Abstract
Background and objective
There is uncertainty about the beneficial effects of exercise intervention for kidney transplant recipients. The purpose of our meta-analysis is to estimate the efficacy of exercise intervention in kidney transplant recipients.
Methods
A database search according to the PICOS framework was performed for all published randomized, double-blind, placebo-controlled trials (RCTs) about exercise intervention for kidney transplant recipients. The databases involved include PubMed, Embase, and Cochrane Library.
Results
A total of 16 RCTs (involving 827 patients) in compliance with inclusion criteria were included in our study. The results demonstrated that adequate exercise intervention improved statistically in creatinine clearance [mean difference (MD) = − 0.29, 95% confidence interval (CI) − 0.46 to − 0.11, p = 0.001], serum urea (MD = − 21.57, 95% CI − 35.84 to − 7.29, p = 0.003), VO2 peak (MD = 3.20, 95% CI 1.97–4.43, p < 0.00001), high-density lipoprotein-cholesterol (HDL-C) (MD = 0.21, 95% CI 0.04–0.37, p = 0.01), 60-s sit to stand test (60-STS) (MD = 14.47, 95% CI 8.89–20.04, p < 0.00001), 6-min walk distance (6-MWD) (MD = 91.87, 95% CI 38.34–145.39, p = 0.0008), and 6-min walk test (6-MWT) (MD = 44.08, 95% CI 20.30–67.87, p = 0.0003) of patients after kidney transplantation. No between-groups differences (p > 0.05) were observed for anthropometric characteristics, body composition, serum cytokine levels, and quality of life short form-36 questionnaire (SF-36).
Conclusions
In kidney transplant recipients, appropriate exercise intervention improved renal function, cardiopulmonary function, physical performance.
Trial registration
The PROSPERO registration number is CRD42022357574.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplantation is currently the most desired treatment option for patients suffering from end-stage renal disease (ESRD). Compared with other treatments, kidney transplantation has some advantages in improving survival and quality of life [1, 2]. Despite this, patients generally experience multiorgan dysfunction following the procedure, after allograft transplantation. In addition, patients after kidney transplantation often require lifelong immunosuppressants to prevent graft rejection. These immunosuppressive drugs often result in adverse events such as muscle weakness, osteoporosis, and cardiovascular disease [3, 4]. Therefore, postoperative management of kidney transplantation plays a crucial role after renal transplantation.
In recent years, with the promotion of the enhanced recovery after surgery (ERAS) concept, the effect of appropriate exercise intervention for postoperative patients has gradually begun to receive attention. Exercise is recognized as an effective non-pharmacological intervention that is generally categorized as aerobic, anaerobic, and flexibility exercises. The health benefits of exercise have been demonstrated in healthy people and people with chronic diseases [5]. Related studies have also shown that exercise interventions are effective in patients with solid organ transplants, including heart, kidney, lung, and liver transplants [6]. Although exercise interventions are considered beneficial, routine exercise intervention programs for renal transplant recipients are not used as part of standard clinical care. Besides, the evidence on the impact of exercise intervention on kidney transplant recipients is limited. The few available studies have only focused on the effects of exercise intervention on several aspects of exercise tolerance, cardiorespiratory fitness, and quality of life in kidney transplant recipients [7,8,9].
This systematic review and meta-analysis aimed to compare the changes of the exercise intervention group and control group, to fully assess the effects of an exercise intervention on kidney transplant recipients.
Methods
Search strategy
We searched the PubMed, Embase, and Cochrane Library databases using kidney transplantation, exercise training, and randomized controlled trials as keywords. Depending on PICOS (populations, interventions, comparators, outcomes, and study designs) strategy, four authors independently conducted the searches. Table 1 shows the search strategy. This meta-analysis has been registered on PROSPERO with the number CRD42022357574. PRISMA 2020 checklist is shown in the supplementary material.
Inclusion criteria
The RCTs included in this study were required to fulfill all of the following inclusion criteria: (1) the study analyzed the effect of exercise intervention for kidney transplant recipients was analyzed; (2) the study contained valued data that could be analyzed and related outcome index; (3) full-text content was accessible; (4) the study was an RCT. The population inclusion criteria for RCTs were more stringent than other prospective and retrospective studies.
Quality assessment
Studies were categorized according to the Cochrane Risk of Bias Tool for Randomized Trials [10], version 2 (RoB2), recommended by the Cochrane Handbook for the Systematic Evaluation of Interventions [11], version 6.2. According to RoB2, we categorized studies into three levels: low risk of bias, moderate risk of bias, and high risk of bias.
