Abstract
Purpose
Partial bladder outlet obstruction is a multifactorial urological condition in which hypoxia plays a significant role. We recently investigated hypoxia’s role as a single stressor and found that hypoxia induced an intense inflammatory and profibrotic switch in bladder smooth muscle cells (bSMCs). With the immunomodulatory capacity of mesenchymal stem cells (MSCs), we aimed to investigate if the hypoxia-signaling pathways can be mitigated using MSCs.
Methods
Bladder smooth muscle cells were cultured in 3% oxygen tension for 72 h with either the direct or indirect co-culture with bone marrow derived MSCs. High pore density transwells were used for indirect co-cultures. Total RNA was extracted for gene expression analysis and the Mesoscale multiplex assay was used for secreted cytokines and growth factor measurements. Total collagen contents were determined using the Sirius Red collagen assay.
Results
Hypoxia induced increase of HIF3α, VEGF, TGFβ1, TNFα, IL-1β, IL-6, αSMA, and total collagen expression and decreased IL-10 levels in bSMCs. Both direct and indirect MSCs co-cultures inhibited > 50% of hypoxia-induced TGFβ1 and IL-6 expression (p < 0.005) in a HIF-independent manner. Also, both MSCs co-culture techniques induced > 200% increase in IL-10 protein (p < 0.005) and inhibited hypoxia-induced αSMA, collagen I and III transcripts as well as total collagen proteins (p < 0.0001). Contrastingly, the hypoxia-induced IL-1β and TNFα were inhibited by only the direct co-cultures (p < 0.05).
Conclusions
MSCs co-culture with bSMCs potently mitigates hypoxia-induced inflammatory and profibrotic pathways. This work has elucidated the role of cell–cell contact and paracrine immunomodulatory mechanisms of MSCs action and opened avenues for therapeutic intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia is known to play a significant role in the pathophysiology of several conditions including partial bladder outlet obstruction (pBOO), renal, cardiovascular, and respiratory diseases [1]. pBOO represents a condition whereby increased resistance to urine outflow results in increased muscular contractility, hypertrophy, and a decreased mucosal perfusion. Some characteristic features include increased urine storage pressure, high hydrodynamic pressure, and exaggerated resistance inducing significant damage to bladder tissues [2]. Ultimately, it may lead to a fibrotic bladder state where the normal trilaminar smooth muscle architecture is replaced by a non-compliant, disorganized collagenous state.
Characterization of pBOO in animal models has shown that there is an initial inflammatory phase that sets the stage for the subsequent hypertrophic and fibrotic events [3]. Irreversible morphological, biochemical, and functional alterations of the bladder due to pBOO have also been reported. These transformations are believed to be caused by a combination of factors that includes mechanical stretch, hydrodynamic pressure, inflammation, and hypoxia. With the exception of hypoxia, the roles of all of these factors as single stressors have been investigated [4, 5]. However, although, several studies have identified hypoxia in obstructed bladder tissues [3, 6,7,8], the effects of hypoxia as a single stressor was yet to be examined in isolation. We have demonstrated that bladder smooth muscle cells (bSMCs) incubated under hypoxic conditions is sufficient to incite an inflammatory cascade, and a subsequent pro-fibrotic response. These included an increase of the hypoxia inducible factor alpha subunits (HIFα), VEGF, TGFβ1, IL-1B, IL-6, TNFα, a decrease of IL-10 transcripts, as well as increased total collagen production [9].
Mesenchymal stem cells (MSCs) are multipotent adult cells that have multilineage differentiation and immunomodulatory capacities [10]. This has resulted in extensive study and their use in tissue repair and regeneration across various inflammatory and fibrotic conditions. We were able to demonstrate the short-term effectiveness of MSCs in ameliorating inflammatory factors in an animal model of pBOO [11]. Other studies using animal models of pBOO have reported the effectiveness of MSCs in improving urodynamic and molecular parameters [12, 13]. With regard to stem cell therapy, many mechanisms of action have been proposed: cell-to-cell contact, paracrine communication, and cell type-specific differentiation [14]. Despite the huge potential of cell therapy in the treatment of pBOO, its exact mechanism is not clearly understood.
