Abstract
Non-interventional monitoring of small renal mass (SRM) patients provides a means to avoid unnecessary treatment and its risks, preserve renal function, and maintain quality of life without detriment in oncologic outcomes. Active surveillance is distinct from observation due to its intent to avoid metastasis and perform curative delayed intervention (DI) in patients who demonstrate local progression during follow-up, while both options aim to avoid cancer-specific mortality. This chapter describes the definitions, biology, patient factors, tumor factors, practice patterns, and reported oncologic outcomes that underlie active surveillance management of SRM patients, as well as a detailed description and comparison of current consensus guidelines on SRM patient management from national and international urology and medical oncology committees. Utilization of active surveillance has gradually increased over the past two decades, however, substantial urology practice variation underscores that factors related to the provider and healthcare setting may be more important than tumor factors in driving current utilization. Provider-related differences may in turn reflect variable awareness or comfort/certainty regarding active surveillance management regimens and/or oncologic safety. Selection criteria for active surveillance are divided into specific tumor factors and patient factors, with the latter including life expectancy, comorbidity burden, renal function, comfort/anxiety, and medical compliance. Tumor factors include tumor size and cystic nature, with the role of biopsy histology remaining controversial. Regarding monitoring, there is consensus for the early use of multiphasic cross-sectional imaging and long-term transition to ultrasound, with an initial short-term reimaging interval (e.g., 3–6 months) used to rule out rapid growth prior to longer intervals of 6–12 months. Any change in oncologic risk during active surveillance that surpasses treatment risk should trigger DI. Tumor factor triggers for DI can be summarized as (acronym “GLASS”) Growth rate, Longest tumor diameter (LTD), Adverse/unfavorable histology, Stage (i.e., cT3), and Symptoms (hematuria, etc.), where growth rate and LTD represent common triggers, and histology/stage/symptoms represent uncommon triggers. Despite limitations of the current literature, accumulating evidence indicates that active surveillance is a safe initial management strategy for many SRM patients. Reports of SRM metastases while still <4 cm are rare and limited to tumors >3 cm with above-average growth rates (>3 mm/year). Most patients appear to have durable freedom from treatment, particularly those with smaller initial tumor sizes. Much of the “active surveillance” literature continues to be contaminated with observation patients, which may overestimate rates of metastasis among patients who are compliant with an active surveillance program. In summary, active surveillance of SRM patients can allow unnecessary treatment to be safely delayed or avoided, minimizing the impact of associated treatment side effects, and with low-to-negligible oncologic risk. More investigation is needed to standardize objective tumor thresholds for triggering DI, and to define the role of active surveillance in young healthy patients, and its impact on quality of life and health care finance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Small renal masses (SRM) are defined as renal cortical tumors that are less than 4 cm. SRM include both benign neoplasms and renal cell carcinomas (RCC), the latter of which have only rare metastatic potential [1]. Historically, surgery was the main and only curative management option for these tumors. With the contemporary over-utilization of cross-sectional imaging, there has been a significant shift from diagnosis of symptomatic advanced disease with large primary tumors towards incidental detection of asymptomatic clinically localized SRM [2, 3]. While radical nephrectomy was the gold standard management option for decades, the treatment for localized renal tumors, especially for SRM, has more recently shifted towards less aggressive treatments such as partial nephrectomy and thermal ablation due to growing appreciation of treatment morbidity [4, 5] and the value of renal preservation [6]. The additional realization of low SRM metastatic potential and high (20%–40%) rates of benign resection in surgical series [7,8,9,10] have together driven the field to consider even more conservative management with an initial expectant approach, aided by the clinical success of active surveillance for low-risk prostate cancer that established a precedent for a successful “less is more” approach. Thus, the concept of non-interventional monitoring of an SRM patient was born as a means to avoid unnecessary treatment, preserve renal function, remove the risk for surgical complications, and maintain quality of life without detriment in oncologic outcomes [11,12,13,14]. With active surveillance of SRM patients, unnecessary treatment can be delayed or avoided, minimizing the impact of associated treatment side effects and with low-to-negligible oncologic risk. This chapter describes the definitions, biology, patient factors, tumor factors, practice patterns, and reported oncologic outcomes that altogether underlie active surveillance management of SRM patients, as well as a detailed description and comparison of current consensus guidelines on SRM patient management from national and international urology and medical oncology committees.
Active Surveillance Definition
Active surveillance is one of two types of expectant management for SRM patients, the other being observation (i.e., watchful waiting). Active surveillance is distinct from observation due to its intent for curative delayed intervention (DI) in patients who demonstrate local progression during follow-up. In contrast, observation is reserved for patients whose comorbidities or otherwise shortened life expectancy contraindicate any curative intent treatment. Avoidance of cancer-specific mortality is a primary goal with either active surveillance or observation. However, whereas metastasis avoidance is paramount during active surveillance, metastasis may be conceded during observation when the likelihood of death from other causes predominates [15]. The European Association of Urology (EAU) [15] and The American Urological Association (AUA) [11] explicitly endorse distinction between active surveillance and observation, however, much of the “active surveillance” literature is contaminated with observation patients, particularly studies published more than a decade ago that still heavily drive current consensus guidelines.
Small Renal Mass (SRM) Indolence
SRM tumors are heterogenous and include both benign and malignant histologic subtypes. Benign tumors including renal angiomyolipomas or oncocytomas represent 20–40% of SRM [7, 16,17,18]. When malignant, SRM are usually low grade (≤2) and low stage (T1a) RCC, with high-grade (3–4) and/or pT3 tumors comprising only 15–25% of surgical cases [19,20,21,22,23]. Yet regardless of histology, SRM rarely metastasize, and risk of death from other causes is generally higher independent of age, comorbidity, and tumor size [24]. Compared to large (>7 cm) RCCs, SRM RCCs have much less genomic complexity and fewer subclonal events [25]. As tumors grow and evolve, acquisition of late subclonal chromosomal changes leads to divergence of gene expression [25]. Cooperative genomic initiatives such as that from TRACERx support low mutational diversity in SRM tumors [5, 26, 27]. This biology is referred to the “VHL mono driver” evolutionary subtype because other (i.e., non-VHL) driver mutations are uniquely lacking, and it is largely specific to SRM tumors. The VHL mono driver subtype is characterized by limited genetic evolutionary branching and often requires decades to acquire mutations conducive to metastatic potential, yielding excellent long-term survival outcomes [26, 27].
Trends in Active Surveillance Utilization
Utilization of active surveillance across urology practices has gradually increased over the past two decades. While international studies are lacking, analysis of U.S. population registries (SEER, NCDB) suggests that active surveillance rates, once <5%, have increased to ~17–18%, although perhaps static over the most recent decade [28, 29]. During this time, there have been even greater increases in either partial nephrectomy or percutaneous thermal ablation, even among the elderly [30]. Recently, particularly high rates of active surveillance utilization have been described by certain Urology practices, suggestive of evolving comfort among both physicians and patients. In a recent study from Roswell Park Cancer Center, >95% of all SRM patients seen over 5 years by a single urologic oncologist underwent active surveillance management, regardless of patient age or health, representing the first report in which the vast majority of SRM patients deferred immediate treatment [22]. Substantial variation in active surveillance utilization was observed across a variety of academic and private practices in Michigan [31]. These two studies underscore that factors related to the provider and healthcare setting may be more important than tumor factors in driving current active surveillance utilization. Provider-related differences may in turn reflect variable awareness or comfort/certainty regarding active surveillance management regimens and/or oncologic safety, perhaps reflecting a lack of standardization among different consensus group guidelines (see later in the chapter).
Selection of Patients for Active Surveillance
There is strong general agreement, including among different consensus guidelines groups, to select active surveillance patients based on a combination of tumor size, comorbidities, and life expectancy [11, 14, 15, 32,33,34]. Patient selection criteria can be divided into specific tumor factors and patient factors, as described below.