Data extraction
From each included RCT, the following information was extracted: (I) the name of the first author; (II) the time of publication and the type of design; (III) the sample size of each group; (IV) the methods of exercise intervention; (V) the time of intervention; (VI) the outcomes of study: anthropometric characteristics, body composition, renal function, cardiorespiratory function, blood parameters, serum cytokine levels, physical performance and quality of life.
Statistical and meta-analysis
Data were analyzed using Review Manager software (RevMan, version 5.3.0, Cochrane Collaboration) [12]. This study adopted mean difference (MD) for assessing continuous data and adopted odds ratios (ORs) with 95% confidence intervals (CIs) for assessing dichotomous data. We considered studies with p values > 0.05 as homogeneous and conducted the analysis using a fixed-effects model. Conversely, we employed a random-effects for analyzing heterogeneous studies. The present study checked for inconsistency through I2 statistics. The value of p < 0.05 was deemed to be statistically significant.
Results
Characteristics of eligible studies
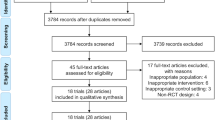
195 articles met the above inclusion criteria and were retrieved from the database. After screening the titles and abstracts, 163 articles were excluded. We reviewed the remaining articles. Among them, 12 studies were removed due to they were not RCTs. Then, 4 studies were eliminated because they missed key information. In the end, 16 RCTs [7,8,9, 13,14,15,16,17,18,19,20,21,22,23,24,25] were included in the final analyses. The flowchart of the selection process is shown in Fig. 1. The details of the included studies are given in Table 2.
Quality of eligible studies
The studies included in our meta-analysis were all RCTs. All studies performed a sample size calculation. Eleven of these RCTs were graded A for quality. Only one study reported an intention-to-treat analysis [13]. No patients were lost during follow-up in the ten studies [7, 8, 13, 15,16,17, 19, 21, 22, 24]. The quality of included studies is shown in Table 3.
Efficacy
We studied the effects of exercise intervention on measurement parameters in kidney transplant patients. Patients in the control group received standard treatment for the same length of time.
Anthropometric characteristics
Body mass index (BMI)
Seven RCTs involving 393 patients compared the differences between the two groups after the intervention in terms of BMI (Supplementary Fig. 1A). Because of p > 0.05, we conducted a fixed-effects model for the study. The results showed no statistical difference in BMI between the two groups after the intervention treatment (MD: 0.12, 95% CI − 0.72 to 0.96, Chi2 = 3.40, p = 0.78).
Waist circumference
Three RCTs reported the changes between the two groups of patients after the intervention in terms of waist circumference (Supplementary Fig. 1B). Since p > 0.05, a fixed-effects model was used to analyze group differences. The model indicated that the MD was 1.50, the 95% CI was − 3.74 to 6.74, the I2 was 25%, and the Chi2 value was 2.67 (p = 0.58). We suggested that the exercise intervention and control groups were similar in terms of the waist circumference of patients.
Hip circumference
Two RCTs analyzed the changes in the hip circumference of 32 patients after the intervention (Supplementary Fig. 1C). A fixed‐effects model was used to assess changes between the two groups, which showed an MD of − 1.19 (95% CI − 6.10–3.72, p = 0.63). There was no significant difference between the two groups concerning hip circumference.
Body composition
Bone mineral density (BMD)
Because of p > 0.05, we employed a fixed-effects model to compare the BMD between the exercise intervention and control groups from two RCTs (Supplementary Fig. 2A). The pooled estimate of MD was − 0.02, 95% CI was − 0.07 to 0.03, I2 was 45%, and Chi2 was 1.80 (p = 0.45). The results showed that the exercise intervention and control groups were similar regarding BMD.
Lean body mass (LBM)
Three RCTs analyzed the differences between the two groups after the intervention in terms of LBM (Supplementary Fig. 2B). Due to p > 0.05, we conducted a fixed-effects model for the study. The results showed no statistical difference in LBM between the two groups after the intervention treatment (MD: 1.21, 95% CI − 2.35 to 4.78, Chi2 = 5.45, p = 0.50).
