Abstract
Here, we show that the development of a conditioned defensive reflex against the context in the terrestrial snail is accompanied by a depolarization shift of the membrane potential and a decrease in the threshold of action potential generation of premotor interneurons LPa3 and RPa3. There were no further significant changes in the membrane potential of the premotor interneurons in snails after reminder (initiation of reconsolidation) with subsequent injection of either the protein synthesis inhibitor anisomycin or saline. The threshold of action potential generation of these neurons decreases after training and remains unchanged after a reminder (initiation of reconsolidation) with subsequent injection of both anisomycin and saline. No significant changes were found in the membrane and threshold potentials of serotonin-containing neurons of the pedal ganglion Pd4 and Pd2 both after training and after a reminder followed by injection of solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the most intriguing and sophisticated functions of the brain is its ability to receive and store information. The mechanism for storing and/or recalling this information is memory [1, 2]. Newly received information undergoes modifications during the process of consolidation before becoming long-term memory [1, 3]. When forming a memory for any event, labile traces first appear, which are consolidated through the synthesis of new proteins into a stable memory [4, 5]. However, this consolidated memory may be then subjected to reorganization or destabilization processes. The process by which reactivated labile memory stabilizes over time is known as memory reconsolidation [4, 6]. This state of the process of memory formation was well expressed by Prof. Dudai in his review “The Restless Engram: Consolidations Never End” [7].
A plethora of experimental evidence shows that learning-related cellular processes are associated with long-term modifications in the efficiency of synaptic transmission and changes in the endogenous properties of the neuron and its membrane [8, 9], while for a long time, the main mechanism of learning was recognized as a change in the efficiency of synaptic transmission. Later evidence of non-synaptic mechanisms appeared [10,11,12]. Within the framework of such ideas, there are a sufficient number of studies on the cellular mechanisms of learning [10, 13,14,15,16]. These studies provide evidence for membrane correlates of learning, which shows the association of behavioural learning outcomes with neuron excitability and electrical characteristics [15, 17,18,19,20,21,22]. The parameters, which govern the excitability of neurons, include the frequency of spikes, the number of spikes in response to an electrical stimulus in silent neurons, and the membrane and threshold potentials. The efficiency of neural networks can be increased by increasing the internal excitability of the neuron [10, 23,24,25].
One widely spread theory of memory formation is that an engram is formed by a group of neurons that are active during learning and then undergo biochemical and physical changes to keep the information in a stable state, which gets activated later during memory recall [26, 27]. The question of the localization of neuronal correlates of associative learning [28] was also investigated using c-fos-active neuron labelling and gene imaging at an early stage [29]. Several studies using modern methods have shown that there is current evidence for engram cells whose activity correlates with memory engrams [2, 26]. The authors pose the question that the application of new technologies will expand our understanding of the consolidation of memory traces at the level of neural circuits [27].
Previously, we have indicated membrane correlates (changes in the membrane and threshold potentials of premotor interneurons) for conditioned defensive reflexes of tapping on the shell and aversion to food, as well as during the formation of long-term sensitization [20, 30]. Therefore, the question arose whether such changes are possible during the development of other types of conditioned reflexes. It is known that the terrestrial snail is able to develop a defensive conditioned reflex to the environment, i.e. context-dependent [31]. Later, it was suggested that this reflex is analogous to declarative memory. We and other authors have shown reconsolidation of long-term contextual memory, with the presentation of a reminder and subsequent inhibition of protein synthesis, in the terrestrial snail when learning the conditioned defensive reflex to the environment described above [32,33,34,35]. Therefore, we aim with this work to compare possible changes in the electrical characteristics of premotor interneurons of the defensive behaviour of the terrestrial snail LPa3 and RPa3, as well as serotonin-containing modulatory neurons Pd2 and Pd4 of the pedal ganglion [36, 37] upon acquiring a conditioned contextual reflex and reconsolidating the memory of this reflex.
2 Materials and Methods
2.1 Experimental Animals
Terrestrial snails Helix lucorum were used in this study. For the experiments, animals of approximately the same weight (about 20–25 g) were selected. The snails were stored in a dormant state in a fridge. Before the experiments, the snails were kept for at least 2 weeks in the glass terrariums in a humid atmosphere at room temperature, with an excess of food. All experimental procedures were conducted in accordance with the guidelines for the care and use of laboratory animals published by the National Institutes of Health and Directive 2010/63/EC of the European Parliament and Council of Europe on September 22, 2010. All groups were housed in separate terrariums that were stored in the same room under the same conditions. All animals developed the conditioned defensive reflex to the situation according to the contextual paradigm “on the ball”.
