Abstract
The barn owl, a nocturnal predator, derives its German name (“Schleiereule”, direct English translation “veil owl”) from the conspicuous ruff that covers the ear openings and gives the head a face-like appearance. The ruff is a specialization for the perception of sound. The densely-ramified reflector feathers forming the border of the ruff direct sound to the ear-openings. We studied the influence of the ruff on the behaviorally relevant sound-localization parameters interaural time difference (ITD) and interaural level difference (ILD). The directionality of the ear was much greater when the ruff was intact than when the reflector feathers were removed. With ruff intact, the distribution of ILDs was oblique and the maximum ITD occurred around 110° of azimuth. When all head feathers were removed, the steepest ILD gradient was much closer to the horizontal axis and ITD was maximal at 90°. Many effects were frequency specific. Thus, the ruff reflects some properties of the human pinna. However, by shifting the point where ITD becomes maximal beyond 90°, the ruff also introduces a break of the front–back symmetry of ITD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barn owls are well known for their conspicuous ruff. The ruff serves as a sound collector (Payne 1971; Coles and Guppy 1988) and is important for sound localization. After ruff-feather removal major errors in elevational sound localization were observed (Knudsen and Konishi 1979). Ruff feathers grow very fast in development, enabling the animals to establish a constant relation between auditory-space coordinates and auditory cues before they leave the nest (Haresign and Moiseff 1988; Knudsen et al. 1994).
The sense of hearing is omnidirectional. Sound localization helps animals to turn towards a sound source and then inspect it more closely with their eyes (Konishi 1973, 2003; Heffner and Heffner 1992). Sound localization is a three-dimensional problem. The location of a sound source may be described in a head-centered spherical coordinate system by three parameters: the horizontal deviation from the mid-sagittal plane or azimuth, the vertical deviation from the horizontal plane or elevation, and the distance between the listener and the sound source. Since the two ears are located at different positions on the head, the sound originating from a source is differentially influenced before it reaches the left and right ear drums. This leads to monaural and binaural cues useful for sound localization. Both monaural and binaural cues are utilized by humans and animals in locating sounds (Wightman and Kistler 1989; Middlebrooks 1992; Rice et al. 1992; Blauert 1997; Populin and Yin 1998; Hofman et al. 1998). Monaural cues may be conspicuous changes in the amplitude spectrum (notches). Interaural level differences (ILD) are a consequence of different influences of the head and outer ear on the sound amplitude, while interaural time differences (ITD) are due to the different distances the sound travels until it reaches the ear drums. In a three-dimensional spherical coordinate system, constant ITDs are found to lie on the surface of a cone. The apex of the cone lies in the centre of the head, while the axis of the cone is parallel to the interaural axis (Blauert 1997). Thus, the ITD is the same for many locations. This classic model of the representation of ITD applies to humans and most animals, but not to barn owls, as we shall demonstrate here.
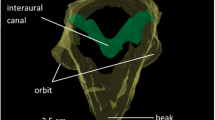
The reason for the deviation from the classic model is the ruff. The ruff is built by two types of feathers: auricular feathers and reflector feathers (Haresign and Moiseff 1988; Koch and Wagner 2002). Auricular feathers are found in the facial disc spanned by the ruff, while reflector feathers form the border of the ruff (Fig. 1). Auricular feathers have a very loose ramification and are acoustically transparent, while reflector feathers are stiff and densely ramified and reflect sounds to the external ear openings (Fig. 1).