Tumor Factors
-
Tumor Size (a.k.a., Longest Tumor Diameter, LTD). The strong association between LTD and metastasis is well established from the surgical literature. Rates of metachronous metastasis approach 0% for SRM <2 cm, and are <1% for SRM <3 cm, and 2–3% for SRM of 3–4 cm, increasing exponentially once a tumor surpasses 4 cm [24, 35,36,37,38]. Surgical series outcomes are consistent with size-specific rates of metastasis reported in active surveillance studies. In a systematic review of early active surveillance series, metastasis never occurred below a primary tumor LTD of 3 cm, and ~90% of all metastases occurred after the primary tumor surpassed 4 cm [39, 40]. More contemporary active surveillance series similarly support a negligible metastatic rate for LTD of <3 cm, and a very low metastatic rate for LTD of 3–4 cm, with the vast majority of reported metastases occurring after the LTD has passed 4 cm (Table 2.1). Accordingly, patients with SRM <2 cm can be considered ideal patients for active surveillance, but accumulating research (see Reported Active Surveillance Outcomes, below) also supports the oncologic safety of active surveillance for patients with SRM up to 4 cm. At present, consensus guidelines panels of the American Urological Association (AUA), National Comprehensive Cancer Network (NCCN), and Canadian Urological Association (CUA) recommend consideration (AUA, NCCN) if not preferential selection (CUA) of active surveillance for all patients with a SRM <2 cm regardless of age or health. The American Society of Clinical Oncologists (ASCO) and European Society of Medical Oncologists (ESMO) both support active surveillance for an SRM up to 4 cm if patients have significant comorbidities and/or short life expectancy. The European Association of Urologists (EAU) guidelines do not currently endorse any specific size threshold for active surveillance patient selection.
-
Cystic vs. Solid. Compared to solid tumors, predominantly cystic tumors (Bosniak III-IV) tend to have more favorable pathology and prognosis, with only rare occurrence of metastasis [51, 52]. Accordingly, Bosniak III-IV SRM are ideal masses for active surveillance. Consensus guidelines panels of the AUA, CUA, and NCCN all support active surveillance as a first line option for SRM up to 4 cm if predominantly cystic, regardless of health or age.
-
Renal Mass Biopsy (RMB) Histology. No imaging modality reliably distinguishes malignant and benign SRMs, and tumor growth rate also is unreliable [12]. RMB can be used to differentiate benign vs. malignant SRM, and also to help detect unfavorable malignant histology (nuclear grade ≥ 3, papillary type 2 RCC, translocation RCC, unclassified/indeterminable RCC subtypes) that may worsen active surveillance candidacy. RMB is thus increasingly used to guide SRM patient management, with general consensus that it is helpful but not required in this setting. RMB accuracy for benign vs. malignant distinction is excellent, with a recent large meta-analysis reporting the diagnostic sensitivity and specificity of core biopsies for malignancy to be 99.1% and 97.7%, respectively [53]. Non-diagnostic biopsies can occur in 5–15% of cases, which should trigger either a second biopsy attempt or histology-agnostic management. Richard et al. showed that, conservatively, 10% of patients could have avoided treatment of tumors with confirmed benign histology [54]. Similarly, high-volume active surveillance centers including Roswell Park Cancer Center and the Canadian RCC Consortium have successfully avoided benign SRM resection using routine RMB [12, 22, 55]. In addition to benign tumor histology identification, RMB provides prognostic information to guide malignant SRM patient management, with high specificity albeit low sensitivity for unfavorable RCC histology (i.e., high nuclear grade and/or papillary non-type 1, unclassified RCC/non-specified subtype or translocation RCC). Unfavorable pathology is infrequent among SRMs, but its occasional presence suggests a higher oncologic risk that may warrant intervention. The prognostic impact in SRM patients of different favorable RCC histologies (low grade clear cell vs. papillary type 1 vs. chromophobe) is more controversial, although the clear cell subtype appears to have faster growth and higher progression/metastasis/intervention rates [22, 55]. Current consensus guidelines generally provide histologic subtype-agnostic recommendations but also commonly support RMB at the discretion of the provider, whenever management may be influenced by the result.
Patient Factors
-
Comorbidities and Age/Life Expectancy. SRM patients are more likely to die of other causes than of kidney cancer, and this is most evident among the elderly or patients with significant comorbidities [56,57,58,59]. Therefore, all major guidelines [11, 14, 15, 32,33,34] favor active surveillance in patients with short life expectancy, although the precise definition of short is often not provided [11, 32, 34]. AUA guidelines [11] favor active surveillance in elderly patients with life expectancy <5 years, while ASCO guidelines [14] consider an absolute indication for patients with life expectancy <5 years; and relative indication for patients with life expectancy <10 years. In young and healthy patients, the role of active surveillance remains more controversial, particularly for SRM of >2 cm. Recently, DISSRM investigators published a subgroup analysis focused on young patients [60]. This study included 224 SRM patients with age ≤60, including 68 (30%) patients electing active surveillance. Tumor sizes in the active surveillance cohort were typically quite small (median 1.5 cm), and the median growth rate was only 0.09 cm/year, with 27% experiencing zero growth. Twenty (29%) active surveillance patients experienced a clinical progression event defined as an elevated growth rate or elective crossover to DI with objective tumor progression, and 13 (19.1%) total patients ultimately crossed over to DI. Local progression-free survival in these young active surveillance patients was 67% at 5 years, and without any metastases [60]. Similarly, a 72% rate of active continuation beyond 5 years was reported in Roswell Park Cancer Center’s recently updated experience of active surveillance recommended to over 200 consecutive progression-free SRM patients without age-related or health-related selection bias, which yielded a relatively young and healthy active surveillance cohort [61]. Thus, active surveillance may be a safe option in young and healthy patients, and also durable in a substantial portion, but further investigation is needed.
-
Renal Function. Renal function is another important factor during consideration of treatment for SRM patients. An estimated loss 10–20% in glomerular filtration rate is expected with conventional nephron-sparing surgery, however, lower losses might be achievable with an enucleation surgical approach [30]. Patients with chronic kidney disease who are at high risk for end-stage renal disease (and associated cardiac/other morbidity) with treatment are ideal candidates for active surveillance.
-
Disease Uncertainty. Disease uncertainty in either the patient or physician can generate anxiety that may swing the risk/benefit balance towards definitive treatment. Historically, this factor has had a predominant role in the management of SRM patients. The impact of the provider and healthcare is supported by the highly variable rates of active surveillance management across different providers. The Michigan Urological Surgery Improvement Collaborative (MUSIC) initiative recently observed widely variable rates of active surveillance management among 13 urology practices, ranging from 0% to 68% [31]. At Roswell Park Cancer Center, nearly all SRM patients seen over a 5-year period were recommended active surveillance. Intriguingly, despite the highly conservative nature of this approach, almost all of these patients agreed to active surveillance after initial informed counseling (95% vs. 1% immediate treatment vs. 4% unknown), including a 100% rate of active surveillance election among patients following up at Roswell Park [22]. These findings underscore the close interconnection between the healthcare provider/setting, anxiety and informed counseling, which altogether continues to have a significant impact on SRM patient management decisions.
Active Surveillance Imaging and Monitoring
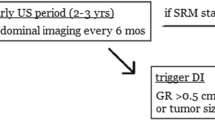
The foundation of active surveillance monitoring is periodic renal mass imaging and staging for metastatic progression, with or without adjunct use of RMB [62]. Many prospective active surveillance pathways report the use of multiphasic cross-sectional imaging initially, but transition in the long-term to ultrasound of more indolent masses in order to minimize risks related to radiation and contrast exposure [22, 55, 63]. Short-term interval reimaging of the tumor (typically within 3–6 months) is uniformly endorsed to rule out rapid growth, followed by progressively longer intervals of 6–12 months [12, 19, 22, 63]. The Roswell Park Cancer Center pathway incorporates size-based interval for initial reimaging, with an initial 3-month vs. 6-month scan recommended for tumors >3 cm vs. ≤3 cm, respectively, given the higher metastatic potential of the former [22]. Baseline chest imaging to rule out pulmonary metastasis is universally recommended [36]. However, the utility of subsequent chest monitoring is more controversial, since the metastatic risk approaches 0% in the absence of significant SRM growth [39]; and there are well established psychologic, medical, and financial harms to incidental pulmonary findings. Some active surveillance centers therefore condone omission of repeat chest imaging unless there is (1) an abnormality on baseline imaging, (2) significant SRM growth, or (3) plans for DI, particularly since 20% of chest imaging tests reveal other abnormalities that are typically non-actionable [62, 64].
