Abstract
Purpose
To evaluate follow-up strategies for active surveillance of renal masses and to assess contemporary data.
Methods
We performed a comprehensive search of electronic databases (Embase, Medline, and Cochrane). A systematic review of the follow-up protocols was carried out. A total of 20 studies were included.
Result
Our analysis highlights that most of the series used different protocols of follow-up without consistent differences in the outcomes. Most common protocol consisted in imaging and clinical evaluation at 3, 6, and 12 months and yearly thereafter. Median length of follow-up was 42 months (range 1–137). Mean age was 74 years (range 67–83). Of 2243 patients 223 (10%) died during the follow-up and 19 patients died of kidney cancer (0.8%). The growth rate was the most used parameter to evaluate disease progression eventually triggering delayed intervention. Maximal axial diameter was the most common method to evaluate growth rate. CT scan is the most used, probably because it is usually more precise than kidney ultrasound and more accessible than MRI. Performing chest X-ray at every check does not seem to alter the clinical outcome during AS.
Conclusion
The minimal cancer-specific mortality does not seem to correlate with the follow-up scheme. Outside of growth rate and initial size, imaging features to predict outcome of RCC during AS are limited. Active surveillance of SRM is a well-established treatment option. However, standardized follow-up protocols are lacking. Prospective, randomized, trials to evaluate the best follow-up strategies are pending.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small renal masses (SRM) are usually defined as incidentally discovered enhancing renal masses < 4 cm in maximal diameter with imaging consistent with cT1aN0M0 disease [1]. Incidental detection of SRM has become more common due to widespread use of ultrasound and cross-sectional imaging [2, 3]. SRM have a clinically diverse spectrum of disease due to the different natural history of renal tumors based on histology and size [4, 5]. All treatment options, including extirpative surgery (i.e., partial or radical nephrectomy), tissue ablation (i.e., cryotherapy and radiofrequency ablation), and active surveillance (AS), have been proved to be effective in the management of SRM [1, 6,7,8]. AS is defined as the initial monitoring of tumor size by serial abdominal imaging (US, CT, or MRI) with delayed intervention reserved for tumors showing clinical progression during follow-up [9]. Elderly and comorbid patients with incidental SRM have a low RCC-specific mortality and significant competing-cause mortality. In properly selected patients, AS can be an attractive treatment strategy. To date, optimal protocols and imaging modalities are still lacking on follow-up strategies for SRM [1]. Several groups have suggested different follow-up strategies for AS and Delayed Intervention (DI). The current review aimed to evaluate follow-up strategies for AS SRM.
Evidence acquisition
Search strategy
A comprehensive search of the Embase, Medline, and Cochrane databases was performed.
The search strategy included English language articles reporting on renal masses and surveillance from January 2000 to July 2020. The search terms are “surveillance”, “renal mass”, “renal cell carcinoma”, “kidney cancer”, and “elderly”.
Study selection
The results of the literature search were screened preliminarily by one reviewer (GR) using titles and abstracts’ duplicates have been eliminated. A secondary screening was performed (NP, CMM). The full texts and references of potentially appropriate literature were searched for further screening as recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Studies were considered relevant to the current systematic review when they included adult patients (age > 18 years) diagnosed with an SRM and enrolled on AS to compare growth rates and oncologic outcomes.
Inclusion criteria
Both prospective and retrospective studies evaluating adult patients (> 18 years old) who underwent AS and or delayed intervention (DI) for small renal masses were included. The following data were required: baseline demographics of patients; English language; schedule of follow-up and follow-up methods, and study enrolled at least 10 patients.
Exclusion criteria
The exclusion criteria were as follows: reviews or editor letters and single case report; non-English language publications; studies without consistent information on follow-up protocol and studies with insufficient or unconfirmed information.
Data extraction
Complete data, including the first author, publication year, number of patients, median age of patients, median FU, FU range, FU scheme, rate of delayed intervention, and metastatic progression, were extracted from the selected papers.