Renal function
Creatinine
Six RCTs involving 317 patients reported the changes between the two groups of patients after the intervention in terms of creatinine (Fig. 2A). Since p = 0.05, a fixed-effects model was used to analyze group differences. The model revealed that the MD was − 0.29, the 95% CI was − 0.46 to − 0.11, the I2 was 54%, and the Chi2 value was 10.90 (p = 0.001). We concluded that creatinine was greatly improved in the exercise intervention group than in the control group.
Urea
Two RCTs analyzed the changes in the area of 28 patients after the intervention (15 in the exercise intervention group and 13 in the control group) (Fig. 2B). We performed a fixed-effects model to analyze differences between groups, due to p > 0.05. The model revealed that the MD was − 21.57, the 95% CI was − 35.84 to − 7.29, the I2 was 0%, and the Chi2 value was 0.62 (p = 0.003). Significant improvements in urea were found in the exercise intervention group.
Estimated glomerular filtration rate (eGFR)
Three RCTs were included in our study. A random-effects model showed that there was no difference between the exercise intervention group and the control group in eGFR (MD: 16.16, 95% CI − 3.98 to 36.29, Chi2 = 9.33, p = 0.12, Fig. 2C).
Cardiorespiratory function
Systolic blood pressure
Because of p < 0.05, we employed a random-effects model to compare the systolic blood pressure between the exercise intervention group and control group from six RCTs (Supplementary Fig. 3A). The pooled estimate of MD was − 1.53, 95% CI was − 4.70 to 1.64, I2 was 0%, and Chi2 was 3.34 (p = 0.34). The results showed that the exercise intervention and control groups were similar regarding systolic blood pressure.
Diastolic blood pressure
Six RCTs involving 258 patients analyzed the differences between the two groups after the intervention in terms of diastolic blood pressure (Supplementary Fig. 3B). Due to p > 0.05, a fixed-effects model was utilized for analyzing data. The results showed no statistical difference in diastolic blood pressure between the two groups after the intervention treatment (MD: − 0.03, 95% CI − 2.25 to 2.19, Chi2 = 4.52, p = 0.98).
Heart rate
Because of p < 0.05, we compared the heart rate between the exercise intervention and control groups from four RCTs by a random-effects model (Supplementary Fig. 3C). The pooled estimate of MD was − 2.32, 95% CI was − 9.26 to 4.62, I2 was 79%, and Chi2 was 14.12 (p = 0.51). The results showed that the exercise intervention and control groups were similar in heart rate.
Peak oxygen uptake (VO2peak)
Six RCTs involving 251 patients (136 patients in the exercise intervention group, and 115 patients in the control group) reported the changes between the two patients after the intervention regarding VO2peak (Supplementary Fig. 3D). Since p > 0.05, a fixed-effects model was used to analyze group differences. The model revealed that the MD was 3.20, the 95% CI was 1.97–4.43, the I2 was 6%, and the Chi2 value was 5.34 (p < 0.00001). We concluded that the exercise intervention group recorded a statistically significant improvement regarding the VO2peak.
Blood parameters
Total cholesterol
Five RCTs analyzed the changes in total cholesterol of 335 patients after the intervention (Supplementary Fig. 4A). A fixed‐effects model was utilized to evaluate differences between the two groups, which showed an MD of − 0.06 (95% CI − 0.33 to 0.21, p = 0.65). These results reflect no significant effect on kidney transplant recipients of total cholesterol with exercise intervention.
High-density lipoprotein-cholesterol (HDL-C)
Four RCTs reported differences in HDL-C of 229 patients after the intervention (Supplementary Fig. 4B). A fixed-effects model was used to conduct the analysis, due to p > 0.05. The model revealed that the MD was 0.21, the 95% CI was 0.04–0.37, the I2 was 0%, and the Chi2 value was 0.62 (p = 0.01), confirming greater improvements in HDL-C in the exercise intervention group.
Hemoglobin
Because of p > 0.05, we analyzed the hemoglobin between the exercise intervention and control groups from three RCTs using a fixed-effects model (Supplementary Fig. 4C). The pooled estimate of MD was 0.23, 95% CI was − 0.10 to 0.56, I2 was 0%, and Chi2 was 0.88 (p = 0.18). The exercise intervention group had a similar hemoglobin compared to the control group.
Serum cytokine levels
Tumor necrosis factor-α (TNF-α)
Two RCTs were included in our study. A random-effects model suggested that there was no difference between the exercise intervention group and the control group in TNF-α (MD: − 1.44, 95% CI − 4.39 to 1.51, Chi2 = 8.17, p = 0.34, Supplementary Fig. 5A).