Two days before the training session and during the training (5 days), the animals were deprived of food. The deprivation of food in snails during the formation of conditioned reflexes is a standard technique; it is not related to the metabolism of certain substances but is determined by the necessity of the active state of the animal [33,34,35]. Electrophysiological measurements were performed on isolated nervous system preparations the next day after the end of training. Prior to the dissection procedure, the snails were anaesthetized by immersion for 30 min in ice–water mixture.
2.2 Defensive Reaction Testing
Before the experiments and on the day after the training, the amplitude of the defensive response of the ommatophore retraction in response to tactile stimulation was measured as an indicator of the formed long-term memory. During the experiments, the amplitude of the defensive response of ommatophore retraction in response to tactile stimulation was recorded [35, 38]. The tactile stimulation consisted of the tangential movement of a brush along the skin of the dorsal side of the front leg of the snail. The hair of the brush touched the skin of the animal for about 1 cm and was moved at an approximate speed of 1 cm/s. Each test consisted of 5 presentations of a tactile stimulus (the interval between tests was 7–10 min), which were first presented on a flat surface (i.e. in a situation different from the learning environment) [33], and then, the animals were moved to the ball. Tests were performed visually and recorded on video. At the preliminary stage of preparing the experiments, we compared the accuracy of visual recording with the use of objective video recording with three cameras (orthogonal arrangement). The final difference between the visual assessment of ommatophore reactions and the assessment using cameras was multidirectional; its value was no more than 5%. In a preliminary visual assessment, the full retraction of the ommatophores in response to tactile stimulation was considered as 100% of the ommatophore retraction amplitude in response to tactile stimulation. The magnitude of the reaction was recorded as 0% when there was no reaction. The values 25%, 50%, and 75% were recorded for intermediate reactions proportionally. Since the increase in the amplitude of the ommatophore retraction is the result of the formation of a conditioned reflex, it was evaluated as an indicator of the defensive conditioned reflex.
2.3 Contextual Reflex Conditioning
In the first experimental series, all snails developed a contextual conditioned defensive reflex (CCDR) in accordance with the “on the ball” contextual paradigm (for a detailed description of the procedure, see [35, 39]). For this purpose, the animals were placed in an experimental setup (on a ball). The training involved presenting electrical stimulation as an unconditioned stimulus when the snail was in a certain context—on a ball. Every day for 5 days, the snails were presented with 5 electrical stimuli per day (electrical stimuli, 1–2 mA, 1 s, 50 Hz, rectangular pulses, 10 ms, applied using two macroelectrodes). The intensity of the stimulating current was sufficient to evoke a protective response associated with retraction of the anterior part of the body and did not exceed 2 mA [40]. The used current did not cause damage to the skin of animals, which is manifested in the form of pigmented areas that form after stimulation with high levels of electrical current [41]. Before the experiments and on the day after the training, the amplitude of the defensive response of the ommatophore retraction in response to tactile stimulation was investigated as an indicator of the formed long-term memory.
2.4 Reconsolidation of Long-Term Memory
In the second experimental series, the long-term memory formed after the training was reconsolidated in the training context for a number of the trained snails as previously described (see [35, 39]). To study reconsolidation, a “reminder” of the learning context was used. This reminder consisted of placing the animal for 20 min in the training context—on the ball. The animals were rigidly fixed by their shell to a bracket and at the same time had the freedom of movement on the surface of a ball floating in the water, as they were during the training. However, the animals received neither tactile nor electrical stimuli. Then, after the reminder procedure, protein biosynthesis was inhibited by anisomycin (AN) (anisomycin (2-[p-methoxybenzyl]-3,4-pyrrolidinediol 3-acetate, Sigma) [42]. Anisomycin dissolved in 0.2 ml of physiological saline solution (SS) was injected at the dose of 16 mg/kg (or 0.4 mg/per snail). Control animals were injected with 0.2 ml of SS. On the following day after the “reminder”, the amplitude of the defensive response was again tested. The significant decrease in the level of the defensive reaction observed in this case reflects the presence of the process of reconsolidation of contextual memory, which depends on protein synthesis [4]. Behavioural experiments were carried out using a double-control method.