Assessment of the risk of bias
The risk of bias was minimized by the different revision of papers made by three authors (GR, NP, and CMM).
Final study selection
Using these search criteria, an initial selection of 1534 articles was considered. After exclusion of case series, review articles, and articles without follow-up protocol, we narrowed down to 110 studies which were selected for abstract screening. Finally, after the removal of duplicates, conference proceedings, abstracts, and non-English texts, 68 abstracts were reviewed. After a comprehensive review process, 20 full-text articles were included in this review, in accordance with the aforementioned inclusion criteria. A PRISMA flowchart is represented in Fig. 1. Primary outcome of interest was to evaluate method and frequency on follow-up protocols for SRM AS. Secondary outcomes included rate of delayed intervention and cancer-related mortality.
Evidence synthesis
Overall characteristics
Twenty studies were selected during the systematic search and their characteristics are detailed in Tables 1 and 2. Seven studies reported prospective collection with clear inclusion criteria and well-described follow-up. The remaining studies were based on retrospective reports.
Follow-up protocols
Follow-up protocols varied widely among the series, as shown in Table 2. Most common protocol was to perform imaging and clinical evaluation at 3, 6, and 12 months and yearly thereafter. Six studies performed a complete evaluation (CT/MRI + clinical evaluation and blood tests) every 6 month during the total length of follow-up (range 6–248 months). Overall, Abdominopelvic CT scan with iv iodine contrast was the most commonly used both for baseline and follow-up evaluation in accordance with the literature. Kidney ultrasound was used in 11 series, varying from a rate of 2% to 100% of patients; Fernando [12] and Pierorazio [20] reported 100% of FU through kidney ultrasound after an initial cross-sectional imaging at baseline. Moreover, all the seven prospective series performed kidney ultrasound. Four of the retrospective manuscripts reported the use of ultrasound (Table 2). Contrast enhanced ultrasound were not reported in any of the selected articles. MRI was performed in 12 studies (6–34%) (Table 2); Two series used magnetic resonance, because the first renal mass diagnosis was on MRI. Four prospective studies reported MRI evaluations at baseline and follow-up. Other series did not report the percentage of patients and/or the number of follow-up performed by MRI.
Characteristics of selected studies
The characteristics of the studies included are presented in Table 1. The follow-up was described in all the studies with a median length of 42 (range 1–137) months. Mean age was 74 years (range 67–83). Of 2243 patients, 223 (10%) died during the follow-up and 19 (0.8%) patients died of kidney cancer. After enrollment of AS, 50 patients (2.2%) were diagnosed with metastasis during follow-up. Rate of delayed intervention, metastatic patients, and related deaths were reported in all the studies except one (Parker) and are listed in Table 1. Overall 421 patients (19%) underwent delayed intervention, as shown in Table 2. It should be noted that two series described a retrospective collection of patients who could not undergo surgery immediately; therefore, the two studies reported a 100% rate of delayed intervention between 9 and 97 months. The delayed intervention rate was consistent among the series (range 0–30%). No differences were reported about different timing of follow-up and different rate of DI.
No study reported the rationale of their frequency of follow-up schedules.
No series reported difference in efficacy of detecting growth rate between follow-up methods.
Chest investigation
Chest X-ray (CXR) was performed as routine in 9 studies. Only Lamb 2004 [10] performed CXR at every follow-up. Four series performed CXR during baseline evaluation and two others performed CXR at baseline and at 1 year follow-up. Chest CT was not performed as a routine exam in any series. Haramis [11] and Fernando [12] performed Chest CT if metastatic disease was suspected or detected.
Growth rate
All the selected studies used the growth rate (GR) of the solid mass to evaluate disease progression. Maximal axial diameter was used to evaluate growth rate (GR) in 90% of the studies. As previously stated, CT scan was the most used also to determine the growth rate of the index lesion. Fernando [12] and Pierorazio [13] scheduled the follow-up performing kidney ultrasound in 100% of the patients and using CT or MRI as per physician’s choice in case of uncertainty or changes in ultrasound findings.