Role of Renal Mass Biopsy (RMB) in Active Surveillance
RMB is increasingly used to aid SRM patient management by identifying occasional benign or unfavorable histology, with a general consensus that RMB is helpful but not required. Complications from RMB are uncommon and limited primarily to a 1% incidence of clinically significant bleeding, with historical concerns of tumor seeding being largely dismissed due to rarity. RMB usage has grown recently due to growing consensus of a high diagnostic rate and excellent safety profile [53, 65], with some contemporary active surveillance cohorts such as those of Roswell Park and the Canadian RCC Consortium reporting RMB utilization rates of >50% [12, 22, 66]. RMB is often deferred until LTD reaches >2 cm, given the negligible oncologic risk and lower technical success rates of biopsy at smaller sizes [11, 18, 22]. However, some programs, such as at Roswell Park and Bologna (Italy), also utilize RMB in smaller SRM (i.e., <2 cm) with a rapid growth rate (>5 mm/year) to rule out benign tumor histology prior to committing to DI conversion [20, 22]. The DISSRM consortium did not historically utilize RMB, but has evolved to selectively recommend RMB to active surveillance patients with rapid tumor growth or patient-specific LTD sizes (e.g., >2, >3 or >4 cm) [67].
Triggers for Delayed Intervention (DI) During Active Surveillance
An increase in oncologic risk during active surveillance that surpasses the treatment risk is an absolute indication for conversion to DI. The oncologic-treatment risk balance is assessed largely by the same tumor factors and patient factors that drive initial selection of active surveillance patients, with the important exception that tumor growth kinetics revealed during active surveillance can be additionally utilized to improve risk assessment [22]. Historically, the assessment of this risk balance has been largely subjective, which has challenged standardization efforts [21, 63]. In some series, specific objective thresholds are mentioned, but rates of patient’s meeting these thresholds are not reported [12, 63], while many patients who meet these thresholds are not converted to DI, perhaps due to common contamination of active surveillance cohorts with unhealthy patients more suited to watchful waiting [39, 40]. While disease uncertainty and patient anxiety continue to drive high variability in contemporary DI rates (11–50%) [22, 27, 35, 36, 68,69,70]; DI is increasingly triggered by objective tumor factors rather than subjective patient factors, reflecting increased present-day comfort with the concept of treatment deferral for SRMs [12, 19, 21, 46, 47, 49, 50, 55, 68, 74, 76].
Tumor Factors
Despite patient-related factors greatly impact the DI rates even in the contemporary studies, tumor related factors are the main referred factors for progression criteria for intervention (PCI), aka DI triggers. PCI standardization has been challenged by inconsistent usage of and variability in proposed thresholds [21, 22, 27, 35, 47, 49, 61, 71,72,73,74,74], yielding substantial variability in reported PCI rates (9–30%) [22, 27, 69]. Roswell Park Cancer Center has proposed tumor PCI to fall within 5 categories under the acronym “GLASS”: 1- Growth rate; 2- Longest tumor diameter; 3- Adverse (i.e., unfavorable) biopsy histology; 4-Stage (i.e., radiographic infiltration); 5-Symptoms. Based on both incidence and likely impact, growth rate and longest tumor diameter can be considered major PCI, whereas other categories can be considered minor PCI. Roswell Park Cancer Center excludes patients with benign RMB histology from meeting PCI, avoiding unnecessary DI in approximately 15% of SRM patients on active surveillance [22].
Major “GLASS” PCI
-
Growth rate. Numerous retrospective studies support a significant association of tumor growth rate with RCC grade [22, 48, 68, 76] and metastatic potential [20, 39, 46,47,48,49,50, 69]. Faster growing tumors also appear to be more likely to have clear cell histology [55]. The systematic review by Smaldone et al. of >800 patients from early active surveillance series identified a median growth rate of 6.5 mm/year among metastatic patients compared to 2.5 mm/year in non-metastatic patients [39]. Rapid primary tumor growth is the most common predefined PCI used in contemporary active surveillance series [21, 22, 35, 46, 55, 71,72,72]. Of the approximately 3 dozen reported cases to date of metastasis during active surveillance for which the primary tumor growth rate is also provided (Table 2.1) [19, 39, 41,42,43, 45,46,47,48,49,50, 75], the vast majority had a rapid primary tumor growth rate >5 mm/year, suggesting this otherwise uncommon feature (only ~15% of all SRMs) to be useful for predicting metastasis. Furthermore, there has not yet been a reported metastasis on active surveillance for a SRM that remains <4 cm in size with a growth rate ≤3 mm/year (Table 2.1), suggesting that this majority subset of SRM patients may have negligible metastatic risk as long as they remain in this category. Despite limitations of these data, which include common retrospective study designs introducing potential bias in retrospective growth rate measurement, growth rate may still be the best readily measurable indicator of metastatic risk, in addition to tumor LTD. Current AUA, ASCO, and CUA guidelines recommend a linear growth rate of >5 mm/year as a PCI threshold, which is most commonly studied threshold in the active surveillance literature and met by ~13–18% of active surveillance patients [21, 22, 45, 52, 68, 76]. The Roswell Park team [22] has proposed size-stratified growth rate PCI: for SRMs <3 cm, a growth rate threshold of 5 mm/year is used; however, for SRMs with LTD ≥3 cm, they endorse a more conservative growth rate threshold of only >3 mm/year to trigger DI, due to a 2–3% rate of metastasis at this size and multiple reports of metastasis for LTD of 3–4 cm with a growth rate of 4–5 mm/year but not ≤3 mm/year (see Table 2.1); as well as the high likelihood that SRMs with LTD >3 cm and confirmed GR >3 mm/year will ultimately meet a size-based PCI threshold (i.e., LTD >4 cm) within only 1–3 years anyway.
-
Longest Tumor Diameter. The association between LTD and metastasis in both surgical and active surveillance series was described earlier (see above: Selection of Patients For Active Surveillance / Tumor Factors) including a negligible metastatic rate below 3 cm, and infrequent metastasis between 3 and 4 cm (Table 2.1) [46, 47, 49, 50, 55, 67]. At present, 4 cm is accordingly the most commonly used PCI threshold size for triggering DI in reported active surveillance series. In the Roswell Park cohort [22], 9% of patients developed LTD >4 cm, including 30% of PCI cases and 50% of DI cases; whereas only 25% of DI cases in the Canadian RCC Consortium cohort were triggered by LTD >4 cm [12]. In contrast, the AUA guidelines [11] endorse an LTD threshold of >3 cm to trigger DI in non-hereditary active surveillance patients. One rationale for this lower size threshold is that patients with LTD surpassing 3 cm have high risk to progress to LTD >4 cm shortly thereafter, as Menon et al. [22] observed that only half of patients surpassing 3 cm LTD remained PCI-free 3 years later. However, use of >3 cm as a predefined PCI in active surveillance series is rarely reported, and likely overtreats many patients, particularly those with slow growth (<3 mm/year) for which metastasis rates appear to be negligible.
Minor “GLASS” PCI
Very few series have utilized minor PCI to date, so their value as DI triggers remains unclear. Roswell Park observed that only <3% vs. 30% of SRM patients met minor PCI vs. major PCI, respectively, during active surveillance.
-
Adverse (Unfavorable) Histology: As described above (see Selection of Patients For Active Surveillance/Tumor Factors/Renal Mass Biopsy), RMB has low sensitivity but high specificity for adverse/unfavorable RCC histology, defined as grade ≥3, papillary non-type 1 RCC, translocation RCC, or unclassified/indeterminable RCC subtypes. Given the higher metastatic risk of unfavorable histology in surgical series [23], patient with unfavorable histology on RMB should be considered for discontinuation of active surveillance.
-
Stage (Invasion/Infiltration): Upstaging from cT1a to cT3a is rarely observed in active surveillance series but is known independent prognostic variable for metastasis in surgical series. Therefore, active surveillance patients with new radiologic findings of tumor infiltration sufficient for cT3a upstaging should be considered for DI given the potentially higher metastatic risk. In the Roswell Park cohort only one patient (1%) developed cT3 disease who also progressed by both growth rate and LTD. [22] Similarly, the Canadian Consortium reported only one (1%) patient with DI triggered by tumor thrombus [12].
