junction. J Neurophysiol 62:239–246
Dixon D, Atwood HL (1989b) Conjoint action of phosphatidylinositol and adenylate cyclase systems in serotonin-induced facilitation at the crayfish neuromuscular junction. J Neurophysiol 62:1251–1259
Dixon D, Atwood HL (1989c) Adenylate cyclase system is essential for long-term facilitation at the crayfish neuromuscular junction. J Neurosci 9:4246–4252
Dyakonova VE, Schürmann FW (1999) Effect of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften 86:435–437
Edwards DH, Kravitz EA (1997) Serotonin, social status and aggression. Curr Opin Neurobiol 7:812–819
Evoy WH, Kennedy D (1967) The central nervous organization underlying control of antagonistic muscles in the crayfish. I. Types of command fibers. J Exp Zool 165:223–238
Fujimoto S, Hirata B, Nagayama T (2011) Dominance hierarchy-dependent behavioural plasticity of crayfish avoidance reactions. J Exp Biol 214:2718–2723
Fuxjager MJ, Mast G, Becker EA, Marler CA (2009) The ‘home advantage’ is necessary for a full winner effect and changes in post-encounter testosterone. Horm Behav 56:214–219
Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA (2010) Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA 107:12393–12398
Gascoigne L, McVean A (1991) Nwueomodulatory effects of acetylcholine and serotonin on the sensitivity of leech mechanoreceptors. Comp Biochem Physiol C 99:369–374
Haller J, Millar S, Kruk MR (1998) Mineralocorticoid receptor blockade inhibits aggressive behaviour in male rats. Stress 2:201–207
Harris-Warrick RM (1985) Amine modulation of extension command element-evoked motor activity in the lobster abdomen. J Comp Physiol A 156:875–884
Harris-Warrick RM, Kravitz EA (1984) Cellular mechanisms for modulation of posture by octopamine and serotonin in the lobster. J Neurosci 4:1976–1993
Herberholz J, Mccurdy C, Edwards DH (2007) Direct benefits of social dominance in juvenile crayfish. Biol Bull 213:21–27
Herberholz J, Sen MM, Edwards DH (2003) Parallel changes in agonistic and non-agonistic behaviors during dominance hierarchy formation in crayfish. J Comp Physiol A 189:321–325
Hofmann HA, Benson ME, Fernald RD (1999) Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci USA 96:14171–14176
Hsu Y, Earley RI, Wolf LL (2006) Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camn Philos Soc 81:33–74
Huber R (2005) Amines and motivated behaviors: a simpler systems approach to complex behavioral phenomena. J Comp Physiol A 191:231–239
Huber R, Pankspp JB, Yue Z, Delago A, Moore P (2001) Dynamic interactions of behavior and amine neurochemistry in acquisition and maintenance of social rank in crayfish. Brain Behav Evol 57:271–282
Huber R, Smith K, Delago A, Isaksson K, Kravitz EA (1997) Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci USA 94:5939–5942
Issa FA, Drummond J, Cattaert D, Edwards DH (2012) Neural circuit reconfiguration by social status. J Neurosci 32:5638–5645
Jellies J, Larimer JL (1986) Activity of crayfish abdominal-positioning interneurons during spontaneous and sensory-evoked movements. J Exp Biol 120:173–188
Kennedy D, Evoy WH, Dane B, Wanawalt JT (1967) The central nervous organization underlying control of antagonistic muscles in the crayfish. I. Coding of position by command fibers. J Exp Zool 165:239–248
Kravitz EA (1988) Hormonal control of behavior: amines and the biasing of behavioral output in lobster. Science 241:1775–1781
Larimer JL, Jellies J (1983) The organization of flexion-evoking interneurons in the abdominal nerve cord of the crayfish, Procambarus clarkii. J Exp Zool 226:341–351
Larimer JL, Moore D (1984) Abdominal positioning interneurons in crayfish : projections to and synaptic activation by higher CNS centers. J Exp Zool 230:1–10
Larson ET, Summers CH (2001) Serotonin reverses dominant social status. Behav Brain Res 121:95–102
Livingstone MS, Harris-Warrick RM, Kravitz EA (1980) Serotonin and octopamine produce opposite postures in lobsters. Science 208:76–79
Ma PM, Beltz BS, Kravitz EA (1992) Serotonin-containing neurons in lobsters: their role as gain-setters in postural control mechanisms. J Neurophysiol 68:36–54
El Manira A, Rossi-Dur C, Clarac F (1991) Serotonin and proctolin modulate the response of a stretch receptor in crayfish. Brain Res 541:157–162
Miller TN, Clements K, Ahn S, Park C, Ji EH, Issa FA (2017) Social status-dependent shift in neural circuit activation affects decision making. J Neurosci 37:2137–2148
Momohara Y, Aonuma H, Nagayama T (2018) Tyraminergic modulation of agonistic outcomes in crayfish. J Comp Physiol A 204:465–473
Momohara Y, Kanai A, Nagayama T (2013) Aminergic control of social status in crayfish agonistic encounters. PLoS ONE 8:e74489
Momohara Y, Minami H, Kanai A, Nagayama T (2016) Role of cAMP signalling in winner and loser effects in crayfish agonistic encounters. Eur J Neurosci 44:1886–1895
Momohara Y, Yoshida M, Nagayama T (2015) Serotonergic modulation of social status-dependent behavioural plasticity of the crayfish avoidance reaction. J Comp Physiol A 201:1063–1074
Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S (1986) A critical period for macromolecular systhesis in long-term heterosynaptic facilitation in Aplysia. Science 234:1249–1254