Interleukin-6 (IL-6)
Two RCTs analyzed levels of IL-6 (Supplementary Fig. 5B). Pooled results from a fixed-effects model suggested that the exercise intervention group did not differ significantly from that of the control group regarding levels of IL-6 (MD = − 0.70, 95% CI − 1.56 to 0.17, p = 0.11).
Physical performance
60-s sit-to-stand test (60-STS)
Two RCTs recorded the differences in 60-STS of 62 patients after the intervention (Fig. 3A). We employed a fixed-effects model to compare differences between groups, due to p > 0.05. The model revealed that the MD was 14.47, the 95% CI was 8.89–20.04, the I2 was 52%, and the Chi2 value was 2.07 (p < 0.00001). The exercise-trained patients had obvious improvement in the 60-STS.
6-Minute walk distance (6-MWD)
Three RCTs analyzed the changes in 6-MWD of 201 patients after the intervention (Fig. 3B). A random‐effects model was utilized to evaluate differences between the two groups, which showed an MD of 91.87 (95% CI 38.34–145.39, p = 0.0008). The results suggested a statistically significant improvement in the exercise intervention group regarding the 6-MWD.
6-Minute walk test (6-MWT)
Two RCTs analyzed the changes in 6-MWT of 169 patients after the intervention (Fig. 3C). A fixed‐effects model was utilized to evaluate differences between the two groups, which showed an MD of 44.08 (95% CI 20.30–67.87, p = 0.0003). The results suggested that the exercise intervention group was significantly superior to the control group in 6-MWT.
Handgrip strength
Because of p < 0.05, we used a random-effects model to analyze the handgrip strength between the exercise intervention and control groups from two RCTs (Fig. 3D). The pooled estimate of MD was 3.66, 95% CI was − 5.80 to 13.11, I2 was 75%, and Chi2 was 4.02 (p = 0.45). These results reflect no statistical difference in handgrip strength between the two groups after the intervention treatment.
Quality of life short form-36 questionnaire (SF-36)
Physical function score
Four RCTs reported the changes between the two patients after the intervention regarding physical function score (Fig. 4A). Since p < 0.05, a random-effects model was employed to analyze group differences. The model revealed that the MD was 6.93, the 95% CI was − 4.76 to 18.61, the I2 was 81%, and the Chi2 value was 15.54 (p = 0.25). The results suggested that the exercise intervention and control groups were similar in physical function scores.
Social function score
Four RCTs analyzed the changes in social function scores of 170 patients after the intervention (Fig. 4B). We performed a random-effects model to analyze differences between groups. The model revealed that the MD was 9.22, the 95% CI was − 5.35 to 23.80, the I2 was 80%, and the Chi2 value was 15.28 (p = 0.21). There was no significant difference between the two groups about the social function score.
Role-physical score
Four RCTs analyzed differences in role-physical scores (Fig. 4C). Pooled results from a fixed-effects model suggested that the exercise intervention group had no significant effect on the role-physical score (MD = 4.94, 95% CI − 4.76 to 14.63, p = 0.32).
Mental composite score
Our study included three RCTs (Fig. 4D). A fixed-effects model suggested that the MD was − 0.17, the 95% CI was − 4.80 to 4.46, the I2 was 0%, and the Chi2 value was 0.83 (p = 0.94), thus indicating that there was no significant difference between the two groups about the mental composite score.
General health score
Three RCTs analyzed the changes in the general health score of 139 patients after the intervention (Fig. 4E). We performed a random-effects model to analyze differences between groups. The model revealed that the MD was 7.90, the 95% CI was − 10.66 to 26.47, the I2 was 87%, and the Chi2 value was 14.87 (p = 0.40). There was no significant difference between the two groups about the general health score.
Body pain score
Three RCTs analyzed differences in body pain scores (Fig. 4F). Pooled results from a random-effects model suggested that the exercise intervention group had no significant effect on the body pain score (MD = 17.00, 95% CI − 9.13 to 43.14, p = 0.20).
Vitality score
Our study included three RCTs (Fig. 4G). A random-effects model suggested that the MD was 16.45, the 95% CI was − 7.82 to 40.71, the I2 was 93%, and the Chi2 value was 30.10 (p = 0.18), thus indicating that there was no significant difference between the two groups about the vitality score.