-
Symptoms: SRM appear to be almost always asymptomatic. Symptoms that are classically related to RCC tumors, such as gross hematuria, retroperitoneal bleeding/pain, and paraneoplastic syndrome, appear to be rarely if ever observed in SRM patients. To date, the DISSRM Registry [21, 63] had identified no cases of gross hematuria during active surveillance, while the Roswell Park cohort [22] reported one case and the Canadian RCC Registry [12] identified three cases, although bleeding was not clearly related to the SRMs. It is important that other reasons for new symptoms be ruled out before considering DI in SRM patients on active surveillance.
Patient Factors
Although conversion to DI is increasingly triggered by tumor factors (i.e., tumor PCI), only a few contemporary active surveillance centers such as at Roswell Park Cancer Center or University of Bologna have reported tumor PCI development as the most common reason for treatment [20, 22]. Instead, most DI cases in the contemporary active surveillance reports are still performed due to patient factors without PCI development [12, 18, 44, 46, 68, 76]. In the multicenter prospective registries of DISSRM and Canadian RCC Consortium, around 50% or more of patients who crossover to DI do so without tumor PCI development [12, 63].
-
Patient Preference/Anxiety. At present, patient preference due to anxiety remains the most common patient factor triggering DI, underscoring the persistence of disease uncertainty in contemporary SRM management [12, 21, 44, 46, 68]. The DISSRM team has strived to quantify the impact of anxiety on SRM patients and the durability of active surveillance, demonstrating statistical associations with general quality of life, cancer-specific quality of life, and distress [69]. However, in a structured active surveillance program, mental health scores appear to improve over time, as patient comfort may increase with demonstration of tumor indolence during surveillance (e.g., slow/no growth) [70]. As discussed above, the provider and health care setting likely have a major role in patient acceptance and tolerance of active surveillance, as reflected by very low rates of DI due to anxiety reported by some active surveillance centers (e.g., Roswell Park—1%; University of Bologna—4%) [20, 22]. To minimize unnecessary conversion to DI, in depth discussions from the provider may be necessary to empower the patient with knowledge regarding details such as planned PCI thresholds and expected outcomes, such as slow or potentially zero growth, negligible metastatic risk in the absence of PCI development, and excellent likelihood for freedom from treatment for at least 5 years (see below, Reported Active Surveillance Outcomes). Additionally, the provider should emphasize the ability to intervene with DI well in advance of missing a window for cure.
-
Life Expectancy. Whereas PCI development should be an absolute indication for DI in young/healthy patients, continued active surveillance using modified (less stringent) PCI or conversion to observation may be appropriate in elderly/comorbid patients with limited life expectancy. Future metastasis will be clinically insignificant when life expectancy is limited (e.g., <5 years) [71]. On the other hand, improved patient health during active surveillance (e.g., resolution of an acute health issue such as stented coronary artery disease) may increase life expectancy and swing the risk-benefit balance towards treatment [46]. The Roswell Park active surveillance program recently described an algorithm that integrates PCI triggers with life expectancy estimations to guide decision making regarding DI conversion [22].
-
Other Patient Factors. Although an uncommon scenario, patients with end-stage renal disease may require resection to be eligible for renal transplantation [49, 62]. Risk for patient non-compliance is rarely reported for DI conversion, but is perhaps under-utilized since many reported metastases during active surveillance have been ascribed to lost patient follow-up that resulted in large primary tumor sizes [39, 49]. Unrelated additional surgery has also been reported as a DI trigger [72], but this reason is generally not endorsed. Similarly, concern for losing a window to perform nephron-sparing treatment should not trigger early DI, since active surveillance does not appear to compromise the ability for nephron-sparing treatment [20,21,22, 44, 47, 49, 55, 68, 76].
Research Support for Active Surveillance
Early research in active surveillance was guided by pioneering studies from the National Cancer Institute on hereditary RCC (particularly VHL syndrome), which accounts for ~5% of all RCC cases. The long-term dialysis risk of these patients due to bilateral/multifocal tumor intervention(s) necessitated more conservative management such as active surveillance. Moreover, prior surgical series indicated the metastatic potential of VHL syndrome-related RCC to be closely related to primary tumor size, with metastasis never observed with tumors <3 cm [38]. Therefore, a threshold tumor size of 3 cm was adopted as a trigger for intervention, and active surveillance thus grew into routine practice for renal tumors <3 cm in patients with VHL syndrome and certain other hereditary RCC syndromes [38, 73]. This pioneering work revealed the oncologic safety of active surveillance in treatment risk-adverse SRM patients, and set the stage for extrapolation of this management into sporadic (non-hereditary) RCC patients, as described below.
Systematic Reviews
Early research into active surveillance management for sporadic renal tumors included predominantly patients who were unfit for surgery, at a time when thermal ablation was not yet widely available as a less invasive option. Many of these patients were, by current definitions, “observation” patients rather than “active surveillance” patients, since curative DI was never intended due to significant comorbidity and/or limited life expectancy. Nevertheless, this early work was pivotal in revealing clinically indolent behaviors of SRM, namely their generally slow (and frequently zero) growth, and their very low metastatic potential including a near-zero incidence with tumor sizes <3 cm, paralleling the hereditary RCC patient active surveillance literature. These early series were excellently summarized in a comprehensive systemic review and pooled subset analysis by Smaldone et al. in 2012, which included active surveillance studies published between 1966 and 2010 [39]. In total these investigators identified 18 retrospective active surveillance series comprising 880 patients with 936 “small renal masses” (median size 2 cm), although many masses were in fact >4 cm at active surveillance initiation (range up to 12 cm); and ~1 in 4 patients were likely “observation” patients, given reportedly an unacceptable operative/renal risk with treatment that negated elective treatment; and tiny lesions of necessarily indeterminate nature (e.g., 0.2 cm) appear to have been included. With median follow-up of 27.5 months, the median linear growth rate was 0.25 cm/year (range −1.4 to +2.5 cm/year), and ~1 in 4 tumors showed zero net growth. Six studies comprising 259 patients (284 masses, median age 69 years) provided adequate individualized data for pooled analysis, in which 45% of tumors progressed to DI after a median of 24 months. Patient factors unrelated to tumor growth (e.g., anxiety) were responsible for most (64%) DI conversions, while tumor growth accounted for only a minority (36%). Of all 880 patients, only 18 (2%) progressed to metastasis, and in most of these cases the patients were not candidates for surgery due to health risks, consistent with “observation” status, and with large tumor sizes (commonly >6 cm) at the time of metastasis. As expected, patients who progressed to metastasis had significantly older age (median 78 vs. 69), larger initial tumor size (median 3.1 vs. 2.0 cm), larger final tumor size (median 5.9 vs. 2.7 cm), and faster tumor growth (median 0.65 vs. 0.25 cm/year). Only 2 metastatic patients had a primary tumor size <4 cm at the time of metastasis, and no tumor metastasized when it remained <4 cm with a growth rate of ≤3 mm/year. This large systematic review thus guided more contemporary active surveillance protocols regarding tumor thresholds for conversion to DI related tumor size and growth rate, setting the stage for contemporary active surveillance management in progressively healthier patients with SRM [22].
A more recent systematic review by Mir et al. [40] in 2018 analyzed 28 active surveillance studies (cT1-cT2) published from 2000 to 2017, although only 10 of these studies included exclusively cT1a patients (median tumor size 2 cm). The median age for the cT1a cohort was 72 years, reflecting selection bias for elderly patients. The median follow-up, at 43 months, was longer than that of the Smaldone et al. review [39] and similar to or longer than most reported surgical series. DI rates for these cT1a patients varied widely (1–26%), likely reflecting the predominant influence of patient factors rather than tumor factors. Similar to the Smaldone review, the median tumor growth rate was 0.37 cm/year overall and 0.22 cm/year for cT1a tumors, while faster among patients electing DI (overall 0.73 cm/year, cT1a 0.62 cm/year). Metastasis rates were low (1.4% for cT1a, 2.5% overall) and cancer-specific mortality was 1%. This review substantiated slow tumor growth and low metastasis and cancer-specific mortality, supporting the oncologic safety of active surveillance for patients with SRMs.