Nagayama T (1997) Organization of exteroceptive inputs onto nonspiking local interneurones in the crayfish terminal abdominal ganglion. J Exp Zool 279:29–42
Nagayama T (2002) Serotonergic modulation of nonspiking local interneurones in the terminal abdominal ganglion of the crayfish. J Exp Biol 205:3067–3076
Nagayama T, Hisada M (1987) Opposing parallel connections through crayfish local nonspiking interneurons. J Comp Neurol 257:347–358
Nagayama T, Isogai Y, Namba H (1993b) Physiology and morphology of spiking local interneurones in the terminal abdominal ganglion of the crayfish. J Comp Neurol 337:584–599
Nagayama T, Isogai Y, Sato M, Hisada M (1993a) Intersegmentassl ascending interneurones controlling uropod movements of the crayfish Procambarus clarkii. J Comp Neurol 332:155–174
Nagayama T, Namba H, Aonuma H (1994) Morphological and physiological bases of crayfish local circuit neurones. Histol Histopath 9:791–805
Nagayama T, Sato M (1993) The organization of exteroceptive information from the uropod to ascending interneurones of the crayfish. J Comp Physiol 172:281–294
Nagayama T, Takahata M, Hisada M (1984) Functional characteristics of local non-spiking interneurons as the pre-motor elements in crayfish. J Comp Physiol A 154:499–510
Nagayama T, Takahata M, Hisada M (1986) Behavioural transition of crayfish avoidance reaction in response to uropod stimulation. Exp Biol 46:75–82
Namba H, Nagayama T, Hisada M (1994) Descending control of nonspiking local interneurons in the terminal abdominal ganglion of the crayfish. J Neurophysiol 72:235–247
Namba H, Nagayama T, Takahata M (1997) Non-spiking local interneurones mediate abdominal extension related descending control of uropod motor neurones in the crayfish terminal abdominal ganglion. J Comp Physiol A 180:463–472
Oliveira RF, Silva A, Canario AVM (2009) Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc Ray Soc Lond B Bio 276:2249–2256
Ping P, Zhang J, Huang S, Cao X, Tang X-L, Li RCX, Zheng Y-T, Qiu Y, Clerk A, Sugden P, Han J, Bolli R (1999) PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Physiol 276:H1466–H1481
Rillich J, Schlidberger K, Stevenson PA (2011) Octopamine and occupancy: an aminergic mechanism for intruder-resident aggression in crickets. Proc Biol Sci 278:1873–1880
Sato D, Nagayama T (2012) Development of agonistic encounters in dominance hierarchy formation in juvenile crayfish. J Exp Biol 215:1210–1217
Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R (1994) Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265:1875–1878
Scholz KP, Byrne JH (1987) Long-term sensitization in Aplysia: biophysical correlates in tail sensory neurons. Science 235:685–687
Shea MM, Douglass LW, Mench JA (1991) The interaction of dominance status and supplemental tryptophan on aggression in Gallus domesticus males. Pharm Biochem Behav 38:567–591
Song C-K, Herberholz J, Edwards DH (2006) The effects of social experience on the behavioral response to unexpected touch in crayfish. J Exp Biol 209:1355–1363
Stevenson PA, Hofmann HA, Schoch K, Schildberger K (2000) The fight and flight responses of crickets depleted of biogenic amines. J Neurobiol 43:107–120
Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA (2010) GABAB receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci 30:11771–11780
van Erp AMM, Miczek KA (2000) Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci 20:9320–9325
van Harreveld A (1936) A physiological solution for freshwater crustaceans. Proc Soc Exp Biol Med 34:428–432
von Holst D, Hutzelmeyer H, Kaetzke P, Khaschei M, Schonheiter R (1999) Social rank, stress, fitness, and life expectancy in wild rabbits. Naturwissenschaften 86:388–393
Vyshedskiy A, Delaney KR, Lin J-W (1998) Neuromodulator enhance transmitter release by two separate mechanisms at the inhibitor of crayfish opener muscle. J Neurosci 18:5160–5169
Watanabe S, Momohara Y, Minami H, Nagayama T (2016) Two types of orienting behaviour during agonistic encounters in the crayfish Procambarus clarkii (Decapoda: Cambaridae). J Crust Biol 36:147–153
Wiersma CAG, Hughes GM (1961) On the functional anatomy of neuronal units in the abdominal cord of the crayfish, Procambarus clarkii (Girard). J COmp Neurol 116:209–228
Wilson EO (1975) Sociobiology. Harvard University Press, Cambridge
Winberg S, Nilsson GE, Olsen KH (1992) Changes in brain serotonergic activity during hierarchic behavior in Arctic charr (Salvelinus alpinus L.) are socially induced. J Comp Physiol A 170:93–99
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sport, and Culture to TN (16K07432). We would like to thank Uni-edit for editing and proofreading this manuscript.
Author information
Authors and Affiliations
Contributions
TA and TN designed the experiments. TA conducted the experiments and performed the statistical analyses. TA and TN discussed the results, wrote the paper and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of Yamagata University (Japan).
Data availability statement
Data are available upon request by contacting the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abe, T., Nagayama, T. Enhancement of synaptic responses in ascending interneurones following acquisition of social dominance in crayfish. J Comp Physiol A 207, 415–428 (2021). https://doi.org/10.1007/s00359-021-01481-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-021-01481-7