Role-emotional score
Because of p > 0.05, we analyzed the role-emotional score between the exercise intervention and control groups from three RCTs using a fixed-effects model (Fig. 4H). The pooled estimate of MD was − 1.21, 95% CI was − 11.69 to 9.27, I2 was 0%, and Chi2 was 0.40 (p = 0.82). The exercise intervention group had a similar role-emotional score compared to the control group.
Discussion
Many patients with ESRD eventually ultimately require kidney transplantation to stay alive. Although advances have been made in surgical procedures, many difficult clinical issues remain in the management of patients during post-transplant. The incidence of postoperative cardiovascular disease (CVDS) is 4–6 times higher in kidney transplant recipients than in the general population [26, 27]. It is currently the leading cause of death in kidney transplant recipients [28]. Relevant studies have reported that kidney transplant recipients are also at increased risk of dyslipidemia, possibly related to using immunosuppressive drugs such as cyclosporine, glucocorticoids, and sirolimus [29]. In addition, patients after kidney transplantation often present with significant motor dysfunction [30]. Therefore, post-transplant management is important for patient recovery, and adjuvant treatment strategies may have important prognostic potential.
Exercise intervention therapy is a rehabilitation method that focuses on functional exercise. Over recent years, exercise training has generated interest as an adjunctive treatment strategy for surgical procedures. Many studies pointed out that perioperative exercise intervention in patients with gastrointestinal tumors can reduce the risk of complications [31] and shorten postoperative hospital stays [32]. Cavalheri et al. [33] suggested training can improve exercise capacity and quadriceps strength in patients after lung cancer surgery. Besides, many studies also analyzed the effects of exercise intervention programs on patients after solid organ transplantation. Raphael et al. [34] found that exercise intervention can significantly improve heart transplantation patients' peak heart rate and aerobic capacity. Langer et al. [35] concluded that exercise training improves functional recovery in postoperative uncomplicated lung transplant patients and that postoperative exercise intervention programs should be strongly encouraged in elderly lung transplant recipients. Stefan [36] demonstrated that exercise training is safe for liver transplant recipients, improves physical functional aspects, and may benefit cardiopulmonary and muscle health. Emily et al. [37] proved that an exercise training intervention can improve exercise capacity and quality of life in patients after lung transplantation.
VO2peak is an important measure to evaluate cardiorespiratory fitness [17]. It is commonly used to analyze various patients' aerobic work capacity [38, 39] and physical exercise effects [40]. In addition, some research confirmed excellent test–retest reliability for VO2peak [41, 42]. Our study considered VO2peak as an index to evaluate cardiopulmonary function in patients after kidney transplantation.
The SF-36 is a commonly used tool to evaluate subjective health-related quality of life [43]. It is now widely available for evaluating the quality of life in various diseases. The SF-36 comprises eight items, each reflecting different health aspects: physical function, role-physical, body pain, general health, vitality, social function, role-emotional, and mental health. In the present study, we used the SF-36 scores to analyze the patients’ quality of life.
Our study included 16 RCTs containing 827 patients. We analyzed the effects of exercise intervention in kidney transplant recipients from eight dimensions, including anthropometric characteristics, body composition, renal function, cardiorespiratory function, blood parameters, serum cytokine levels, physical performance, and SF-36 scores. Analysis of the results revealed that exercise intervention had some positive effects on improving renal function. Specifically, patients in the exercise intervention group showed significantly improved creatinine and urea than the control group. In terms of cardiorespiratory function, patients following the exercise intervention program had significant superiority in improving VO2 peak. Moreover, exercise intervention induced improvements in HDL-C, 60-STS, 6-MWD, and 6-MWT. And no difference was found between groups in terms of anthropometric characteristics, body composition, serum cytokine levels, and SF-36 scores. These findings laid a theoretical foundation for introducing exercise intervention in kidney transplant recipients.
In contrast to previously published studies [44, 45], we found that exercise intervention had shown advantages in improving renal function and dyslipidemia in kidney transplant recipients. These findings may be more clinically relevant. Additionally, this study analyzed the efficacy of exercise intervention in patients with kidney transplants from multiple dimensions and involved more evaluation indicators, offering more comprehensive results. Finally, our study, with more RCTs and a larger sample size, included, may provide more reliable results.
There are some shortcomings in the present study. First, the intervention duration and exercise programs of each RCT included were not unified, which may also result in bias of results. Second, given that many studies in recent years have reported on the effects of exercise intervention on renal transplant recipients, our study was limited by its lack of novelty. Therefore, we will continue the research topic and focus on the latest RCTs to address this.