2.5 Intracellular Recording
Analysis of the electrical characteristics was carried out in the readily identifiable giant premotor interneurons of the defensive reflex LPa3 and RPa3 [43] and also of serotonin-containing neurons of the pedal ganglion Pd4 and Pd2, which modulate this reflex [36, 37]. The isolated nervous system was placed in a saline solution of the following composition: NaCl—80 mM, KCl—4 mM, CaCl2—10 mM, MgCl2—6 mM, and NaHCO3—5 mM (or Tris—5 mM) (pH—7.6–7.8). Measurements were carried out at room temperature (18–21 °C) using intracellular glass microelectrodes filled with 2.5 M KCl and having a resistance of 5–10 MΩ. The following parameters of nerve cells were recorded: membrane resting potential—Vm (initial value before the beginning of each electrical stimulation)—and a threshold of action potential generation—Vt (threshold potential).
2.6 Data Analyses
Contextual conditioned defensive reflex was developed in 50 snails: 20 of them were dissected to study the electrical characteristics of premotor interneurons, and neurons of the pedal ganglion were measured the next day. Twenty naïve snails were used as the control group for the electrophysiological measurements. The 30 remaining snails of the CCDR group were divided as follows: 15 snails were used to study the possibility of reconsolidation after a reminder and the subsequent inhibition of protein synthesis while the other 15 were reminded with subsequent saline injection as reconsolidation control.
In the electrophysiological experiments, measurements were made for 20 premotor interneurons in the control group, 20 in the post-CCDR group, 13 premotor interneurons in the post-reconsolidation group, and 14 premotor interneurons in the reconsolidation control group. Electrical characteristics were also measured in 19 neurons of the pedal ganglion of the control group, 18 neurons of the pedal ganglion of the CCDR group, 14 neurons of the pedal ganglion of the group after reconsolidation, and 19 neurons of the pedal ganglion of the reconsolidation control group.
The results are shown as mean ± SEM. Graphical illustrations and significance were obtained using the statistical software SigmaStat32. To compare the electrophysiological data, one-way ANOVA was used. For behavioural data, the comparison was done via one-way repeated measures ANOVA. Independent Student’s t-test was used for comparison of withdrawal reaction on the ball and on the flat surface. The statistical significance criterion was p<0.05.
3 Results
3.1 Elaborating a Contextual Conditioned Defensive Reflex
To develop the contextual conditioned defensive reflex, the animal was presented with an electrical stimulus as an unconditioned stimulus for 5 days while the snail was in a certain context—on the ball. Testing in the context of learning (on a ball) showed a significant (p<0.001), more than 10-fold increase in defensive responses of ommatophore withdrawal in response to tactile stimulation. On the other hand, when testing on a flat surface, there was no significant increase in the amplitude of defensive reactions (Fig. 1). A significant (p<0.001) difference was observed in the results when testing animals in different contexts (on the flat surface, i.e. in a neutral environment, and on the ball which is the training environment), which indicate the development of the contextual conditioned reflex to the training environment.
The level of the defensive reaction (the amplitude of the ommatophore withdrawal reaction) of snails in two contexts, on a ball and on a plane for snails trained according to the “5 stimuli in 5 days” protocol, with reminder followed by injection of anisomycin (AN) or saline solution (SS). T1, initial testing before the beginning of training; T2, testing 1 day after the elaboration of conditioned reflex (learning); T3–T7, testing after injecting substances and reminders on the 8th–12th day after training and reminder. Arrows indicate Reminder (time of Reminder), SS (time of injection of SS), and AN (time of injection of AN). Asterisks and hash signs indicate a significant difference in the amplitude of response of ommatophore withdrawal in response to T3–T7 vs. amplitude of response of ommatophore withdrawal in response to T2 (###p<0.001) vs. amplitude of response of ommatophore withdrawal in response to T1 (***p<0.001). One-way repeated measures ANOVA was used. The vertical axis shows the value of defensive reaction in response to the conditioned stimulus (the amplitude of reaction of ommatophore withdrawal), in % to the maximum. The horizontal axis shows the course (protocol) of the experiment: T1, T2, T3–T7, SS, AN, and Reminder
3.2 Reconsolidation of Long-Term Memory of the Contextual Conditioned Defensive Reflex
After elaborating and testing the contextual conditioned defensive reflex to the environment, the animals were reminded of the learning environment 1 day after testing and then injected with the protein synthesis inhibitor anisomycin. This is a commonly used method for investigating memory reconsolidation of the developed conditioned reflex [4, 33]. Testing 1 to 5 days (T3–T7) after the reminder and the subsequent injection of anisomycin showed a significant (p<0.001) decrease in memory for the conditioned reflex to the level of 20% (2/3 reduction from the initial value of 60% (T2)) (Fig. 1). This result demonstrates a disruption in memory reconsolidation, a process that was initiated but failed due to inhibition of protein synthesis. At the same time, snails that received an injection of physiological saline after a reminder demonstrate the preservation of the contextual conditioned defensive reflex to the learning environment (Fig. 1).