Feather types on the barn owl’s head. Three types of feathers are shown (Koch and Wagner 2002). The contour feather (top) is the typical contour feather found everywhere on the head and body except in the ruff; it shows no specialization. Auricular feathers (left) fill the ruff. This feather type has a reduced ramification so that it becomes acoustically transparent but still is effective in preventing the ruff from becoming dirty. Reflector feathers (right) form the border of the ruff. This feather type is very densely ramified and, thus, is able to influence the path of the sound. Scale bars 1 cm
Barn owls use ITDs to locate sound sources in azimuth (Moiseff and Konishi 1981; Moiseff 1989a, b; Poganiatz et al. 2001; Bala et al. 2003). In this species, ILDs are a major cue for elevational sound localization (Moiseff 1989a; Egnor 2001; Poganiatz and Wagner 2001). Measurements of barn owl skulls (width about 4.5 cm) suggest a lower limit for the range of physiological ITDs of about 130 μs in air, while measurements with microphones placed near the eardrum resulted in values clearly above those estimates (Moiseff 1989; Keller et al. 1998; Poganiatz et al. 2001). In addition, Poganiatz et al. (2001) observed that the maximum of ITD occurred at more than 90° in azimuth, which is not consistent with the classic model of a front–back symmetrical representation of ITD (Blauert 1997). The reasons for the shift of the ITD extrema beyond ±90° of azimuth were not clear so far. We speculated that the ruff may play an important role. Therefore, we studied the contribution of the ruff feathers to the distribution of ITDs and ILDs across space in adult barn owls.
Materials and methods
Five adult barn owls (4 dead, 1 living) were included in this study. All owls came from the breeding colony of the institute. Anesthesia was induced and maintained by administering Valium (1 mg/kg/h) and Ketanest (20 mg/kg/h). Atropine sulfate (0.05 mg i.p.) was given at the start of the anesthesia to prevent salivation. A brass head plate had been fixed to the skull under anesthesia in all animals. The living animal was anesthetized during the measurements described here. It was sacrificed after the measurements were completed. The procedures were approved by the Regierungspräsidium Köln.
Set up
Measurements were performed in a sound-attenuating chamber (IAC 403A, Industrial Acoustics, Niederkrüchten, Germany). A broadband loudspeaker (MacAudio ML-103E) was positioned along a custom-built semicircular track (90 cm radius). The track defined a three-dimensional spherical coordinate system with 0° azimuth and 0° elevation directly in front of the owl (Fig. 2). Since the distance between animal and speaker was constant, only two parameters (azimuth, elevation) were variable. In our graphs these two parameters were plotted in a two-dimensional Cartesian coordinate system with the origin (0° azimuth, 0° elevation) in the center of the graph.
The coordinate system used. The owl is in the center of a two-dimensional spherical coordinate system. Azimuth is the horizontal deviation from the mid-sagittal plane. Elevation is the vertical deviation from the horizontal plane. The origin (0° azimuth, 0° elevation), marked with a black dot, is directly in front of the owl. Lines in the figure have a distance of 30°. Solid lines refer to frontal hemisphere; dotted lines refer to rear hemisphere
Speaker rotation was possible from −160 (left) to +170° (right) in azimuth and +80 (top) to −70° (bottom) in elevation. Data were taken with 5, 10 or 20° of resolution. The legs, wings and body of the animals were wrapped in cloth. The animals were supported by a thin rod (∼1 cm diameter), formed to follow the back of the animal to minimize acoustic reflections. The center of the owl’s head was fixed in the center of the track, so that the midpoint between the ear channels was as close to the center as possible. Four measurements per animal were made sequentially: (1) ruff intact, (2) auricular feathers removed, (3) in addition reflector feathers removed, (4) all feathers on the head removed.
Sound delivery and measurements
Audio hardware (Tucker–Davies Technologies, System II) was used for sound delivery and recording. Transfer characteristics of the ears (‘signals’) were measured with sound sweeps covering a broad frequency range (20 Hz to 16 kHz, logarithmically rising, 500 ms duration, 5 ms rise/fall time, 5 repetitions) (for more details see Keller et al. 1998; Poganiatz et al. 2001; Müller und Massarani 2001). We fitted two microphones (Sennheiser KE4 211-2) with a flexible probe tube (outer diameter 1.4 mm, inner diameter 1.2 mm, length 25 mm). The tubes were inserted 15 mm into the ear channel, thus ending near the ear drums (Keller et al. 1998; Poganiatz et al. 2001). The microphone signal was amplified, filtered (20 kHz cutoff frequency, 60 dB roll-off within 3 kHz) and recorded for 510 ms, beginning with the start of the stimulus. A reference measurement without the owl in place was performed to remove influences of the setup on our data. For the reference measurement, the microphones with the tubes were fixed in the center of the setup, arranged in parallel at a horizontal distance of 1 cm and pointed towards 0 azimuth and 0 elevation.