We hypothesized that the co-culture of bSMCs with MSCs in a reduced oxygen tension will mitigate the inflammatory and pro-fibrotic cascade. Furthermore, to elucidate the mechanisms of action; both direct and indirect co-culture techniques were used.
Materials and methods
Cell culture
Human bSMCs purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) were cultured in smooth muscle growth medium supplemented with 10% fetal bovine serum, 1× smooth muscle growth factors, 1× 1000 U/ml penicillin, and 1000 mg/ml streptomycin from ScienCell Research Laboratories (CA). The phenotype of the cells was confirmed by culture characteristics coupled with the expression of bladder smooth muscle markers; high molecular weight caldesmon, desmin, α smooth muscle actin (αSMA) as well as the absence of light molecule weight caldesmon as previously established [9, 15, 16].
Human bone marrow derived mesenchymal stem cells (MSCs) were isolated from bone marrow aspirates of surgically discarded material obtained from the iliac crest of six donors. These were obtained by approval and a waiver of informed consent of the ethics committee of the University of Alberta (Edmonton, Canada). MSCs were isolated and expanded as previously reported [17]. Briefly, bone marrow mononuclear cells (BMMCs) were isolated from the aspirates using Histopaque-1077 (Sigma-Aldrich Canada Co, Ontario, Canada). Then, 15 million BMMCs were cultured in 150 cm2 tissue culture flask. Culture medium was alpha-minimal essential medium (α-MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 88.5 U/ml penicillin–streptomycin, 0.26 g/ml l-glutamine, 8.8 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 885 × 10−6 mol/l sodium pyruvate (Life Technologies, Burlington, Canada), and 5 ng/ml fibroblast growth factor-2 (FGF-2) (Neuromics, Edina, MN, USA). After 7 days, culture medium on the adherent nucleated cells was changed and culture was expanded to passage 2. The mesenchymal stem cell phenotype of the cells was confirmed by the positive expression of markers: CD151, CD105, CD90, CD73, CD44, and the absence of CD34 and CD14 using flow cytometry.
Direct and indirect co-cultures
To examine the effects of MSCs co-culture on hypoxia-induced inflammatory and fibrotic pathways, six-well plates were seeded with 6 × 105 human bladder smooth muscle cells (SMCs) with either the direct or indirect addition of 3 × 105 MSCs. Indirect co-cultures were set up in a transwell system in which the SMCs and MSCs were physically separated by a high pore density (0.4 µm) transwell inserts (Becton–Dickinson, New Jersey, USA). This limited interaction between the two cell types to the diffusion of soluble factors across the membranes. Cells were incubated in hypoxia defined as 3% O2 tension, 5% CO2, and 92% N2 at 37 °C for 72 h. Normoxia control conditions were also defined as 21% O2, 5% CO2, and 74% N2 at 37 °C for 72 h.