3.3 Electrophysiological Data
The next step was analyzing the electrical characteristics (Vm and Vt) in the readily identifiable giant premotor interneurons of the defensive reflex LPa3 and RPa3 [43] and serotonin-containing neurons of the pedal ganglion Pd4 and Pd2, which modulate this reflex [36, 37]. The analysis was carried out in naive snails, snails after learning the contextual reflex, and snails after memory reconsolidation of the conditioned contextual reflex (or rather, its initiation).
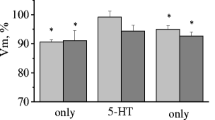
The results showed a significant (p<0.01) decrease—about 5 mV—in the membrane potential in LPa3 and RPa3 neurons after training (Fig. 2A). No further significant changes in Vm were found in the snails after the reminder (after starting the reconsolidation process compared to post-CCDR level) with subsequent injection of either anisomycin or saline. The membrane potential of premotor interneurons remains depolarized after reconsolidation (p<0.05). The threshold potential of these neurons decreases (p<0.05) after acquiring the CCDR and remains unchanged (decreased) after the reminder with subsequent injections of both anisomycin and saline (Fig. 3A). In other words, Vm and Vt remain significantly reduced after the reminder relative to the initial level before training (Figs. 2 and 3). After training, no significant changes were found in the membrane and threshold potentials of the modulatory serotonin-containing neurons of the pedal ganglion Pd4 and Pd2. After the reminder (starting of reconsolidation), with the subsequent injection of both anisomycin and saline, no further changes in Vm and Vt were observed (Figs. 2B and 3B).
Membrane potential (−Vm) of premotor interneurons of withdrawal behaviour LPa3 and RPa3 (A) and membrane potential (−Vm) of serotonin-containing neurons of the pedal ganglion Pd4 and Pd2 (B) of control and trained snails. SS, control snails (only injected by saline solution (SS)); Learning, snails trained to the contextual conditioned defensive reflex; Learning+(Rem+SS), snails trained to the contextual conditioned defensive reflex and then receiving the reminder with injection of SS; Learning+(Rem+AN), snails trained to the contextual conditioned defensive conditioned reflex and then receiving the reminder with injection of the protein synthesis blocker anisomycin (AN). The vertical axis shows the value of potential, in mV. Asterisks (*, **) indicate a significant difference (p<0.05, p<0.01, one-way ANOVA)
Threshold potential (Vt) of premotor interneurons of withdrawal behaviour LPa3 and RPa3 (A) and threshold potential (Vt) of serotonin-containing neurons of the pedal ganglion Pd4 and Pd2 (B) of control and trained snails. SS, control snails (only injected by saline solution (SS)); Learning, snails trained to the contextual conditioned defensive reflex; Learning+(Rem+SS), snails trained to the contextual conditioned defensive reflex and then receiving the reminder with injection of SS; Learning+(Rem+AN), snails trained to the contextual conditioned defensive reflex and then receiving the reminder with injection of the protein synthesis blocker anisomycin (AN). The vertical axis shows the value of potential, in mV. Asterisks (*) indicate a significant difference (p<0.05, one-way ANOVA)
4 Discussion
When discussing the mechanisms of learning and the formation of long-term memory, we face a dilemma: how to combine the need to preserve long-term memory, on the one hand, with its modification (plasticity) during learning, on the other hand? Considering the mechanisms of non-synaptic plasticity, including the membrane (cellular) correlates of learning, again confronts us with the same problem. This concerns particularly long-term changes in postsynaptic neurons during and after learning [9, 13, 16].