Data analysis
To obtain the transfer function for a given position, the fast Fourier transformation (FFT) of the recorded signal was divided by the FFT of the reference measurement. An inverse FFT of that quotient gave the impulse response for that position. Interaural time difference (ITD) was derived from a cross correlation of the right and left impulse responses at a given position. The gain was calculated as the average level of the transfer function in the region of 1–12 kHz relative to the reference measurement. Interaural level difference (ILD) was calculated by subtracting the left from the right gain. The area on the unit sphere is measured as solid angle (unit: steradian, sr). The whole sphere has a solid angle of 4π sr. To calculate directionality, we measured the solid angle where the gain was within 3 dB of the highest gain of that ear. We refer to this measure of directionality as 3-dB area.
The ITD was positive for right-ear leading sounds. The ILD was positive for right-ear louder sounds. The ITD (ILD) span was the difference between the most positive ITD (ILD) and the most negative ITD (ILD). The ITD extrema refer to the most positive or negative ITDs, respectively. The ITD extrema difference was the azimuthal range from the most negative to the most positive ITD. We also wanted to determine the steepest changes of ITD (ILD) in the frontal and rear hemisphere. Since iso-ITD lines are parallel and almost vertical, we calculated the steepest gradient of ITD by measuring the change of ITD with azimuth at zero elevation. We call this the slope of ITD and measured it in the frontal region (from −40° to +40°) and the rear region (from −140° to +140°). To find the steepest gradient of ILD we used a more complicated method, because the largest changes of ILD usually occurred along an axis that was inclined relative to the azimuthal axis. We first found by trial and error that a circular patch of 30° was optimal for the analysis. We then moved the center of this patch along the zero ILD line, which typically had the shape of an S, with a slightly curved part in the middle, followed by sharp changes in orientation. We again found by trial and error that the best position for finding the steepest gradient was in the center of the slightly curved part. The maximal gradient along the plane within this patch was defined to be the slope of ILD. The orientation of the straight line defining the slope to the horizon was defined at the orientation of the ILD slope. For the calculation of parameters in frequency bands we filtered the transfer functions with a third order 1/3 octave band pass filter prior to calculation of the parameter.
For statistical test we used the Student’s t test. In the figures a P value smaller than 0.01 is denoted with a double asterisk (**), a P value smaller than 0.05 with a single asterisk (*).
Results
The measurements with the five owls yielded similar results. Therefore, sample data plots are shown from one bird (Figs. 3, 5), while summary plots include data from all ears and birds (Figs. 4, 6, 7, 8, 9).
The ruff as a sound collector
When the ruff was intact, the barn owl’s ears were highly directional. The ears exhibited the highest gain in front of the animal (Fig. 3a, c). Signals from the front were amplified by more than 20 dB compared to signals from lateral positions. The point of maximal sensitivity was about 10–30° from the front, with the left ear pointing slightly down (Fig. 3a) and the right ear pointing slightly up (Fig. 3c). Directionality was measured as the size of the area enclosed by the contour line characterizing a 3 dB loss of gain with respect to the maximum gain (“3 dB-area”). In the normal situation, this area had a size of 0.4 sr or about 1/30 of the unit sphere (Fig. 3a, c).
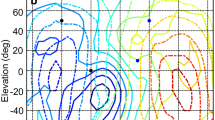
Sensitivity profile of the barn owl’s ear. Data from the left (a, b) and right (c, d) ears of the living owl. a, c ruff intact (data recorded at 10° resolution); b, d reflector feathers removed (data recorded at 20° resolution). The lines are contour lines at 1 dB distance obtained by interpolation. The circles with the plus or minus signs denote the position of the extrema. The shaded area encloses the solid angle where the gain is not more than 3 dB below the maximum gain (3 dB-area). Note that the 3 dB-area increases dramatically and shifts laterally after reflector-feather removal. Lines with dots mark the 0 dB gain with respect to the measurement taken without a bird
When, first, the auricular feathers were removed, the gain did not change (Fig. 4a), while the 3 dB-area became slightly narrower (Fig. 4b). A mean decrease of about 6 dB in gain was observed after the additional removal of the reflector feathers (Fig. 4a). The change was significant compared to the normal situation (P<0.01). When the reflector feathers were removed, directionality decreased as well (Fig. 4b). This decrease was reflected by a tenfold increase of the 3-dB area. The change was again significant compared to the normal situation (P<0.01). When, in the last step, the rest of the head feathers were removed as well, the situation did not change much compared to the earlier removal of the reflector feathers (Figs. 3, 4). Thus, the owls’ ears dramatically changed their receiving characteristics with the removal of the feathers. We next tested how feather removal influenced binaural parameters known to be important for sound localization.