Active Surveillance Series with Prospective Management Pathways
A comprehensive summary of published contemporary active surveillance series (>50 patients, minimum) is provided in Table 2.2. Conclusions are limited by the commonly retrospective study designs and heterogeneous active surveillance management strategies even within a single active surveillance center. A major challenge to interpreting this literature has been the scarcity of prospectively applied active surveillance approaches, particularly with regard to imaging approaches (modality/intensity/frequency) and objective tumor PCI thresholds for triggering DI. Recently, several centers have described the use of prospectively applied active surveillance pathways, with or without required protocol enrollment. These series differ in nuances of their prospective pathways, but collectively provide strong support for the durable safety of active surveillance.
-
The Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) Registry described by Pierorazio et al. in 2015 [63] and updated by Gupta et al. in 2019 [21] includes a large cohort of non-randomized SRM patients who were prospectively enrolled with the primary outcome measure of oncologic outcome between active surveillance vs. surgery. DI was recommended for renal masses with growth rate >0.5 cm/year or size >4 cm. Per most recent update, a total of 727 SRM patients were enrolled, including 371 (51%) electing active surveillance with median follow-up of 23.6 months, during which 46 (12.4%) crossed over to DI. Median tumor size in the active surveillance cohort was only 1.7 cm and median age was 71 years, reflecting expected selection biases. Compared to the surgical cohort, active surveillance patients were significantly older, unhealthier, and more likely to have smaller and multiple tumors. No progression to metastasis or cancer-specific deaths occurred in active surveillance patients, whereas two deaths occurred in the surgery group (p = 0.8); and 33 (8.9%) patients died secondary to other causes. Although the median follow-up interval for surveillance patients was only 23.6 months, 25% of this cohort had at least 5-year follow-up, and the 5-year DI-free survival rate was 78%. Hence, most active surveillance patients had durable avoidance of treatment and without any apparent compromise in oncologic outcome. While overall rates of PCI were not reported, half of DI cases were ascribed to PCI while around half were due to patient preference, underscoring the persistent role for anxiety in truncating surveillance and perpetuating overtreatment.
-
The prospective registry of the Renal Cell Cancer Consortium of Canada includes active surveillance patients from eight centers, the initial outcomes of which were detailed by Jewett et al. in 2011 [12]. From 2004 to 2009, 178 patients with 209 incidentally detected SRMs who unfit for surgery due to advanced age, comorbidities, or treatment refusal were enrolled (median age 74 years, median size 2.1 cm). Patients were excluded if they had less than a 2-year life expectancy, SRM diagnosis >12 months prior to enrollment, systemic therapy for other malignancies, or a known hereditary RCC syndrome. Tumor progression was prospectively defined as size ≥4 cm, doubling of volume in ≤12 months, or metastasis. With a median follow-up of 29 months, 27 (15%) patients progressed, including 13 (48%) due to size, 12 (44%) due to doubling rate, and 2 (7%) due to metastases. Only 9 of 25 (36%) patients who progressed locally underwent DI, suggesting likely “observation” status at the time of progression. Median tumor growth was only 0.13 cm/year, and ~1 in 3 tumors did not grow. More recently, this consortium described outcomes of active surveillance (2004–2015) for a 134 SRM patient subgroup with biopsy-proven RCC (median age 70 years, median size 2.3 cm) [55]. This report is significant because it focused exclusively on malignant SRM, exploiting this consortium’s common if not routine use of renal mass biopsy, in contrast to other high-volume active surveillance centers in the U.S. or Europe (other than Roswell Park Cancer Center, see below). The 5-year PCI rate was 54%, with the majority of PCI cases having clear cell histology (73%). The median growth rate was 0.17 cm/year in the first year and 0.19 cm/year over the first 3 year, although a mathematically predicted 12-year growth rate was 0.28 cm/year. The growth rate with clear cell history was significantly higher than with other RCC subtypes, which commonly had no growth (median 2.5 vs. 0.2 mm/year, respectively). Moreover, metastases occurred in 6 (4%) patients, all with clear cell histology, and most of which had local progression. Importantly, 2 patients had metastasis without “significant” local progression, although the specific sizes and growth rates were not reported for these 2 cases. Overall, 29 patients died, including 3 due to metastases, 23 due to other causes, and 3 due to unknown causes.
-
More recently, Roswell Park Comprehensive Center has described a unique clinical practice of a single urologic oncologist, in which active surveillance was recommended universally over ~5 consecutive years to all SRM patients lacking evidence of local progression at the time of presentation, a practice which resulted in >95% of all newly presenting SRM patients undergoing active surveillance [22]. Thus there was no health- or age-related selection bias, which is novel among reported active surveillance series. This cohort is the first reported consecutive patient series in which the vast majority of SRM patients deferred immediate treatment. Per their prospective management pathway, DI was recommended only if tumor PCI developed during active surveillance. PCI thresholds were prospectively defined using the GLASS criteria described above. However, for the growth rate threshold, the researchers utilized a size-stratified cut-off, including growth rate >5 mm/year for SRM size ≤3 cm, but >3 mm/year for SRM size >3 cm. Similarly, initial repeat cross-sectional imaging (CT or MRI) and staging chest X-ray (CXR) were recommended after 6 months for SRM size <3 cm, but after 3 months for SRM size >3 cm. Additional serial imaging was then obtained every 6 months until tumor stability was observed (<3 mm/year over a 2–3-year period). Patients with low oncologic risk (tumor stability or benign histology on biopsy) and/or high treatment risk were switched to annual ultrasound monitoring after at least 3 years of cross-sectional imaging. Patients who met >1 progression criteria were offered treatment if life expectancy was >15 years or were converted to observation if life expectancy was <5 years. Overall, of the 128 patients electing active surveillance, 75% remained DI-free at 3 years and none metastasized. In their recently updated report of 201 patients with median follow-up of 40 months [61], the 5-year PCI-free and DI-free survival rates were 60% and 71%, respectively. Worse PCI-free and DI-free survival was associated with the initial tumor size and clear cell RCC biopsy histology, supporting the importance of histologic subtype as also reported by the Canadian RCC Consortium (see above). DI resections were enriched for pT3 and/or nuclear grade 3–4 malignant pathology (55% of DI cases), including no benign resections, suggesting that their prospectively applied PCI thresholds may be effective at identifying more aggressive SRM cases for treatment, given that only 15–25% of SRM generally harbor adverse pathology. Importantly, only 1 active surveillance patient had crossed over to DI without PCI development, indicating excellent overall tolerance of the “universal” active surveillance approach at their cancer center. No patient developed metastasis, supporting the oncologic safety of this unique approach.
-
The Michigan Urological Surgery Improvement Collaborative (MUSIC) recently initiated a prospective kidney mass registry for all patients with newly presenting cT1 renal masses, with a goal to assess initial management decisions across a diverse range of urology practices [31]. From September 2017 to April 2019, patients with cT1a or cT1b renal masses were studied from 13 practices. The terminology of observation was used in this study instead of active surveillance. Out of 965 patients, an initial observation period was employed in 48% (n = 459), with individual practice rates ranging widely from 0% to 68%. As expected, patients managed with observation (vs. immediate treatment) were significantly older (71.2 vs. 62.8 year) and had smaller tumors (2.3 vs. 3.4 cm). Observation was used for 53.5% of cT1a renal masses, 29.9% of cT1b renal masses, and 42.5%, 53.7%, and 63.9% of radiographically solid, Bosniak III–IV cystic, and indeterminate cT1RMs, respectively. Factors significantly associated with observation in multivariable analysis included lesion type (Bosniak III–IV vs. solid), tumor stage (cT1a vs. cT1b), and higher age. DI was performed in only 3.1% (14 patients) of the observation patients in a median follow-up of 24.6 months. In univariate analyses, physicians were more likely to observe a cT1 renal mass if they practiced in a non-academic setting (52.9% vs. 42.8%, p = 0.002) and if RMB was not performed (49.2% vs. 39.5%, p = 0.022); however, these associations were not maintained in multivariable analyses. This early work is informative to capture the current large variation in active surveillance utilization among contemporary academic and private practices.