Conclusions
Our meta-analysis concluded that appropriate exercise intervention can improve renal function, cardiopulmonary function, dyslipidemia, physical performance, and quality of life in renal transplant recipients. The patients should be encouraged to participate in an exercise training intervention after kidney transplantation. These findings will assist clinicians in developing and applying exercise rehabilitation programs specifically designed for kidney transplant recipients as part of standard medical care. In addition, our study will help bridge the gap in knowledge about the importance of exercise intervention programs in kidney transplant patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ESRD:
-
End-stage renal disease
- ERAS:
-
Enhanced recovery after surgery
- RCTs:
-
Randomized controlled trials
- PICOS:
-
Populations, interventions, comparators, outcomes, and study designs
- SF-36:
-
Short form-36 questionnaire
- MD:
-
Mean difference
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
- BMI:
-
Body mass index
- BMD:
-
Bone mineral density
- LBM:
-
Lean body mass
- eGFR:
-
Estimated glomerular filtration rate
- HDL-C:
-
High-density lipoprotein-cholesterol
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- 60-STS:
-
60-Second sit-to-stand test
- 6-MWD:
-
6-Minute walk distance
- 6-MWT:
-
6-Minute walk test
- CVDS:
-
Cardiovascular disease
References
Jansz TT, Go MHY, Hartkamp NS, Stöger JL, Celeng C, Leiner T et al (2018) Health-related quality of life compared between kidney transplantation and nocturnal hemodialysis. PLoS One 13(9):386–394. https://doi.org/10.1016/j.xkme.2021.01.010
Schold JD, Buccini LD, Goldfarb DA, Flechner SM, Poggio ED, Sehgal AR (2014) Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin J Am Soc Nephrol 9(10):1773–1780. https://doi.org/10.2215/CJN.02380314
Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45(10):2163–2172. https://doi.org/10.1016/j.biocel.2013.05.036
Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N (2009) Risk of fractures after renal transplantation in the United States. Transplantation 87(12):1846–1851. https://doi.org/10.1097/TP.0b013e3181a6bbda
Gordon EJ, Prohaska T, Siminoff LA, Minich PJ, Sehgal AR (2005) Needed: tailored exercise regimens for kidney transplant recipients. Am J Kidney Dis 45(4):769–774. https://doi.org/10.1053/j.ajkd.2005.01.002
Janaudis-Ferreira T, Mathur S, Konidis S, Tansey CM, Beaurepaire C (2016) Outcomes in randomized controlled trials of exercise interventions in solid organ transplant. World J Transplant 6(4):774–789. https://doi.org/10.5500/wjt.v6.i4.774
Riess KJ, Haykowsky M, Lawrance R, Tomczak CR, Welsh R, Lewanczuk R et al (2014) Exercise training improves aerobic capacity, muscle strength, and quality of life in renal transplant recipients. Appl Physiol Nutr Metab 39(5):566–571. https://doi.org/10.1139/apnm-2013-0449
Pooranfar S, Shakoor E, Shafahi M, Salesi M, Karimi M, Roozbeh J et al (2014) The effect of exercise training on quality and quantity of sleep and lipid profile in renal transplant patients: a randomized clinical trial. Int J Organ Transplant Med 5(4):157–165
Senthil Kumar TG, Soundararajan P, Maiya AG, Ravi A (2020) Effects of graded exercise training on functional capacity, muscle strength, and fatigue after renal transplantation: a randomized controlled trial. Saudi J Kidney Dis Transpl 31(1):100–108. https://doi.org/10.4103/1319-2442.279929
Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Higgins JPT, Thomas J, Chandler J (2021) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021), Cochrane. www.training.cochrane.org/handbook
Higgins JP, Green S (eds) Cochrane handbook for systematic reviews of interventions. Version 5.3.0. The Cochrane Collaboration. www.cochranehandbook.org
Onofre T, Fiore Junior JF, Amorim CF, Minamoto ST, Paisani DM, Chiavegato LD (2017) Impact of an early physiotherapy program after kidney transplant during hospital stay: a randomized controlled trial. J Bras Nefrol 39(4):424–432. https://doi.org/10.5935/0101-2800.20170075