Monaural characteristics of the barn owls’ ears. a The maximal gain decreased with the removal of the reflector feathers. b The size of the 3 dB-area (measured in solid angle, unit in steradian, sr), increased after reflector-feather removal. Below b: drawings of an owl’s appearance during each of the measurements. Lines connect mean values. Data are from five owls and 10 ears. Different symbols denote different animals. Statistical changes by the feather removal compared with the normal situation are denoted by asterisks (**P<0.01, *P<0.05)
Influence of the ruff on the representation of ITD
Iso-ITD contour lines varied mainly with azimuth (Fig. 5a–c). Interestingly, the maximum ITD occurred beyond 90° of azimuth when the ruff was intact (Fig. 5a). This was observed in all animals. The azimuthal difference of ITD extrema, the range from the most negative to the most positive ITD, varied between 200° and 240° in the individual animals (Fig. 6b). The ITD spans ranged from about 500 to about 600 μs (Fig. 6a) in the five animals measured. These ITD spans are about twice as large as those estimated from head size. The relation between ITD and azimuth, measured as slope of the regression line, was between 2.5 and 3 μs/degree in the frontal hemisphere (Fig. 6c). This relation had a considerably larger value in the rear hemisphere (Fig. 6c) in the untreated condition.
Contour plots of ITDs and ILDs. Note the changes of the position of the ITD extrema (+, −, in circles) (a–c) and the changes of the ILD distribution across space (d–f) with feather removal. Contour lines of ITDs are separated by 50 μs, while contour lines of ILDs are separated by 2 dB. Contour lines were obtained by interpolation. Lines with dots indicate the 0 ITD (ILD) contour lines. Other symbols are as in Fig. 3. Data are from the living bird
Influence of the ruff on ITD. a Ranges of the ITDs (most positive ITD minus most negative ITD, “ITD span”), b the azimuthal distance of ITD extrema. Both parameters decreased with reflector-feather removal and again after all head feathers were removed. c The relation between ITD and azimuth in the front (open symbols) and rear hemisphere (closed symbols). After reflector feather removal the slopes in front of the animal and on its back became more similar. Lines connect mean values. Data are from five owls. Statistical changes of the slopes in the rear hemisphere by feather removal are denoted by asterisks on the upper panel margin. Asterisks in the lower part of panel c show the results of the tests of the difference between the slopes in front and in the back for every step of feather removal. Symbols are the same as in Fig. 4
No change in the azimuthal difference between ITD extrema, ITD spans, or the slopes was seen when the auricular feathers were removed (Fig. 6a). However, removal of reflector feathers caused a shift of the azimuthal position of extrema towards ±90° and a decrease in the azimuthal difference of ITD extrema to nearly 190° (Figs. 5b, 6a, b). The relation between ITD and azimuth in the frontal hemisphere did not change (Fig. 6c). However, after reflector-feather removal the slope value was dramatically decreased for the rear hemisphere (Fig. 6c). These changes were even more pronounced when all head feathers were removed (Figs. 5, 6), suggesting that the contour feathers might also contribute to the representation of ITDs. After the removal of all head feathers, the angular distance was close to 180°, as is observed in animals lacking a ruff (Figs. 5c, 6b). The relation between ITD and azimuth was equal in the frontal and rear hemisphere (Fig. 6c).