RT-PCR
At the end of the specified incubation period, cells were transferred on ice before the subsequent procedures. Culture medium was immediately harvested and stored at – 80 °C and total RNA was extracted using RNeasy Mini kit (Qiagen, CA, USA). The Quantitect Reverse Transcription kit (Qiagen, CA, USA) was used for first strand complementary DNA (cDNA) production. Quantitative real-time PCR was carried out in a Biorad CFX96 Real time system (Kallang, Singapore) using Kapa Sybr Fast qPCR Kit (Kapa Biosystems, Boston, USA), cDNA samples, and oligo-dt primers specific for target genes: HIF1α forward sequence (5′–3′) TTC ACC TGA GCC TAA TAGTCC, reverse sequence (5′–3′); CAA GTC TAA ATC TGT GTCCTG. HIF3α forward sequence (5′–3′): TTC TCC TTG CGC ATGAAGAGTACG, reverse sequence (5′–3′); TCT GCG CAG GTG GCT TGT AGG VEGF forward sequence (5′–3′) CTA CCT CCA CCATGC CAAGT, reverse sequence (5′–3′); GCA GTA GCT GCG CTG ATAGA; TGFβ1 forward sequence (5′–3′) TGGAAGTGGATCCACGCGCCCAAGG, reverse sequence (5′–3′); GCAGGAGCGCACGATCATGTTGGAC; αSMA forward sequence (5′–3′) CCG ACC GAATGC AGA AGGA, reverse sequence (5′–3′); ACAGAGTATTTGCGCTCCGAA, TNFα forward sequence (5′–3′) CTT CTC CTT CCT GAT CGTGG, reverse sequence (5′–3′); GCT GGT TAT CTC TCA GCTCCA, collagen 1 forward sequence (5′–3′) CAG CCG CTT CAC CTA CAGC, reverse sequence (5′–3′); TTTTGTATTCAATCACTGTCT TGCC, collagen 3 forward sequence (5′–3′)TGAAAGGACACAGAGGCTTCG, reverse sequence (5′–3′); GCA CCATTC TTA CCA GGC, 18S forward sequence (5′–3′) CGG CTA CAT CCA AGG AA, reverse sequence (5′–3′); GCT GGA ATT ACC GCG GCT, interleukin 1β forward sequence (5′–3′) ACA GAT GAA GTG CTC CTT CCA, reverse sequence (5′–3′); GTC GGA GAT TCG TAG CTG GAT, interleukin 6 forward sequence (5′–3′)TGGTCTTTTGGAGTTTGAGGTA, reverse sequence (5′–3′); AGGTTTCTGACCAGAAGAAGGA, interleukin 10 forward sequence (5′–3′) CCCTGGGTGAGAAGCTGAAG, reverse sequence (5′–3′); CACTGCCTTGCTCTTATTTTCACA, and Β actin forward sequence (5′–3′) AAGCCACCCCACTTCTCTCTAA, reverse sequence (5′–3′); AATGCTATCACCTCCCCTGTGT.
Forty cycles of denaturing at 95 °C (2 s) and annealing/extension at 60 °C (30 s) followed initial 3 min enzyme activation at 95 °C. Gene expression of the experimental groups relative to the normoxia-incubated SMCs controls were normalized using 2 endogenous controls; beta actin, and 18S.
Cytokine measurement
The multiplex assays (U-PLEX) for biomarker group 1 (Meso Scale Diagnostics, Rockville, MD, USA) enabled the concurrent measurement of protein levels of cytokines and growth factors in harvested culture media. Briefly, each specific biotinylated antibody of cytokines of interest was coupled to a linker, pooled, and used to coat the high electrode multi-spot microplates. Samples were run alongside a serially diluted multi-calibrator standard. These standards consisted of a cocktail of cytokines and growth factor proteins of known concentrations. Following incubation in a composite of SULFO-TAG-labeled detection antibodies, quantities of analytes in samples were determined on a Sector Imager 6000 Plate Reader (Meso Scale Diagnostics).
Sirius Red collagen detection assay
Total soluble collagen secreted into culture media by cells was determined using the Sirius red technique. Manufacturer’s instructions for the Sirius Red Collagen Detection kit (Chondrex, Inc, Redmond, WA, USA) were followed. Briefly, collagen content in samples and in a serially diluted standard were precipitated by the addition of Sirius red solution, collected, purified, and then resolubilized. Optical densities of the pure collagen samples were read at 520 nm using a microplate reader.
Statistical analysis
Graphpad prism 6.0 (Graphpad Prism INC, CA, USA) was used to analyze data. Results represent data from at least three independent experiments which are presented as mean ± SEM. One-way analysis of variance (ANOVA) and the Student’s T test with Bonferroni adjustments were used to evaluate differences between groups. p value of < 0.05 was accepted as statistically significant (Fig. 1).