Delayed intervention
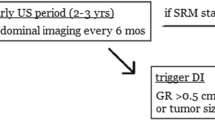
Delayed intervention is defined as surgery (radical or partial nephrectomy) and/or radiofrequency postponed at least 6 months following the diagnosis. Two series reported 100% of DI (Li 2012 [14] and Crispen 2009 [15] see Table 1), because the design of the study was a retrospective evaluation of patients who did not receive immediate surgical treatment and started an active surveillance. Overall 421 (19%) patients underwent delayed intervention (13% without the two series with 100% rate of delayed intervention). The delayed intervention rate was consistent among the series, with 0–30% rate of DI (Table 1). The most common trigger for delayed intervention was a growth rate > 0.5 cm/yr.
Renal biopsy was performed in a small representation of patients (< 30% see Table 1). No renal biopsy was scheduled in the follow-up protocol in any article of the series.
Discussion on different regimens of follow-up and current evidence
Within the literature guidelines [1] (AUA, EAU), AS remains an important therapeutic option for selected patients. It may be ideal to spare the surgical intervention with its complications in those patients who carry significant comorbidity profiles. Our analysis highlights that most of the series used different protocols of follow-up without consistent differences in the outcomes, as shown in Table 1. This reinforces that many patients can remain on active surveillance for a prolonged period of time regardless of the FU protocol. Furthermore, there was a considerable difference between prospective and retrospective studies as the follow-up was not standardized. Of 2243 patients, only 19 (0.8%) died because of renal cancer and only 50 had cancer-related metastasis (2.2%). These results might be explained by several reasons (elder patients, high rate of delayed intervention); however, it does not seem to correlate with the FU scheme.
In addition to initial tumor size, the natural history of SRM can be characterized by their growth kinetics [16]. The change in maximum tumor diameter over time, known as linear tumor growth [17, 18], is used as a parameter to evaluate disease progression and decide on delayed intervention during AS [19,20,21]. It is expressed by variations of cm/year, and usually, two evaluations per year should be enough to determine significant variations of tumor growth [20]. Disease progression is considered when mass grows more than 0.5 cm/year or over 4 cm, due to the higher rate of metastasis if one or both of these conditions are present [6]. Outside of growth rate and initial size, imaging features to predict outcome of RCC during AS are limited [22]. For those placed on AS, the American Urological Association (AUA), the National Comprehensive Cancer Network (NCCN), and the European Association of Urology (EAU) guidelines recommend abdominal imaging with CT or MRI within 6 months from initiation of AS and subsequent imaging (CT, MRI, or ultrasound) performed annually thereafter for as long as the patient is on active surveillance [23]. During this time, the size of the lesion should be closely monitored, as growth kinetics is a commonly cited surrogate for metastatic potential and decision for further intervention. No differences are reported regarding the method of follow-up in any guideline. Although the growth rates for SRM may be highly variable, several studies in our series have reported a mean growth rate varying between 0.3 and 0.4 cm/year [24,25,26]. A systematic review showed lesions on AS that progressed to metastatic disease demonstrated a significantly higher growth rate (0.8 cm/year) compared to those with favorable outcomes (0.3 cm/year) [27]. Anyway, despite general consensus in the literature about certain criteria to trigger delayed intervention, there are no formal guidelines on the topic.
Performing CXR at every check does not seem to alter the clinical outcome during AS. In fact, CXR is known to be a low yield diagnostic tool for detecting pulmonary metastasis in patients treated for RCC [28, 29] and does not seem to affect the outcomes of active surveillance if performed at every FU step [30]. Ideally, follow-up imaging should be performed with a consistent modality to limit inter-modality variability in mass characterization and size measurements [16]. CT scan is the most used exam mainly because it is usually more precise than kidney ultrasound and more accessible than MRI [31]. Abdominal CT provides information on function and morphology of the contralateral kidney during enrollment [32], primary tumor extension; enlargement of locoregional LNs; condition of the adrenal glands and other solid organs.