Influence of the ruff on the representation of ILD
The ILD is the most important parameter for localization in elevation (Moiseff 1989; Egnor 2001; Poganiatz and Wagner 2001). We calculated the ILD as the difference of left and right ear amplifications. The representation of ILD is known to be complex and frequency-dependent (Coles and Guppy 1988; Olsen et al. 1989; Brainard et al. 1992; Keller et al. 1998; Euston and Takahashi 2002). The spatial distribution of ILDs in the frequency range from 1 to 12 kHz exhibited a main extremum and usually one more local extremum (Fig. 5d). Two extrema occurred in the frontal hemisphere. One was located around 30° to the right; the other was found about 30° to the left. The minimum was pointing down and occurred in the left hemisphere, while the maximum, pointing upwards, occurred in the right hemisphere (Fig. 5d). Thus, in normal animals the lines connecting positions with equal ILDs were not vertical, but tilted. This led to a steep ILD gradient in the frontal regions. The steepest gradient had an orientation of some 65°–85° in different animals (Fig. 7b). The value of the steepest gradient was between 0.3 and 0.4 dB/degree, meaning that within 30°, ILD changed by about 10 dB (Figs. 5, 7).
Influence of the ruff on ILD. a The ILD span increased slightly after the removal of the reflector feathers. The orientation of the largest gradient of ILDs in the front rotated towards the horizontal axis (b). The slope decreased (c). Lines connect mean values. Data are from five owls. Different symbols denote different owls. Other symbols are the same as in the legend to Fig. 6
When the auricular feathers were removed, the ILD span became slightly smaller, but the orientation and slope did not change (Fig. 7). As with the ITD, a major change was seen after the removal of the reflector feathers (Figs. 5e, 7). The steepest gradient of ILD now occurred at an orientation between about 20° and 50°, i.e. much closer to the horizontal plane. Surprisingly, the decreases in both directionality and gain (Figs. 3, 4) lead to an increase of the ILDs available to the owl (Fig. 7a). This was due to the much greater range over which ILDs increased (decreased) when the reflector feathers were removed (Figs. 5e, 7). The additional removal of all head feathers did not further influence ILD span, orientation and slope (Figs. 5f, 7).
The influence of frequency on the representation of gain, ITD and ILD
ILDs vary with frequency (Payne 1971; Moiseff 1989; Brainard et al. 1992; Keller et al. 1998; Euston and Takahashi 2002). Our data demonstrate that the dependence of ILDs on frequency resembled the dependence of gain on frequency (compare Fig. 8a with b). The highest ILDs occurred for the highest frequencies. After reflector feather removal, the gain was reduced by about10 dB in the frequency bands above about 2 kHz. This reduction in gain was significant (P<0.01) for all frequency bands. However, in the 1/3 octave bands a consistent effect on ILD spans was not observed with head-feather removal (Fig. 8b). The most pronounced influence of head-feather removal occurred in the 7 and 8 kHz band, in which the ILD span increased by more than 10 dB with reflector-feather removal.
Frequency dependence of ILD. The gain (a), like the ILD-span (b), is frequency dependent; both are highest for high frequencies. Removal of the feathers mainly had a frequency dependent effect on the gain above 3 kHz (a) and on ILD span in the 7 and 8 kHz bands (b). Data are from five owls. Other symbols are the same as in the legend to Fig. 6
A significant frequency-specific effect was also seen for the ITD (Fig. 9). We measured the ITD span in 1/3 octave frequency bands and observed a shallow dip in the ITD span around 3 kHz. This dip occurred in all birds and was significant (P<0.01; Fig. 9a). Likewise, the slope of the ITD representation in the frontal 40° differed between frequencies. For the lowest frequencies the highest slopes were measured (about 3.5 μs/degree). The slopes decreased to about 2.5–3 μs/degree for frequencies above 3 kHz (Fig. 9b).
Frequency dependence of ITD. a The ITD-span showed a dip around 3 kHz. b The slope was maximal for low frequencies. c Comparison of ITDs in frequency bands for positions having equal ITDs in the ruff intact situation. The ITD difference within 1/3 octave frequency bands for the positions with about ±100 μs ITD, one in the front and one in the back hemisphere was calculated. The respective azimuthal values were 40° and 160° on both the left and right sides. The grey area illustrates the behaviorally measured threshold (see text). Open symbols and the continuous line show measured data and mean with standard deviation for a pair of locations on the right (e. g. +40° and +160° of azimuth); filled symbols and dashed line for locations on the left of an animal (e.g. −40° and −160° of azimuth). Data are from five owls. Different symbols in c denote different owls
The ITD differences between frequency bands might be a way to solve front back confusions with ITD alone. Tests were carried out for six different pairs of locations in the horizontal plane having ITDs of ±50, 60, 100 (Fig. 9c), 120, 150 or 200 μs. We took pairs of positions that had a corresponding ITD, when calculated in the frequency range of 1–12 kHz. One position was in the frontal hemisphere and the second position in the posterior hemisphere on the same side of the animal. For these positions we calculated the ITD difference within 1/3 octave frequency bands.