Culture micrographs and characterization of cells. a Normal human bladder smooth muscle cell culture at passage 4 showing the typical spindle-shaped elongated cells. The formation of cellular masses of parallel arranged cells became noticeable after day 3 of culture. Note also the characteristic “hill” and “valley” growth pattern. b Isolated human bone marrow-derived mesenchymal stem cell culture at passage 2, showing plastic-adherent spindle-shaped fibroblast-like cells. Micrographs were taken with an inverted microscope using ×20 objective lens. c Bladder smooth muscle cell characterization data showing the expression of heavy molecular weight caldesmon (Cad H), αSMA and desmin. The smooth muscle cells lacked the expression of light molecular weight caldesmon (Cad L)
Results
Pro- and anti-inflammatory cytokines
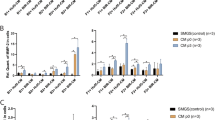
Co-culture of bSMCs with MSCs gave varying effects on cytokines’ expression; Hypoxic incubation of a monoculture of bSMCs increased TGFβ1 transcript levels by 4.6-fold (p < 0.0001). However, transcript levels were reduced by more than 50% in both the direct and transwell co-cultures with MSCs (p < 0.005 for both). Similarly, IL-6 transcripts increased by 6.1-fold in hypoxia-incubated bSMCs. Nonetheless, both direct and indirect co-culture with MSCs under hypoxia resulted in a substantial reduction to baseline normoxic control levels (p < 0.05 for both). IL-6 protein in the hypoxia-incubated bSMCs monoculture was 24% higher than normoxic controls (p < 0.005). Levels were significantly reduced by 27 and 24% in the direct and transwell co-cultures (p < 0.005 for both), respectively (Fig. 2a).
MSC co-culture significantly inhibits inflammatory response to hypoxia and increases anti-inflammatory cytokine production: a both direct and indirect co-cultures were effective in inhibiting TGFβ1 and IL-6 expression under hypoxia. b Bladder smooth muscle cells (bSMCs) responded to hypoxia by a downregulation of IL-10. Both the direct and indirect MSC co-culture induced a significant increase in the level of this cytokine. SMC [N]: bSMC monoculture controls incubated in normoxia (21% O2). SMC [H]: bSMC monoculture incubated in hypoxia. One-way ANOVA and the Student’s t test with Bonferroni corrections were used to statistically evaluate the differences between the hypoxia-incubated SMC monoculture and the co-culture groups (*p < 0.05, **p < 0.005)
Transcript levels of IL-10, the anti-inflammatory and anti-fibrotic cytokine were downregulated by 0.25-fold in the hypoxic bSMCs monoculture (p < 0.05). Nonetheless, there was an increase of 2.3- and 3.25-fold in the direct (p < 0.050) and indirect (p < 0.005) co-cultures, respectively. Likewise, the hypoxia incubation of bSMCs also resulted in a 69% reduction in IL-10 protein (p < 0.005). Nevertheless, both the direct and transwell co-culture techniques resulted in an upregulation of 241 and 252%, respectively, above the hypoxia-incubated bSMCs levels (p < 0.005 for both, Fig. 2b).
Both TNFα and IL-1β gave similar effects where transcript levels rose by 3.8- and 3.0-fold, respectively, in bSMCs monoculture exposed to 72 h of hypoxia (p < 0.005 for both). The direct co-culture with MSCs reduced transcript levels by approximately 50% (p < 0.005) for both genes. Transcript levels of both cytokines remained elevated in the indirect co-cultures. TNFα protein level was 61.6 ± 1.2 pg/ml in hypoxia-incubated bSMCs cultures while that in normoxia-incubated controls was 37.5 ± 5.0 pg/ml (p < 0.005). Level of this protein was reduced in the direct co-cultures to 30.6 ± 3.8 pg/ml (p < 0.005) but remained elevated in transwell cultures at 59.8 ± 6.7 pg/ml compared to levels in hypoxic bSMCs. IL-1β protein was 122.1 ± 4.4 pg/ml in normoxic bSMCs and increased to 152.7 ± 2.24 pg/ml in hypoxic bSMCs (p < 0.05). A direct co-culture with MSCs reduced protein levels to 93.10 ± 5.02 pg/ml (p < 0.005) but levels increased in the transwell cultures to 212.2 ± 22.56 pg/ml (p < 0.005) (Fig. 3).