Karelis AD, Hébert MJ, Rabasa-Lhoret R, Räkel A (2016) Impact of resistance training on factors involved in the development of new-onset diabetes after transplantation in renal transplant recipients: an open randomized pilot study. Can J Diabetes 40(5):382–388. https://doi.org/10.1016/j.jcjd.2015.08.014
Hernández Sánchez S, Carrero JJ, Morales JS, Ruiz JR (2021) Effects of a resistance training program in kidney transplant recipients: a randomized controlled trial. Scand J Med Sci Sports 31(2):473–479. https://doi.org/10.1111/sms.13853
Kouidi E, Vergoulas G, Anifanti M, Deligiannis A (2013) A randomized controlled trial of exercise training on cardiovascular and autonomic function among renal transplant recipients. Nephrol Dial Transplant 28(5):1294–1305. https://doi.org/10.1093/ndt/gfs455
Greenwood SA, Koufaki P, Mercer TH, Rush R, O’Connor E, Tuffnell R et al (2015) Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: a 12-week pilot randomized controlled trial (the exercise in renal transplant [ExeRT] trial). Am J Kidney Dis 66(4):689–698. https://doi.org/10.1053/j.ajkd.2015.06.016
Hemmati N, Kazemi S, Jamshidian-Tehrani N, Roozbeh J, Koushkie Jahromi M, Salesi M et al (2022) Effects of exercise training on immunological factors in kidney transplant recipients; a randomized controlled trial. Res Sports Med 30(1):80–91. https://doi.org/10.1080/15438627.2021.1906671
Lima PS, de Campos AS, de Faria NO, Ferreira TCA, Amorim CEN, Stone WJ et al (2021) Effects of combined resistance plus aerobic training on body composition, muscle strength, aerobic capacity, and renal function in kidney transplantation subjects. J Strength Cond Res 35(11):3243–3250. https://doi.org/10.1519/JSC.0000000000003274
Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM et al (2002) A randomized trial of exercise training after renal transplantation. Transplantation 74(1):42–48. https://doi.org/10.1097/00007890-200207150-00008
Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M et al (2003) Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis 42(2):362–369. https://doi.org/10.1016/s0272-6386(03)00673-5
Tzvetanov I, West-Thielke P, D’Amico G, Johnsen M, Ladik A, Hachaj G et al (2014) A novel and personalized rehabilitation program for obese kidney transplant recipients. Transplant Proc 46(10):3431–3437. https://doi.org/10.1016/j.transproceed.2014.05.085
Juskowa J, Lewandowska M, Bartłomiejczyk I, Foroncewicz B, Korabiewska I, Niewczas M et al (2006) Physical rehabilitation and risk of atherosclerosis after successful kidney transplantation. Transplant Proc 38(1):157–160. https://doi.org/10.1016/j.transproceed.2005.12.077
O’Connor EM, Koufaki P, Mercer TH, Lindup H, Nugent E, Goldsmith D et al (2017) Long-term pulse wave velocity outcomes with aerobic and resistance training in kidney transplant recipients—a pilot randomised controlled trial. PLoS ONE 12(2):e0171063. https://doi.org/10.1371/journal.pone.0171063
Zhang P, Liu S, Zhu X, Liu H, Zeng L, Yan J et al (2023) The effects of a physical exercise program in Chinese kidney transplant recipients: a prospective randomised controlled trial. Clin Kidney J 16(8):1316–1329. https://doi.org/10.1093/ckj/sfad065. (eCollection 2023 Aug)
Oterdoom LH, de Vries AP, van Ree RM, Gansevoort RT, van Son WJ, van der Heide JJ et al (2009) N-terminal pro-B-type natriuretic peptide and mortality in renal transplant recipients versus the general population. Transplantation 87(10):1562–1570. https://doi.org/10.1097/TP.0b013e3181a4bb80
Aakhus S, Dahl K, Widerøe TE (2004) Cardiovascular disease in stable renal transplant patients in Norway: morbidity and mortality during a 5-yr follow-up. Clin Transplant 18(5):596–604. https://doi.org/10.1111/j.1399-0012.2004.00235.x
Ojo AO (2006) Cardiovascular complications after renal transplantation and their prevention. Transplantation 82(5):603–611. https://doi.org/10.1097/01.tp.0000235527.81917.fe
Holdaas H, Fellström B, Cole E, Nyberg G, Olsson AG, Pedersen TR et al (2005) Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant 5(12):2929–2936. https://doi.org/10.1111/j.1600-6143.2005.01105.x