For example, it was found that the primary cellular correlates for associative learning in the mollusc Hermissenda are changes in the properties of type B photoreceptors. More specifically, depolarization accumulates with each subsequent experience [44]. This accumulation of depolarization turned out to be specific to combined stimuli and subsequent orientation of the nervous system relative to the centre of rotation. A significant learning effect is also an increase in the input resistance of type B photoreceptors, an increase in the frequency of evoked spikes, and the duration of action potentials [45]. Both animal training and in vitro training produced a marked increase in voltage- and Ca2+-dependent K+ currents across the membrane of the type B photoreceptors [46].
We have shown that the formation of conditioned reflexes and long-term sensitization (LTS) of the defensive reflex in the terrestrial snail is accompanied by a depolarization shift in the membrane potential and a decrease in the threshold potential of the premotor defensive behaviour interneurons LPa3 and RPa3 [17]. We found that training in the context of serotonin depletion by the neurotoxin 5,7-dihydroxytryptamine, which did not lead to the formation of LTS, was not accompanied by changes in electrical characteristics [47]. Investigating the efficiency of synaptic transmission after LTS formation in Aplysia showed that it did not change for the LPl17 interneuron–motoneuron pair, but the efficiency increased for the sensory neuron–motoneuron pair [18]. This means that the change in excitability is specific to certain elements of the neural network. Later, it was found that the excitability of neuron B51, which is responsible for decision-making in the eating behaviour network, on the contrary, decreases [48].
Consolidation can be examined at two levels of description and analysis: the cellular/synaptic level, which occurs within the first minutes or hours after training and occurs in all memory systems studied, and the systematic level, where consolidation takes much longer and is required [3, 34, 49]. The current central dogma of synaptic consolidation is that it involves the activation of intracellular signalling cascades, leading to post-translational modifications, modulation of gene expression, and synthesis of gene products [50, 51]. Memory is maintained by a specific ensemble of neurons distributed throughout the brain that forms a unique memory trace. However, only a fraction of suitable neurons is recruited into a particular memory. They are active during learning and then undergo biochemical and physical changes to keep information stable and get activated during memory formation and recall [26, 27]. We assume that during these processes, they are accompanied by changes in the parameters of key neurons, including electrical characteristics.
These studies show the relevance of the search for membrane (cellular) correlates, both in the consolidation of memory and in its reconsolidation. Our results show that membrane correlates are observed for the contextual defensive reflex and include a decrease in the membrane and threshold potentials of premotor interneurons LPa3 and RPa3 after learning. These data are consistent with similar changes in the membrane and threshold potentials of premotor interneurons after the development of other conditioned defensive reflexes, which we obtained earlier [17, 29]. This fact is new and relevant since there is an assumption that this conditioned reflex is analogous to declarative memory [43]. These altered electrical characteristics remained at the same level after reconsolidation trigger failure, although no defensive responses were observed. At the same time, no significant changes in membrane and threshold potentials were found in serotonin-containing neurons of the pedal ganglion Pd4 and Pd2 relative to both their level after training and after a reminder followed by anisomycin injection (starting reconsolidation).
Thus, here we have identified the membrane correlates of learning for the contextual conditioned defensive reflex, expressed by a decrease in the values of the membrane and threshold potentials of premotor interneurons LPa3 and RPa3 after learning. We have also shown that there were no further changes in the membrane characteristics of premotor interneurons LPa3 and RPa3 after reconsolidating the contextual memory in the snail through a reminder, both in the case of inhibiting protein synthesis which leads to a decrease in memory (negative reconsolidation) and in the case of positive reconsolidation of memory and its preservation. It has also been shown that after developing both the reflex and positive or negative reconsolidation of memory, there were no changes in the membrane characteristics of the serotonin-containing neurons of the pedal ganglion Pd4 and Pd2, modulatory neurons on which the formation of this reflex depends.
References
McGaugh, J. L. (2000). Memory: A century of consolidation. Science, 287, 248–251.
Asok, A., Leroy, F., Rayman, J. B., & Kandel, E. R. (2019). Molecular mechanisms of the memory trace. Trends Neurosci., 42(1), 14–22. https://doi.org/10.1016/j.tins.2018.10.005
Dudai, Y. (2004). The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol., 55, 51–86.