We describe our procedures for pairs having an ITD of ±100 μs corresponding to about ±40° of azimuth in the frontal hemisphere. Behavioral measurements suggested mean azimuthal localization acuity of 4.7° at 40° of azimuth (Knudsen et al. 1979). This acuity corresponds to 13.3 μs of ITD (shaded area in Fig. 9c). We found that the ITD difference in the single frequency bands (exception the 3 kHz band) was not larger than this threshold, when the mean of all five animals was considered (Fig. 9c). The values for single animals exceeded in some animals this threshold at some frequencies, however. The maximum ITD difference in a single frequency band we found was 50 μs. The analysis of the other pairs of ITDs yielded similar results. These data were obtained in the ruff intact situation. When the data from this situation were compared with data in which the reflector feathers or all feathers were removed, the general observations were similar, but the scatter in those conditions was smaller.
Discussion
It was observed that the reflector feathers had the greatest effect on the gain, as well as on the representation of ITD and ILD at the owl’s ear drum. The reflector feathers cause the ears to become more spatially selective, more sensitive, extend the range of physiological ITDs, and shift the position of the maximal ITDs backwards in space. Many of these effects were frequency specific. We shall discuss the possible consequences of the action of the ruff in the following.
Influence of the ruff on the ears’ sensitivity and directionality, and on ILD
Barn owls have a hearing threshold that is about 10 dB lower than the human hearing threshold (Konishi 1973; Wagner 1993; Dyson et al. 1998). Part of this is due to the action of the ruff as is demonstrated by a loss of the gain after reflector-feather removal. The high directionality observed before reflector-feather removal is an ideal precondition for an excellent sound-localization system. Indeed, barn owls also have a very precise sound-localization not only in the horizontal, but also in the vertical plane (Knudsen et al. 1979; Bala et al. 2003). The effect of head-feather removal on the frequency-dependence of the gain showed the expected picture (Brainard et al. 1992; Keller et al. 1998): a greater effect was seen for high frequencies than for low frequencies.
The high directionality with the maximum of the left ear pointing to the left and downward and the maximum of the right ear pointing up and to the right caused a steep gradient of ILDs in normal birds along an oblique angle. Although the gradient was steepest along an oblique angle and not along the vertical axis, this still meant that the owl has a two-dimensional grid available for sound localization in which each location is specified by a unique combination of ITD and ILD (see also Moiseff 1989a). This gradient may be regarded as the basis for the localization capabilities in the vertical plane. So far, it had been shown that ILDs are a very important cue for elevational sound localization in the barn owl (Moiseff 1989b; Egnor 2001; Poganiatz and Wagner 2001). Knudsen and Konishi (1979) observed large elevational errors after feather removal when auditory targets were presented in frontal auditory space. We found that the total range of ILDs available to the owl increased slightly after reflector-feather removal. The spatial distribution of ILDs changed from a steep, oblique gradient to a shallow, almost horizontally-oriented gradient. Thus, it seems that it is not the range of ILDs but the slope and orientation of ILDs that determines the sound–localization precision. Experiments should be carried out to test the role of the spatial distribution of ILDs on the barn owl’s sound-localization precision. Barn owls are known to exhibit some plasticity after manipulation of auditory parameters (Knudsen 1999; Gold and Knudsen 2000). It would be interesting to see whether owls could relearn to associate ITD and ILD with spatial parameters as humans can (Hofman et al. 1998).
The frequency-specific effects on ILDs in untreated barn owls were similar to earlier observations (Brainard et al. 1992; Keller et al. 1998). As was the case in the ILDs measured over the total frequency range, reflector-feather removal increased the ILD span in some frequency bands, specifically, in the 7 and 8 kHz band.