Eleven studies (55%) performed US during follow-up; Fernando [20], Schiavina [12], and Pierorazio [33] followed every patient by kidney ultrasound, while CT or MRI were used at the discretion of the physician in case of uncertainty or changes in ultrasound findings. US can be time -saving, accessible, and precise enough on growth rate, unfortunately burdened by being operator-dependent, difficult to reproduce and unreliable to detect disease progressions. US is considered the preferred imaging modality for the screening of RCC (sensitivity and specificity of 82–83% and 98–99%, respectively [22]).
Twelve studies (60%) performed MRI during the AS; MRI is able to perform precise axial imaging on patients who are allergic to iv iodine contrast [22, 34] and it is an option for CKD patients with GFR 30–60 mL/(min 1.73 m2) who cannot undergo CT with iodine contrast (recommended GFR > 60 mL/(min 1.73 m2) [35]. MRI appears to have a good sensitivity and specificity on RCC diagnosis, but it can be expensive and time-consuming [36]. MRI should not be considered a perfect replacement for CT during staging and follow-up as CT's sensitivity for pulmonary nodules is clearly superior [37]. Less common use of MRI, in comparison to CT (see Table 2), stems from its higher associated costs, its decreased accessibility, and poorer patient tolerance [38]. Future developments in imaging technology, including the identification of predictive imaging biomarkers, can be promising advances that may help predict patient outcomes [39].
The type and timing of follow-up was poorly described in most of the series. Indeed, a comparison between different outcomes regarding different methods of follow-up was not reported.
On the basis of these studies and current guidelines, most common enrollment into AS is with initial abdominal axial imaging (CT or MRI) and chest imaging. The most used initial follow-up imaging is every 6 months in the early AS period (2 years), while a longer time interval (every 12 months) for the late AS period assuming radiographic stability of the SRM. Regarding SRM progression the vast majority of patients underwent DI when tumor GR > 0.5 cm/year or absolute tumor size > 4 cm.
Randomized, longer term, prospective studies are required to find the best intervals that reduce the harms, costs, and invasiveness of serial imaging while minimizing the risk of undertreating masses that harbor metastatic potential.
Conclusions
Active surveillance of SRM is a well-established treatment option. However, standardized follow-up protocols are lacking. Prospective, randomized, trials to evaluate the best follow-up strategies are pending.
Data availability
We declare that all materials and all data are available and do not involve plagiarism.
References
Finelli A, Ismaila N, Bro B et al (2017) Management of small renal masses: American society of clinical oncology clinical practice guideline. J Clin Oncol 35(6):668–680. https://doi.org/10.1200/JCO.2016.69.9645
Volpe A (2016) The role of active surveillance of small renal masses. Int J Surg 36(Pt C):518–524. https://doi.org/10.1016/j.ijsu.2016.06.007
Kane CJ, Mallin K, Ritchey J et al (2008) Renal cell cancer stage migration: analysis of the national cancer data base. Cancer. https://doi.org/10.1002/cncr.23518
Chawla SN, Crispen PL, Hanlon AL et al (2006) The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. https://doi.org/10.1016/S0022-5347(05)00148-5
Gill IS, Aron M, Gervais DA, Jewett MAS (2010) Clinical practice. Small renal mass. N Engl J Med 362(7):624–634. https://doi.org/10.1056/NEJMcp0910041
Smaldone MC, Kutikov A, Egleston BL et al (2012) Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. https://doi.org/10.1002/cncr.26369
Jewett MAS, Rendon R, Lacombe L et al (2015) Canadian guidelines for the management of small renal masses (SRM). J Can Urol Assoc. https://doi.org/10.5489/cuaj.2969