Painter PL, Luetkemeier MJ, Moore GE, Dibble SL, Green GA, Myll JO et al (1997) Health-related fitness and quality of life in organ transplant recipients. Transplantation 64(12):1795–1800. https://doi.org/10.1097/00007890-199712270-00029
Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R et al (2018) Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 267(1):50–56. https://doi.org/10.1097/SLA.0000000000002293
Ahn KY, Hur H, Kim DH, Min J, Jeong DH, Chu SH et al (2013) The effects of inpatient exercise therapy on the length of hospital stay in stages I-III colon cancer patients: randomized controlled trial. Int J Colorectal Dis 28(5):643–651. https://doi.org/10.1007/s00384-013-1665-1
Cavalheri V, Burtin C, Formico VR, Nonoyama ML, Jenkins S, Spruit MA et al (2019) Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev 06(17):6. https://doi.org/10.1002/14651858.CD009955.pub3
Perrier-Melo RJ, dos Santos Figueira FAM, Guimarães GV, da Cunha Costa M (2018) High-intensity interval training in heart transplant recipients: a systematic review with meta-analysis. Arq Bras Cardiol 110(2):188–194. https://doi.org/10.5935/abc.20180017. (Epub 2018 Feb 19)
Langer D, Burtin C, Schepers L, Ivanova A, Verleden G, Decramer M et al (2012) Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant 12(6):1584–1592. https://doi.org/10.1111/j.1600-6143.2012.04000.x. (Epub 2012 Mar 5)
De Smet S, O’Donoghue K, Lormans M, Monbaliu D, Pengel L (2023) Does exercise training improve physical fitness and health in adult liver transplant recipients? A systematic review and meta-analysis. Transplantation 107(1):e11–e26. https://doi.org/10.1097/TP.0000000000004313. (Epub 2022 Oct 4)
Hume E, Ward L, Wilkinson M, Manifield J, Clark S, Vogiatzis I (2020) Exercise training for lung transplant candidates and recipients: a systematic review. Eur Respir Rev 29(158):200053. https://doi.org/10.1183/16000617.0053-2020
Lavie CJ, Milani RV, Mehra MR (2004) Peak exercise oxygen pulse and prognosis in chronic heart failure. Am J Cardiol 93(5):588–593. https://doi.org/10.1016/j.amjcard.2003.11.023
O’Neill JO, Young JB, Pothier CE, Lauer MS (2005) Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 111(18):2313–2318. https://doi.org/10.1161/01.CIR.0000164270.72123.18
Nourry C, Deruelle F, Guinhouya C, Baquet G, Fabre C, Bart F et al (2005) High-intensity intermittent running training improves pulmonary function and alters exercise breathing pattern in children. Eur J Appl Physiol 94(4):415–423. https://doi.org/10.1007/s00421-005-1341-4
Kyle SB, Smoak BL, Douglass LW, Deuster PA (1989) Variability of responses across training levels to maximal treadmill exercise. J Appl Physiol (1985) 67(1):160–165. https://doi.org/10.1152/jappl.1989.67.1.160
van’t Hul A, Gosselink R, Kwakkel G (2003) Constant-load cycle endurance performance: test-retest reliability and validity in patients with COPD. J Cardiopulm Rehabil 23(2):143–150. https://doi.org/10.1097/00008483-200303000-00012
Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30(6):473–483
Oguchi H, Tsujita M, Yazawa M, Kawaguchi T, Hoshino J, Kohzuki M et al (2019) The efficacy of exercise training in kidney transplant recipients: a meta-analysis and systematic review. Clin Exp Nephrol 23(2):275–284. https://doi.org/10.1007/s10157-018-1633-8
Chen G, Gao L, Li X (2019) Effects of exercise training on cardiovascular risk factors in kidney transplant recipients: a systematic review and meta-analysis. Ren Fail 41(1):408–418. https://doi.org/10.1080/0886022X.2019.1611602
Acknowledgements
All authors have no acknowledgments to disclose.
Funding
No specific funding.
Author information
Authors and Affiliations
Contributions
HXP and LP designed the research, interpreted the data and revised the paper. ZDX, YLQ, XBW and ZX performed the data extraction and carried out the meta-analysis. ZDX and YLQ drafted the paper. All of the authors approved the submitted and final versions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, D., Yu, L., Xia, B. et al. Systematic review and meta-analysis of the efficacy of exercise intervention in kidney transplant recipients. World J Urol 41, 3449–3469 (2023). https://doi.org/10.1007/s00345-023-04673-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04673-9