Influence of the ruff on ITD
ITD is a robust parameter for azimuthal sound localization. However, one problem in using ITDs for sound localization is front–back confusion. In the classic model (Blauert 1997), ITDs are equal for positions lying on the surface of a cone, centered in the middle of the interaural axis and extending parallel to the axis. In humans, the pinna helps with disentangling front–back confusions mainly by effecting ILD and spectral characteristics as do head movements (Wightman and Kistler 1999). A similar effect is seen, for example, in the chinchilla (Heffner et al. 1996).
The most important influence of the ruff on the representation of ITD was the increase of the ranges of ITDs available to the owl. In normal barn owls, the maxima (and minima) of ITD occurred beyond ±90°. This breaks the front–back symmetry of the ITD observed in ruff-less animals. Thus, the ‘cone of confusion’ (Blauert 1997), the points in space that give equal ITDs at the ear drums, is not centered around ±90° of azimuth and is not symmetrical but distorted.
Behavioral experiments demonstrate that the owl does not have problems with front–back confusions (Poganiatz et al. 2001). If our conjecture is correct that the ruff helps to solve front–back confusions, animals in which the reflector feathers were cut should have problems to discriminate between locations having the same ITDs in the front and the back. HRTFs obtained after removal of reflector feathers or all head feathers may be used in behavioral experiments with normal animals to test whether one function of the reflector feathers is to help to disentangle front–back confusion.
Comparison with other systems
One may wonder why other animals do not have such a conspicuous ruff as owls do. Indeed, some species of nocturnal birds or birds hunting in environments where prey is invisible, like frogmouths, nighthawks or harriers have indications of a ruff. The popular literature ascribes the ruff in these species a function in sound localization. This has, however, been questioned (Calford et al. 1985). To our best knowledge, the function of the ruff in these species has not been investigated quantitatively. No ruff is found in mammals, but they may use the pinna instead.
We think that a very precise sound-localization system most important for owls. In order to survive, owls must catch at least two mice a night mainly be using their sense of hearing. This heavy dependence on sound localization is also reflected in an increase of brain structures involved in processing of auditory information. Other animals often use sound localization to bring an auditory target in the visual field, where the auditory target can then be inspected more closely by the visual system (Heffner and Heffner 1992). In addition, mammals may move their pinnae, and, thus, induce some flexibility in the representation of ITD (Middlebrooks 1992; Rice et al. 1992).
Abbreviations
- ITD:
-
Interaural time difference
- ILD:
-
Interaural level difference
- FFT:
-
Fast Fourier transformation
References
Bala AD, Spitzer MW, Takahashi TT (2003) Prediction of auditory spatial acuity from neural images on the owl’s auditory space map. Nature 424:771–774
Blauert J (1997) Spatial hearing. MIT, Cambridge
Brainard MS, Knudsen EI, Esterly SD (1992) Neural derivation of sound source location: resolution of spatial ambiguities in binaural cues. J Acoust Soc Am 91:1015–1027
Calford MB, Wise LZ, Pettigrew JD (1985) Coding of sound location and frequency in the auditory midbrain of diurnal birds of prey, families Accipitridae and Falconidae. J Comp Physiol A 157:149–160
Coles RB, Guppy A (1988) Directional hearing in the barn owl (Tyto alba). J Comp Physiol A 163:117–133
Dyson ML, Klump GM, Gauger B (1998) Absolute hearing thresholds and critical masking ratios in the European barn owl: a comparison with other owls. J Comp Physiol A 182:695–702
Egnor SER (2001) Effects of binaural decorrelation on neural and behavioral processing of interaural level differences in the barn owl (Tyto alba). J Comp Physiol A 187:589–595
Euston DR, Takahashi TT (2002) From spectrum to space: the contribution of level difference cues to spatial receptive fields in the barn owl inferior colliculus. J Neurosci 22:284–293