Kunkle DA, Egleston BL, Uzzo RG (2008) Excise, ablate or observe: the small renal mass dilemma—a meta-analysis and review. J Urol 179(4):1227–1233; discussion 1233–1234. https://doi.org/10.1016/j.juro.2007.11.047
Volpe A, Panzarella T, Rendon RA et al (2004) The natural history of incidentally detected small renal masses. Cancer. https://doi.org/10.1002/cncr.20025
Lamb GWA, Bromwich EJ, Vasey P, Aitchison M (2004) Management of renal masses in patients medically unsuitable for nephrectomy—natural history, complications, and outcome. Urology. https://doi.org/10.1016/j.urology.2004.05.039
Haramis G, Mues AC, Rosales JC et al (2011) Natural history of renal cortical neoplasms during active surveillance with follow-up longer than 5 years. Urology. https://doi.org/10.1016/j.urology.2010.09.031
Fernando HS, Duvuru S, Hawkyard SJ (2007) Conservative management of renal masses in the elderly: our experience. Int Urol Nephrol. https://doi.org/10.1007/s11255-006-9119-0
Uzosike AC, Patel HD, Alam R et al (2018) Growth kinetics of small renal masses on active surveillance: variability and results from the DISSRM registry. J Urol. https://doi.org/10.1016/j.juro.2017.09.087
Li XS, Yao L, Gong K et al (2012) Growth pattern of renal cell carcinoma (RCC) in patients with delayed surgical intervention. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-011-1083-0
Crispen PL, Viterbo R, Fox EB et al (2008) Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. https://doi.org/10.1002/cncr.23268
Liaw CW, Winoker JS, Mehrazin R (2018) Imaging protocols for active surveillance in renal cell carcinoma. Curr Urol Rep 19(10):81. https://doi.org/10.1007/s11934-018-0830-z
Celtik KE, Shah PH, Patel VR et al (2017) Active surveillance for incidental renal mass in the octogenarian. World J Urol. https://doi.org/10.1007/s00345-016-1961-9
Lee SW, Sung HH, Jeon HG et al (2016) Size and volumetric growth kinetics of renal masses in patients with renal cell carcinoma. Urology. https://doi.org/10.1016/j.urology.2015.10.051
Mason RJ, Abdolell M, Trottier G et al (2011) Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol. https://doi.org/10.1016/j.eururo.2011.02.023
Pierorazio PM, Johnson MH, Ball MW et al (2015) Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. https://doi.org/10.1016/j.eururo.2015.02.001
Finelli A, Cheung DC, Al-Matar A et al (2020) Small renal mass surveillance: histology-specific growth rates in a biopsy-characterized cohort. Eur Urol. https://doi.org/10.1016/j.eururo.2020.06.053
Diaz de Leon A, Pedrosa I (2017) Imaging and screening of kidney cancer. Radiol Clin North Am 55(6):1235–1250. https://doi.org/10.1016/j.rcl.2017.06.007
Donat SM, Diaz M, Bishoff JT et al (2013) Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol. https://doi.org/10.1016/j.juro.2013.04.121
Crispen PL, Viterbo R, Boorjian SA et al (2009) Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer. https://doi.org/10.1002/cncr.24338
Rosales JC, Haramis G, Moreno J et al (2010) Active surveillance for renal cortical neoplasms. J Urol. https://doi.org/10.1016/j.juro.2010.01.024
Kouba E, Smith A, McRackan D et al (2007) Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol. https://doi.org/10.1016/j.juro.2006.09.064
Campi R, Sessa F, Corti F et al (2020) Triggers for delayed intervention in patients with small renal masses undergoing active surveillance: a systematic review. Minerva Urol Nefrol. https://doi.org/10.23736/S0393-2249.20.03870-9
Canvasser NE, Stouder K, Lay AH et al (2016) The usefulness of chest X-rays for T1a renal cell carcinoma surveillance. J Urol. https://doi.org/10.1016/j.juro.2016.02.068
Doornweerd BHJ, de Jong IJ, Bergman LM, Ananias HJK (2014) Chest X-ray in the follow-up of renal cell carcinoma. World J Urol. https://doi.org/10.1007/s00345-013-1176-2
Kassiri B, Cheaib JG, Pierorazio PM (2019) Patients with small renal masses undergoing active surveillance-is yearly chest imaging necessary? J Urol. https://doi.org/10.1097/JU.0000000000000079