Summary of Consensus Guidelines
Commonly used guidelines for the management of SRM include those from the American Urological Association (AUA), European Association of Urology (EAU), Canadian Urology Association (CUA), National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO). Summaries of these guidelines are provided below and in Table 2.3. Other national Urological societies including those from England, Japan, Saudi Arabia, and Argentina have published guidelines on the management of RCC, however, these guidelines have not been updated for more than 5 years and are not discussed here.
American Urological Association (AUA) Guidelines
The AUA updated their guidelines on SRM active surveillance in 2021 [11]. The current guidelines make a conditional recommendation (Evidence Level: Grade C) for active surveillance with potential DI as an appropriate initial management for all SRM patients with a tumor that is either <2 cm or predominantly cystic. For larger SRM, the AUA recommends prioritizing active surveillance/expectant management whenever the anticipated risk of intervention or competing risks of death outweigh the potential oncologic risks of the tumor. Tumor radiographic or histologic features that favor active surveillance/expectant management per the AUA include size <3 cm, growth rate <5 mm per year, non-infiltrative appearance, predominantly cystic nature, intralesional fat suggestive for an AML, or favorable histology by renal mass biopsy (RMB). Regarding patient factors, the guidelines favor active surveillance/expectant management in elderly patients with life expectancy <5 years, high comorbidities, excessive perioperative risk, poor functional status, marginal renal function. The AUA guideline also emphasizes the importance of informing patients regarding the possibility of benign rather than malignant tumor.
The AUA guidelines endorse a role for RMB in assessing active surveillance candidacy in patients with a solid or complex cystic renal mass (if adequate solid component) in whom the risk/benefit analysis for treatment is equivocal. For those with predominantly cystic lesions, RMB should be avoided. Regarding imaging modality, the AUA guidelines recommend that the initial scan consist of contrast-enhanced cross-sectional imaging, while subsequent imaging may include the same or an abdominal ultrasound. For imaging interval, the AUA guidelines recommend an initial 3–6-month period to assess interval growth, after which the subsequent imaging interval should be individualized to the patient based on growth rate, tumor biology, risk calculations and shared decision making focusing on goals, risks and triggers for intervention. Regardless of the surveillance intensity, surveillance chest imaging with plain radiography is recommended annually or whenever intervention triggers or symptoms arise. Acknowledging a lack of level 1 supporting evidence, the AUA guidelines recommend that triggers for DI should generally be driven by changes in risk based on a combination of these tumor factors (absolute size >3 cm, median growth rate in excess of 5 mm/year, clinical stage migration, infiltrative appearance, or aggressive histology on RMB) and patient factors (life expectancy, comorbidities), with continual objective reassessments that may include the use of RMB when appropriate. More specifically, DI should be recommended per AUA guidelines whenever substantial interval growth is observed or other clinical/imaging findings suggest that the risk/benefit analysis is no longer equivocal or favorable for AS continuation, although it is not explicitly stated in the guideline what constitutes equivocal or favorable risks, permitting some subjectivity in evaluation.
European Association of Urology (EAU) Guidelines
The EAU guidelines (2022 updated annually) [15] make a weak recommendation to offer active surveillance to frail or comorbid patient with SRM considering the slow tumor growth in most cases and low progression rate to metastatic disease (1–2%). The guideline does not mention any specific size or period for follow-up. The EAU guidelines make a weak recommendation for RMB when active surveillance is under consideration and acknowledge that characterization of histological grade and subtype by is useful to select SRM patients at lower risk of progression that can be managed safely with active surveillance. The EAU guidelines explicitly distinguish the concept of active surveillance from that of watchful waiting, and do not require follow-up imaging for the latter.
Canadian Urology Association (CUA) Guidelines
Recently published guidelines from CUA [34] for managing a patient with an SRM(s) acknowledge that there is no “one-size-fits-all” strategy and emphasize the consideration of shared decision making based on the tumor characteristics, competing medical risks and patient’s values and preferences. As with AUA and EAU guidelines, CUA guidelines differentiate active surveillance from watchful waiting (observation). For SRM <2 cm, these guidelines endorse active surveillance as the preferred strategy over immediate intervention (Conditional recommendation). For SRM 2–4 cm, either active surveillance or definitive treatment (partial nephrectomy or thermal ablation) is endorsed (Conditional recommendation). RMB is recommended whenever the result may alter management, but not for patients who will undergo surgical removal regardless of histology or watchful waiting patients who will not undergo treatment regardless of the RMB result. The panel defines indications for conversion to DI as growth >4 cm and/or consecutive growth rates of >0.5 cm/year (clinical principle). In case of suspected tumor growth on ultrasound, cross-sectional imaging should be performed to confirm growth prior to intervention. The guidelines recommend contrast-enhanced CT or MRI at baseline like other guidelines. Different than other guidelines, acknowledging that the sensitivity for metastasis is low with chest X-ray compared to a chest CT, the guidelines suggest a chest X-ray at the initial imaging of choice, given the low incidence of metastasis and lower harms and cost. If any abnormalities are detected on the chest X-ray, a chest CT should be performed. For patient on follow-up during AS, the panel recommended routine abdominal ultrasound until definitive treatments are no longer considered. Chest X-ray imaging was recommended during follow-up for metastatic staging. The panel was unable to achieve a consensus as to the frequency of abdominal imaging. Similarly, no consensus was reached for the interval of metastatic staging, which varied from for-cause to once a year.
National Comprehensive Cancer Network (NCCN) Guidelines
Similar to AUA guidelines, NCCN guidelines (2022) [32] endorse active surveillance as an option for the initial management of patients with SRM <2 cm or with a predominantly cystic component. For the cT1 masses up to 7 cm, they recommend active surveillance as the primary consideration if there is decreased life expectancy or significant competing risks of death/morbidity from intervention. Unlike AUA and EAU guidelines, the current NCCN guidelines do not differentiate active surveillance from watchful waiting. NCCN guidelines recommend RMB at initiation of active surveillance or at follow-up, as clinically indicated. In order to determine the tumor growth rate, abdominal imaging with contrast-enhanced CT or MRI is recommended within 6 months of active surveillance initiation and every 6 months for the first 2 years; subsequent imaging may be performed annually thereafter with either cross-sectional imaging or ultrasound. The NCCN guidelines state that all three imaging modalities (US, CT, and MRI) accurately predict pathologic tumor size, therefore, best clinical judgment should be used in choosing the imaging modality. For metastatic staging, NCCN guidelines recommend chest x-ray or chest CT at baseline, annually “as clinically indicated,” and whenever intervention is under consideration. However, these guidelines also allow follow-up to be individualized based on “surgical status,” treatment schedules, side effects, comorbidities, and symptoms.
American Society of Clinical Oncology (ASCO) Guidelines
ASCO guidelines [14] (2017) recommend that active surveillance for SRM should be an initial management option for patients who have significant comorbidities and limited life expectancy. They note an absolute indication for patients at high risk for anesthesia/intervention or life expectancy <5 years; and relative indication for patients with significant risk of end-stage renal disease if treated, SRM <1 cm, or life expectancy <10 years. The guideline recommends that a RMB biopsy should be considered for all patients with an SRM when the results may alter management. The guideline recommends a staging chest x-ray and axial abdominal imaging (or ultrasonography) every 3 months in the first year of active surveillance and twice in the second and third years (yearly thereafter), which is notably more frequent than other guidelines endorse. DI triggers supported by ASCO include tumor growth >0.5 cm/year or size >4 cm, depending on the patient’s comorbidities and life expectancy.
European Society of Medical Oncology (ESMO) Guidelines
ESMO guidelines [33] (2019) are mainly focused on metastatic RCC and provide very limited recommendations and discussion on active surveillance. The guidelines recommend active surveillance as an option in elderly patients with significant comorbidities or those with a short life expectancy and SRM measuring <4 cm. RMB is recommended to select patients with SRM for active surveillance, because of the incidence of non-malignant tumors in this setting. The guidelines do not mention any recommendation on follow-up, progression or treatment for patients elected for active surveillance.