Choi, J.-H., Kim, J.-E., & Kaang, B.-K. (2010). Protein synthesis and degradation are required for the incorporation of modified information into the pre-existing object-location memory. Mol. Brain., 3(1). https://doi.org/10.1186/1756-6606-3-1
Nader, K., & Hardt, O. (2009). A single standard for memory: The case for reconsolidation. Nat. Rev. Neurosci., 10, 224–234.
Dudai, Y. (2012). The restless engram: Consolidations never end. Annu. Rev. Neurosci., 35, 227–247.
Balaban, P. M., & Korshunova, T. A. (2011). Networks, cellular and molecular mechanisms of plasticity in simple nerve systems. Usp. Fiziol. Nauk., 42(4), 3–19.
Oh, M. M., & Disterhoft, J. F. (2015). Increased excitability of both principal neurons and interneurons during associative learning. Neuroscientist, 21, 372–384.
Lisman, J., Cooper, K., Sehgal, M., & Silva, A. J. (2018). Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat. Neurosci., 21(3), 309–314. https://doi.org/10.1038/s41593-018-0076-6
Zhang, W., & Linden, D. J. (2003). The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nature Rev. Neurosci., 4, 885–900.
Nikitin, E. S., Balaban, P. M., & Kemenes, G. (2013). Nonsynaptic plasticity underlies a compartmentalized increase in synaptic efficacy after classical conditioning. Current Biol., 23, 614–619.
Chen, S., Cai, D., Pearce, K., Sun, P. Y.-W., Roberts, A. C., & Glanzman, D. L. (2014). Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. eLife, 3, e03896. https://doi.org/10.7554/eLife.03896
Byrne, J. (1987). Cellular analysis of associative learning. Physiol. Rev., 67(2), 329–439.
Crow, T. (2004). Pavlovian conditioning of Hermissenda: Current cellular, molecular, and circuit perspectives. Learn. Mem., 11, 229–238.
Nikitin, E. S., Kiss, T., Staras, K., O’Shea, M., Benjamin, P. R., & Kemenes, G. (2006). Persistent sodium current is a target for cAMP-induced neuronal plasticity in a state-setting modulatory interneuron. J. Neurophysiol., 95, 453–463.
Gainutdinov, K. L., Andrianov, V. V., & Gainutdinova, T. K. (2011). Changes of the neuronal membrane excitability as cellular mechanisms of learning and memory. Usp. Fiziol. Nauk., 42(1), 33–52.
Gainutdinov, K. L., Chekmarev, L. Y., & Gainutdinova, T. H. (1998). Excitability increase in withdrawal interneurons after conditioning in snail. NeuroReport, 9, 517–520.
Cleary, L. J., Lee, W. L., & Byrne, J. H. (1998). Cellular correlates of long-term sensitization in Aplysia. J. Neurosci., 18, 5988–5998.
Mozzachiodi, R., Lorenzetti, F. D., Baxter, D. A., & Byrne, J. H. (2008). Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nature Neurosci., 11, 1146–1148.
Andrianov, V. V., Bogodvid, T. K., Deryabina, I. B., Golovchenko, A. N., Muranova, L. N., Tagirova, R. R., Vinarskaya, A. K., & Gainutdinov, K. L. (2015). Modulation of defensive reflex conditioning in snails by serotonin. Front. Behav. Neurosci., 9, 279. https://doi.org/10.3389/fnbeh.2015.00279
Balaban, P. M. (2017). Molecular mechanism of memory modification. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 67, 131–140.
Bogodvid, T. K., Andrianov, V. V., Deryabina, I. B., Muranova, L. N., Silantyeva, D. I., Vinarskaya, A. K., Balaban, P. M., & Gainutdinov, K. L. (2017). Responses of premotor interneurons to serotonin application in naïve and learned snails are different. Front. Cell. Neurosci., 11, 403. https://doi.org/10.3389/fncel.2017.00403
Daoudal, G., & Debanne, D. (2003). Long-term plasticity of intrinsic excitability: Learning rules and mechanisms. Learn. Mem., 10, 456–465.
Schulz, D. J. (2006). Plasticity and stability in neuronal output via changes in intrinsic excitability: It's what's inside that counts. J. Experim. Biol., 209, 4821–4827.