Gold JL, Knudsen EI (2000) Abnormal auditory experience induces frequency-specific adjustments in unit tuning for binaural localization cues in the optic tectum of juvenile owls. J Neurosci 20:862–877
Haresign T, Moiseff A (1988) Early growth and development of the common barn-owl’s facial ruff. The Auk 105:699–705
Heffner RS, Heffner HE (1992) Visual factors in sound localization in mammals. J Comp Neurol 317:219–232
Heffner RR, Koay F, Heffner HE (1996) Sound localization in chinchillas, III: Effect of pinna removal. Hear Res 99:13–21
Hofman PM, van Riswick JG, van Opstal AJ (1998) Relearning sound localization with new ears. Nat Neurosci 1:417–421
Keller CH, Hartung K, Takahashi TT (1998) Head-related transfer functions of the barn owl: measurement and neural responses. Hear Res 118:13–34
Knudsen EI (1999) Mechanisms of experience-dependent plasticity in the auditory localization pathway of the barn owl. J Comp Physiol A 185:305–321
Knudsen EI, Blasdel GG, Konishi M (1979) Sound localization by the barn owl measured with the search coil technique. J Comp Physiol 133:1–11
Knudsen EI, Konishi M (1979) Mechanisms of sound localization in the barn owl (Tyto alba). J Comp Physiol 133:13–21
Knudsen EI, Esterly SD, Olsen JF (1994) Adaptive plasticity of the auditory space map in the optic tectum of adult and baby barn owls in response to external ear modification. J Neurophysiol 71:79–94
Koch UR, Wagner H (2002) Morphometry of auricular feathers of barn owls (Tyto alba). Eur J Morphol 40:15–21
Konishi M (2003) Coding of auditory space. Ann Rev Neurosci 26:31–55
Konishi M (1973) How the owl tracks its prey. Am Sci 61:414–424
Middlebrooks JC (1992) Narrow-band sound localization related to external ear acoustics. J Acoust Soc Am 92:2607–2624
Moiseff A, Konishi M (1981) Neuronal and behavioral sensitivity to binaural time differences in the owl. J Neurosci 1:41–49
Moiseff A (1989a) Binaural disparity cues available to the barn owl for sound localization. J Comp Physiol A 164:629–636
Moiseff A (1989b) Bi-coordinate sound localization by the barn owl. J Comp Physiol A 164:637–644
Müller S Massarani P (2001) Transfer function measurement with sweeps. J Audio Eng Soc 49:443–471
Olsen JF, Knudsen EI, Esterly SD (1989) Neural maps of interaural time and intensity differences in the optic tectum of the barn owl. J Neurosci 9:2591–2605
Payne RS (1971) Acoustic location of prey by barn owls (Tyto alba). J Exp Biol 54:535–573
Poganiatz I, Nelken I, Wagner H (2001) Sound-localization experiments with barn owls in virtual space: influence of interaural time difference on head-turning behavior. J Assoc Res Otolaryngol 2:1–21
Poganiatz I Wagner H (2001) Sound-localization experiments with barn owls in virtual space: influence of broadband interaural level different on head-turning behavior. J Comp Physiol A 187:225–233
Populin LC, Yin TCT (1998) Behavioral studies of sound localization in the cat. J Neurosci 18:2147–2160
Rice JJ, May BJ, Spirou GA, Young ED (1992) Pinna-based spectral cues for sound localization in cat. Hear Res 58:132–152
Wagner H (1993) Lesion-induced sound-localization deficits in the barn owl. J Neurosci 13:371–386
Wightman FL, Kistler DJ (1989) Headphone simulation of free-field listening. I. Psychophysical validation. J Acoust Soc Am 85:868–878
Wightman FL, Kistler DJ (1999) Resolution of front–back ambiguity in spatial hearing by listener and source movement. J Acoust Soc Am 105:284–2853
Acknowledgments
We thank Frank Endler for help with programming. Eli Nelken and Iris Poganiatz supported us in an early phase of this project. We thank Catherine Carr and Eli Nelken for comments on an earlier version of this manuscript. Petra Nikolay made the drawings of the owl’s head. This research was supported by the DFG (Wa606/13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Campenhausen, M., Wagner, H. Influence of the facial ruff on the sound-receiving characteristics of the barn owl’s ears. J Comp Physiol A 192, 1073–1082 (2006). https://doi.org/10.1007/s00359-006-0139-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0139-0