Conclusion
Despite limitations of the current literature, accumulating evidence indicates that active surveillance is a safe initial management strategy for many SRM patients. Future research should focus on standardization of objective PCI definitions and characterization of long-term active surveillance outcomes, including rates of DI, metastasis, and cancer-specific survival. Additionally, more investigation is needed to define the role of active surveillance in young healthy patients, as well as impacts on quality of life and health care finance.
Abbreviations
- DI:
-
Delayed intervention
- PCI:
-
Progression criteria for intervention
- RCC:
-
Renal cell carcinoma
- RMB:
-
Renal mass biopsy
- SRM:
-
Small renal masses
References
Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med. 2010;362(7):624–34. https://doi.org/10.1056/NEJMcp0910041.
Patel HD, Gupta M, Joice GA, et al. Clinical stage migration and survival for renal cell carcinoma in the United States. Eur Urol Oncol. 2019;2(4):343–8. https://doi.org/10.1016/j.euo.2018.08.023.
Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113(1):78–83. https://doi.org/10.1002/cncr.23518.
Mir MC, Derweesh I, Porpiglia F, et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol. 2017;71(4):606–17. https://doi.org/10.1016/j.eururo.2016.08.060.
Network CGAR. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–9. https://doi.org/10.1038/nature12222.
Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–52. https://doi.org/10.1016/j.eururo.2010.12.013.
Bhindi B, Thompson RH, Lohse CM, et al. The probability of aggressive versus indolent histology based on renal tumor size: implications for surveillance and treatment. Eur Urol. 2018;74(4):489–97. https://doi.org/10.1016/j.eururo.2018.06.003.
Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178(2):429–34. https://doi.org/10.1016/j.juro.2007.03.106.
Pahernik S, Ziegler S, Roos F, et al. Small renal tumors: correlation of clinical and pathological features with tumor size. J Urol. 2007;178(2):414–7; discussion 416-7. https://doi.org/10.1016/j.juro.2007.03.129.
Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–20. https://doi.org/10.1097/01.ju.0000095475.12515.5e.
Campbell SC, Clark PE, Chang SS, et al. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA guideline: part I. J Urol. 2021;206(2):199–208. https://doi.org/10.1097/JU.0000000000001911.
Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60(1):39–44. https://doi.org/10.1016/j.eururo.2011.03.030.
Sebastià C, Corominas D, Musquera M, et al. Active surveillance of small renal masses. Insights Imaging. 2020;11(1):63. https://doi.org/10.1186/s13244-020-00853-y.
Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(6):668–80. https://doi.org/10.1200/JCO.2016.69.9645.
Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399. https://doi.org/10.1016/j.eururo.2022.03.006.
Kim JH, Li S, Khandwala Y, et al. Association of Prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg. 2019;154(3):225–31. https://doi.org/10.1001/jamasurg.2018.4602.
Johnson DC, Vukina J, Smith AB, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol. 2015;193(1):30–5. https://doi.org/10.1016/j.juro.2014.07.102.
Patel HD, Semerjian A, Gupta M, et al. Surgical removal of renal tumors with low metastatic potential based on clinical radiographic size: a systematic review of the literature. Urol Oncol. 2019;37(8):519–24. https://doi.org/10.1016/j.urolonc.2019.05.013.
McIntosh AG, Ristau BT, Ruth K, et al. Active surveillance for localized renal masses: tumor growth, delayed intervention rates, and >5-yr clinical outcomes. Eur Urol. 2018;74(2):157–64. https://doi.org/10.1016/j.eururo.2018.03.011.
Schiavina R, Borghesi M, Dababneh H, et al. Small renal masses managed with active surveillance: predictors of tumor growth rate after long-term follow-up. Clin Genitourin Cancer. 2015;13(2):e87–92. https://doi.org/10.1016/j.clgc.2014.08.006.
Gupta M, Alam R, Patel HD, et al. Use of delayed intervention for small renal masses initially managed with active surveillance. Urol Oncol. 2019;37(1):18–25. https://doi.org/10.1016/j.urolonc.2018.10.001.
Menon AR, Hussein AA, Attwood KM, et al. Active surveillance for risk stratification of all small renal masses lacking predefined clinical criteria for intervention. J Urol. 2021;206(2):229–39. https://doi.org/10.1097/JU.0000000000001714.
Rothman J, Egleston B, Wong YN, et al. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181(1):29–33; discussion 33-4. https://doi.org/10.1016/j.juro.2008.09.009.
Patel HD, Kates M, Pierorazio PM, et al. Survival after diagnosis of localized T1a kidney cancer: current population-based practice of surgery and nonsurgical management. Urology. 2014;83(1):126–32. https://doi.org/10.1016/j.urology.2013.08.088.
Ueno D, Xie Z, Boeke M, et al. Genomic heterogeneity and the small renal mass. Clin Cancer Res. 2018;24(17):4137–44. https://doi.org/10.1158/1078-0432.CCR-18-0214.
Mitchell TJ, Turajlic S, Rowan A, et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx renal. Cell. 2018;173(3):611–623.e17. https://doi.org/10.1016/j.cell.2018.02.020.
Turajlic S, Xu H, Litchfield K, et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell. 2018;173(3):595–610.e11. https://doi.org/10.1016/j.cell.2018.03.043.
Sun M, Abdollah F, Bianchi M, et al. Treatment management of small renal masses in the 21st century: a paradigm shift. Ann Surg Oncol. 2012;19(7):2380–7. https://doi.org/10.1245/s10434-012-2247-0.
Tang Y, Liu F, Mao X, et al. The impact of tumor size on the survival of patients with small renal masses: a population-based study. Cancer Med. 2022;11(12):2377–85. https://doi.org/10.1002/cam4.4595.
Pierorazio PM, Johnson MH, Patel HD, et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol. 2016;196(4):989–99. https://doi.org/10.1016/j.juro.2016.04.081.
Patel AK, Rogers CG, Johnson A, et al. Initial observation of a large proportion of patients presenting with clinical stage T1 renal masses: results from the MUSIC-KIDNEY statewide collaborative. Eur Urol Open Sci. 2021;23:13–9. https://doi.org/10.1016/j.euros.2020.11.002.
Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(1):71–90. https://doi.org/10.6004/jnccn.2022.0001.
Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–20. https://doi.org/10.1093/annonc/mdz056.
Richard PO, Violette PD, Bhindi B, et al. Canadian Urological Association guideline: management of small renal masses—full-text. Can Urol Assoc J. 2022;16(2):E61–75. https://doi.org/10.5489/cuaj.7763.
Umbreit EC, Shimko MS, Childs MA, et al. Metastatic potential of a renal mass according to original tumour size at presentation. BJU Int. 2012;109(2):190–4; discussion 194. https://doi.org/10.1111/j.1464-410X.2011.10184.x.
Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182(1):41–5. https://doi.org/10.1016/j.juro.2009.02.128.
Mano R, Duzgol C, Ganat M, et al. Preoperative nomogram predicting 12-year probability of metastatic renal cancer—evaluation in a contemporary cohort. Urol Oncol. 2020;38(11):853.e1–7. https://doi.org/10.1016/j.urolonc.2020.07.019.
Duffey BG, Choyke PL, Glenn G, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172(1):63–5. https://doi.org/10.1097/01.ju.0000132127.79974.3f.
Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118(4):997–1006. https://doi.org/10.1002/cncr.26369.
Mir MC, Capitanio U, Bertolo R, et al. Role of active surveillance for localized small renal masses. Eur Urol Oncol. 2018;1(3):177–87. https://doi.org/10.1016/j.euo.2018.05.001.
Wong JA, Rendon RA. Progression to metastatic disease from a small renal cell carcinoma prospectively followed with an active surveillance protocol. Can Urol Assoc J. 2007;1(2):120–2. https://doi.org/10.5489/cuaj.57.
Siu W, Hafez KS, Johnston WK, et al. Growth rates of renal cell carcinoma and oncocytoma under surveillance are similar. Urol Oncol. 2007;25(2):115–9. https://doi.org/10.1016/j.urolonc.2006.07.018.