Artinian, L., Zhongm, L., Yangm, H., & Rehderm, V. (2012). Nitric oxide as intracellular modulator: Internal production of NO increases neuronal excitability via modulation of several ionic conductances. Eur. J. Neurosci., 36, 3333–3343.
Tonegawa, S., Liu, X., Ramirez, S., & Redondo, R. (2015). Memory engram cells have come of age. Neuron, 87, 918–931. https://doi.org/10.1016/j.neuron.2015.08.002
Ortega-de San Luis, C., & Ryan, T. J. (2022). Understanding the physical basis of memory: Molecular mechanisms of the engram. J. Biol. Chem., 298(5), 101866. https://doi.org/10.1016/j.jbc.2022.101866
Deng, W., Mayford, M., & Gage, F. H. (2013). Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife, 2, e00312. https://doi.org/10.7554/eLife.00312
Reijmers, L. G., Perkins, B. L., Matsuo, N., & Mayford, M. (2007). Localization of a stable neural correlate of associative memory. Science, 317, 1230–1233.
Beregovoi, N. A., & Gainutdinov, K. L. (1988). Depolarization displacements of the membrane potential of command neurons of the defensive behavior of the grape snail during long-term sensitization. Dokl. AN SSSR, 301(4), 989–992.
Balaban, P. М., & Bravarenko, N. I. (1993). Long-term sensitization and environmental conditioning in terrestrial snails, Experim. Brain Res., 96, 487–493.
Gainutdinova, T. H., Tagirova, R. R., Ismailova, A. I., Muranova, L. N., Gainutdinov, K. L., & Balaban, P. M. (2004). Dependent from protein syntheses reactivation of situational defensive reflex in terrestrial snail. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 54, 795–800.
Balaban, P. M., Vinarskaya, A. K., Zuzina, A. B., Ierusalimsky, V. N., & Malyshev, A. Y. (2016). Impairment of the serotonergic neurons underlying reinforcement elicits extinction of the repeatedly reactivated context memory. Sci Rep., 6, 36933. https://doi.org/10.1038/srep36933
Nikitin, V. P., Solntseva, S. V., Kozyrev, S. A., Nikitin, P. V., & Shevelkin, A. V. (2018). NMDA or 5-HT receptor antagonists impair memory reconsolidation and induce various types of amnesia, Behavioural. Brain Res., 345, 72–82. https://doi.org/10.1016/j.bbr.2018.02.036
Deryabina, I. B., Muranova, L. N., Andrianov, V. V., Bogodvid, T. K., & Gainutdinov, K. L. (2020). Effects of thryptophan hydroxylase blockade by p-chlorophenylalanine on contextual memory reconsolidation after training of different intensity. Intern. J. Mol. Sci., 21, 2087. https://doi.org/10.3390/ijms21062087
Ierusalimskii, V. N., & Balaban, P. M. (2010). Serotonergic system of neurons in the CNS of terrestrial snail: Morphology, ontogenesis, control of behavior. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 60(5), 515–524.
Nikitin, E. S., & Balaban, P. M. (2014). Compartmentalization of non-synaptic plasticity in neurons at the subcellular level. Neurosci. Behav. Physiol., 44, 725–730.
Gainutdinov, K. L., Andrianov, V. V., Bogodvid, T. K., Deryabina, I. B., & Muranova, L. N. (2021). Memory reconsolidation after learning of different depends on the duration of the reminder interval. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 71(6), 856–867. https://doi.org/10.31857/S0044467721060046
Gainutdinova, T. H., Tagirova, R. R., Ismailova, A. I., Muranova, L. N., Samarova, E. I., Gainutdinov, K. L., & Balaban, P. M. (2005). Reconsolidation of a context long-term memory in the terrestrial snail requires protein synthesis. Learn Mem., 12, 620–625.
Shevelkin, A. V. (1992). The facilitation of defensive reactions during food consumption in the snail helix: The participation of glucose and gastrin/cholecystokinin-like peptide. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 42(6), 1235–1249.
Gainutdinov, K. L., & Beregovoi, N. A. (1994). Long-term sensitization in snail: Electrophysiological correlations in command neurons of avoidance behavior. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 44, 307–315.
Ghirardi, M., Benfenati, F., Giovedi, S., Fiumara, F., Milanese, C., & Montarolo, P. G. (2004). Inhibition of neurotransmitter release by a nonphysiological target requires protein synthesis and involves cAMP-dependent and mitogen-activated protein kinases. J. Neurosci., 24, 5054–5062.