Yamashita Y, Ariyoshi A, Sakamoto K (1989) The therapeutic value of lymph node dissection for renal cell carcinoma. Nishinihon J Urol 51:777–781
Gong IH, Hwang J, Choi DK et al (2012) Relationship among total kidney volume, renal function and age. J Urol. https://doi.org/10.1016/j.juro.2011.09.005
Schiavina R, Borghesi M, Dababneh H et al (2015) Small renal masses managed with active surveillance: predictors of tumor growth rate after long-term follow-up. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2014.08.006
Mueller-Lisse UG, Mueller-Lisse UL, Meindl T et al (2007) Staging of renal cell carcinoma. Eur Radiol. https://doi.org/10.1007/s00330-006-0554-1
ACR Committee M on CD and C (2015) Manual on Contrast Media, v10.1
Vogel C, Ziegelmüller B, Ljungberg B et al (2019) Imaging in suspected renal-cell carcinoma: systematic review. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2018.07.024
Platzek I, Zastrow S, Deppe PE et al (2010) Whole-body MRI in follow-up of patients with renal cell carcinoma. Acta radiol. https://doi.org/10.3109/02841851003724846
Krishna S, Murray CA, McInnes MD et al (2017) CT imaging of solid renal masses: pitfalls and solutions. Clin Radiol 72(9):708–721. https://doi.org/10.1016/j.crad.2017.05.003
Gorin MA, Rowe SP, Allaf ME (2015) Nuclear imaging of renal tumours: a step towards improved risk stratification. Nat Rev Urol 12(8):445–50. https://doi.org/10.1038/nrurol.2015.122
Beisland C, Hjelle KM, Reisæter LAR, Bostad L (2009) Observation should be considered as an alternative in management of renal masses in older and comorbid patients. Eur Urol. https://doi.org/10.1016/j.eururo.2008.12.031
O’Connor KM, Davis N, Lennon GM et al (2009) Can we avoid surgery in elderly patients with renal masses by using the Charlson comorbidity index? BJU Int. https://doi.org/10.1111/j.1464-410X.2008.08275.x
Lane BR, Abouassaly R, Gao T et al (2010) Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer. https://doi.org/10.1002/cncr.25184
Parker PA, Alba F, Fellman B et al (2013) Illness uncertainty and quality of life of patients with small renal tumors undergoing watchful waiting: a 2 year prospective study. Eur Urol. https://doi.org/10.1016/j.eururo.2013.01.034
Tang DH, Nawlo J, Chipollini J et al (2017) Management of renal masses in an octogenarian cohort: is there a right approach? Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2017.05.011
Siu W, Hafez KS, Johnston WK, Wolf JS (2007) Growth rates of renal cell carcinoma and oncocytoma under surveillance are similar. Urol Oncol Semin Orig Investig. https://doi.org/10.1016/j.urolonc.2006.07.018
Jewett MAS, Mattar K, Basiuk J et al (2011) Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 60:39–44. https://doi.org/10.1016/j.eururo.2011.03.030
McIntosh AG, Ristau BT, Ruth K et al (2018) Active surveillance for localized renal masses: tumor growth, delayed intervention rates, and >5 yr clinical outcomes[figure presented]. Eur Urol. https://doi.org/10.1016/j.eururo.2018.03.011
Author information
Authors and Affiliations
Contributions
All authors have contributed to the information and material submitted for publication and all authors have read and approved the manuscript. GR: data collection, data analysis, and manuscript writing. NP: data collection, data analysis, and manuscript writing. MCM: data collection, data analysis, and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no direct or indirect commercial financial incentives associated with publishing the manuscript.
Human participants and/or animals
No research involving human participants and/or animals.
Informed consent
No informed consent required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rebez, G., Pavan, N. & Mir, M.C. Available active surveillance follow-up protocols for small renal mass: a systematic review. World J Urol 39, 2875–2882 (2021). https://doi.org/10.1007/s00345-020-03581-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03581-6