Abou Youssif T, Kassouf W, Steinberg J, et al. Active surveillance for selected patients with renal masses: updated results with long-term follow-up. Cancer. 2007;110(5):1010–4. https://doi.org/10.1002/cncr.22871.
Crispen PL, Viterbo R, Boorjian SA, et al. Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer. 2009;115(13):2844–52. https://doi.org/10.1002/cncr.24338.
Rosales JC, Haramis G, Moreno J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183(5):1698–702. https://doi.org/10.1016/j.juro.2010.01.024.
Mason RJ, Abdolell M, Trottier G, et al. Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol. 2011;59(5):863–7. https://doi.org/10.1016/j.eururo.2011.02.023.
Dorin R, Jackson M, Cusano A, et al. Active surveillance of renal masses: an analysis of growth kinetics and clinical outcomes stratified by radiological characteristics at diagnosis. Int Braz J Urol. 2014;40(5):627–36. https://doi.org/10.1590/S1677-5538.IBJU.2014.05.07.
Zhang L, Yin W, Yao L, et al. Growth pattern of clear cell renal cell carcinoma in patients with delayed surgical intervention: fast growth rate correlates with high grade and may result in poor prognosis. Biomed Res Int. 2015;2015:598134. https://doi.org/10.1155/2015/598134.
Paterson C, Yew-Fung C, Sweeney C, et al. Predictors of growth kinetics and outcomes in small renal masses (SRM ≤4 cm in size): Tayside active surveillance cohort (TASC) study. Eur J Surg Oncol. 2017;43(8):1589–97. https://doi.org/10.1016/j.ejso.2017.03.006.
Whelan EA, Mason RJ, Himmelman JG, et al. Extended duration of active surveillance of small renal masses: a prospective cohort study. J Urol. 2019;202(1):57–61. https://doi.org/10.1097/JU.0000000000000075.
Chandrasekar T, Ahmad AE, Fadaak K, et al. Natural history of complex renal cysts: clinical evidence supporting active surveillance. J Urol. 2018;199(3):633–40. https://doi.org/10.1016/j.juro.2017.09.078.
Winters BR, Gore JL, Holt SK, et al. Cystic renal cell carcinoma carries an excellent prognosis regardless of tumor size. Urol Oncol. 2015;33(12):505.e9–13. https://doi.org/10.1016/j.urolonc.2015.07.017.
Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016;69(4):660–73. https://doi.org/10.1016/j.eururo.2015.07.072.
Richard PO, Jewett MA, Bhatt JR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68(6):1007–13. https://doi.org/10.1016/j.eururo.2015.04.004.
Finelli A, Cheung DC, Al-Matar A, et al. Small renal mass surveillance: histology-specific growth rates in a biopsy-characterized cohort. Eur Urol. 2020;78(3):460–7. https://doi.org/10.1016/j.eururo.2020.06.053.
Patel HD, Kates M, Pierorazio PM, et al. Balancing cardiovascular (CV) and cancer death among patients with small renal masses: modification by CV risk. BJU Int. 2015;115(1):58–64. https://doi.org/10.1111/bju.12719.
Sun M, Becker A, Tian Z, et al. Management of localized kidney cancer: calculating cancer-specific mortality and competing risks of death for surgery and nonsurgical management. Eur Urol. 2014;65(1):235–41. https://doi.org/10.1016/j.eururo.2013.03.034.
Kutikov A, Egleston BL, Wong YN, et al. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28(2):311–7. https://doi.org/10.1200/JCO.2009.22.4816.
Psutka SP, Gulati R, Jewett MAS, et al. A clinical decision aid to support personalized treatment selection for patients with clinical T1 renal masses: results from a multi-institutional competing-risks analysis. Eur Urol. 2022;81(6):576–85. https://doi.org/10.1016/j.eururo.2021.11.002.
Metcalf MR, Cheaib JG, Biles MJ, et al. Outcomes of active surveillance for young patients with small renal masses: prospective data from the DISSRM registry. J Urol. 2021;205(5):1286–93. https://doi.org/10.1097/JU.0000000000001575.
Altok M, Menon A, Aly A, et al. Updated outcomes for active surveillance recommended to all small renal mass patients lacking progression criteria for intervention. J Urol. 2022;207(Supplement 5):e266. https://doi.org/10.1097/JU.0000000000002547.09.
Rebez G, Pavan N, Mir MC. Available active surveillance follow-up protocols for small renal mass: a systematic review. World J Urol. 2021;39(8):2875–82. https://doi.org/10.1007/s00345-020-03581-6.
Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015;68(3):408–15. https://doi.org/10.1016/j.eururo.2015.02.001.
Kassiri B, Cheaib JG, Pierorazio PM. Patients with small renal masses undergoing active surveillance-is yearly chest imaging necessary? J Urol. 2019;201(6):1061–3. https://doi.org/10.1097/JU.0000000000000079.
Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol. 2016;195(5):1340–7. https://doi.org/10.1016/j.juro.2015.11.029.
Uzosike AC, Patel HD, Alam R, et al. Growth kinetics of small renal masses on active surveillance: variability and results from the DISSRM registry. J Urol. 2018;199(3):641–8. https://doi.org/10.1016/j.juro.2017.09.087.
Cheaib JG, Alam R, Kassiri B, et al. Active surveillance for small renal masses is safe and non-inferior: 10-year update from the DISSRM registry. Eur Urol Open Sci. 2020;19:e945–6. https://doi.org/10.1016/S2666-1683(20)33210-9.
Bertelli E, Palombella A, Sessa F, et al. Contrast-enhanced ultrasound (CEUS) imaging for active surveillance of small renal masses. World J Urol. 2021;39(8):2853–60. https://doi.org/10.1007/s00345-021-03589-6.
Parker PA, Alba F, Fellman B, et al. Illness uncertainty and quality of life of patients with small renal tumors undergoing watchful waiting: a 2-year prospective study. Eur Urol. 2013;63(6):1122–7. https://doi.org/10.1016/j.eururo.2013.01.034.
Patel HD, Riffon MF, Joice GA, et al. A prospective, comparative study of quality of life among patients with small renal masses choosing active surveillance and primary intervention. J Urol. 2016;196(5):1356–62. https://doi.org/10.1016/j.juro.2016.04.073.
O'Connor KM, Davis N, Lennon GM, et al. Can we avoid surgery in elderly patients with renal masses by using the Charlson comorbidity index? BJU Int. 2009;103(11):1492–5. https://doi.org/10.1111/j.1464-410X.2008.08275.x.
Campi R, Sessa F, Corti F, et al. Triggers for delayed intervention in patients with small renal masses undergoing active surveillance: a systematic review. Minerva Urol Nefrol. 2020;72(4):389–407. https://doi.org/10.23736/S0393-2249.20.03870-9.
Walther MM, Choyke PL, Glenn G, et al. Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol. 1999;161(5):1475–9. https://doi.org/10.1016/s0022-5347(05)68930-6.
Brunocilla E, Borghesi M, Schiavina R, et al. Small renal masses initially managed using active surveillance: results from a retrospective study with long-term follow-up. Clin Genitourin Cancer. 2014;12(3):178–81. https://doi.org/10.1016/j.clgc.2013.11.011.
Rais-Bahrami S, Guzzo TJ, Jarrett TW, et al. Incidentally discovered renal masses: oncological and perioperative outcomes in patients with delayed surgical intervention. BJU Int. 2009;103(10):1355–8. https://doi.org/10.1111/j.1464-410X.2008.08242.x.
Ajami T, Sebastia C, Corominas D, et al. Clinical and radiological findings for small renal masses under active surveillance. Urol Oncol. 2021;39(8):499.e9–499.e14. https://doi.org/10.1016/j.urolonc.2021.04.010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Altok, M., Kauffman, E.C. (2023). Active Surveillance of Patients with Clinically Localized Small Renal Masses. In: McKay, R.R., Singer, E.A. (eds) Integrating Multidisciplinary Treatment for Advanced Renal Cell Carcinoma. Springer, Cham. https://doi.org/10.1007/978-3-031-40901-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-40901-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40900-4
Online ISBN: 978-3-031-40901-1
eBook Packages: MedicineMedicine (R0)