Balaban, P. M. (2002). Cellular mechanisms of behavioral plasticity in terrestrial snail. Neurosci. Biobehav. Rev., 26, 597–630.
Alkon, D. L. (1984). Changes of membrane currents during learning. J. Experim. Biol., 112, 95–112.
Matzel, L. D., Collin, C., & Alkon, D. L. (1992). Biophysical and behavioral correlates of memory storage, degradation, and reactivation. Behav. Neurosci., 106(6), 954–963.
Alkon, D. L., Sakakibara, M., Forman, R., Harrigan, J., Lederhendler, I., & Farley, J. (1985). Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav. Neural. Biol., 44, 278–300.
Gainutdinov, K. L., Andrianov, V. V., & Gainutdinova, T. K. (1999). The action of the neurotoxins 5,6-dihydroxytryptamine and p-chlorphenylalanine on the electrical activity parameters of the command neurons during long-term sensitization and learning in the snail. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 49, 48–58.
Shields-Johnson, M. E., Hernandez, J. S., Torno, C., Adams, K. M., Wainwright, M. L., & Mozzachiodi, R. (2013). Effects of aversive stimuli beyond defensive neural circuits: Reduced excitability in an identified neuron critical for feeding in Aplysia. Learn. Mem., 20, 1–5. https://doi.org/10.1101/lm.028084.112
Zuzina, A. B., & Balaban, P. M. (2015). Extinction and reconsolidation of memory. Zh. Vyss. Nerv. Deiat. Im. I. P. Pavlova., 65(5), 564–576.
Dudai, Y., Karni, A., & Born, J. (2015). The consolidation and transformation of memory. Neuron, 88, 20–32. https://doi.org/10.1016/j.neuron.2015.09.004
Borodinova, A. A., & Balaban, P. M. (2020). Epigenetic regulation as a basis for long-term changes in the nervous system: In search of specificity mechanisms. Biochemistry, 85(9), 994–1010.
Acknowledgements
The authors would like to thank Dr. Dinara Silantyeva for her editorial assistance in the preparation of this manuscript.
Availability of data and material
Not applicable.
Funding
This work was funded by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Author information
Authors and Affiliations
Contributions
Tatiana Bogodvid participated in the development of a conditioned contextual reflex and experiments on the reconsolidation of contextual memory to this reflex, carried out electrophysiological measurements of the electrical potentials of neurons, participated in the interpretation of these results, did the literature search, drafted the paper, and gave the final approval of the manuscript.
Vyacheslav Andrianov carried out electrophysiological measurements of electrical potentials of neurons, processed the results of electrophysiological measurements of electrical potentials of neurons, participated in the interpretation of these results, prepared Figs. 2 and 3, drafted the paper, and gave the final approval of the manuscript.
Lyudmila Muranova participated in the development of a conditioned contextual reflex and experiments on the reconsolidation of contextual memory to this reflex, carried out electrophysiological measurements of the electrical potentials of neurons, participated in the interpretation of these results, and prepared Fig. 1.
Irina Deryabina participated in the development of a conditioned contextual reflex and experiments on the reconsolidation of contextual memory to this reflex, participated in the interpretation of these results, and prepared Fig. 1.
Aliya Vinarskaya participated in the analysis, processing, and interpretation of the results, did the literature search, and participated in the drafting of the manuscript.
Abdulla Chihab participated in the electrophysiological measurements of the electrical potentials of neurons, helped to draft the manuscript, and was responsible for its editing.
Khalil Gainutdinov conceived the study, was responsible for the conception of its design and coordination, drafted the manuscript, did the literature search, and gave the final approval.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental procedures (using anaesthesia methods) are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985), the UK Animals Scientific Procedures Act 1986, or the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Consent for publication
All authors agree to publish the results and approved the final manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bogodvid, T.K., Andrianov, V.V., Muranova, L.N. et al. Changes in the Electrical Characteristics of Premotor Interneurons and Serotonin-Containing Modulator Snail Neurons upon Developing a Contextual Conditioned Reflex and Its Reconsolidation. BioNanoSci. 13, 66–73 (2023). https://doi.org/10.1007/s12668-023-01062-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01062-9