Pro-inflammatory cytokines that were only reduced by the direct co-culture technique and not the indirect: to determine if MSCs co-culture inhibits hypoxia-induced inflammatory cytokine expression, MSC: bSMC co-culture was compared with bSMCs monoculture similarly cultured under hypoxic conditions: hypoxia-induced TNFα and IL-1β levels remained elevated in transwells, whereas levels were significantly mitigated in the direct MSC: bSMC co-cultures. Results represent data from at least three independent experiments which are presented as mean (± SEM)
Pro-fibrotic genes (Fig. 4)
αSMA transcripts increased by 5.2-fold when bSMCs were cultured under hypoxia (p < 0.005) and both the direct and indirect co-culture techniques reduced levels to 1.4- and 1.2-fold, respectively (p < 0.005 for both). Both collagen 1 and 3 mRNA showed a similar pattern of expression in cultures; there was a 9.9-fold increase in collagen 1 transcripts when bSMCs were incubated in hypoxia (p < 0.005) and both the direct and indirect co-culture techniques induced a 78 and 72% decrease in transcript levels, respectively (p < 0.005 for both). Collagen 3 transcripts which increased by 4.5-fold in the hypoxic bSMCs monoculture (p < 0.005) were reduced by 84% when co-cultured directly with MSCs. The indirect co-culture also induced a decrease of 74% in transcript levels (p < 0.005 for both). Total collagen protein secreted into media was 120.4 ± 4.89 pg/ml in the normoxic bSMCs control, but increased significantly to 269.6 ± 36.15 pg/ml when bSMCs were incubated under hypoxia (p < 0.005). However, levels of this protein were reduced to 116.2 ± 17.48 pg/ml in the direct co-culture and to 140.9 ± 27.8 pg/ml in the indirect co-culture (p < 005 for both).
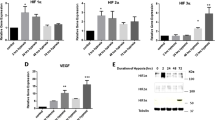
Hypoxic and pro-angiogenic response (Fig. 5)
Culture of bSMCs in 3% hypoxia for 72 h did not significantly increase HIF 1α transcript but resulted in a 4.5-fold increase in the transcript levels of HIF 3α (p < 0.005). Notwithstanding, both HIF 1 and 3α transcript levels remained unaltered by co-culturing with MSCs under hypoxic conditions. Similarly, VEGF transcripts increased significantly by 7.9-fold after 72 h of hypoxic incubation of the bSMCs monoculture (p < 0.005). Co-culturing with MSCs did not significantly affect the elevated levels of this transcript. VEGF protein increased by 35.7% when bSMCs were incubated in hypoxia (p < 0.005) and remained significantly unchanged when co-cultured either directly or indirectly with MSCs under hypoxic conditions.
Discussion
The paracrine immunomodulatory property of stem cells has gained much attention in the past decade especially with regard to the treatment of inflammatory and fibrotic conditions like pBOO. Therefore, the current experiments were designed to investigate the underlying molecular mechanisms.
Even though the transforming growth factor-β1 (TGFβ1) is required for normal cellular growth and differentiation, it is also a known potent mediator of fibrosis. This cytokine is implicated in disease processes such as epithelial-mesenchymal transition, myofibroblast activation, epithelial cell apoptosis, and extracellular matrix production [18]. As a result, numerous studies have investigated strategies for inhibiting TGFβ expression, signaling pathways, and function in order to reduce fibrosis. Physiologically, elevated levels of TGFβ1 correlated with the stage of bladder obstruction in patients and in animal models of pBOO and its inhibition led to a significant reduction in fibrosis [19,20,21,22]. In the current study, the efficient downregulation of TGFβ1 expression by both co-culture techniques reflects the anti-fibrotic nature of MSCs when co-cultured directly or indirectly. A similar effect was seen with the downregulation of IL-6, a pro-inflammatory cytokine. Mechanistically, TGFβ1 has been proposed to have regulatory effects on IL-6 [23]. Thus, the reduced expression of TGFβ 1 in the co-cultures may be important for the inhibition of IL-6 expression.
IL-1β and TNFα are pro-inflammatory cytokines that are expressed in common chronic pathologies such as rheumatoid arthritis, diabetes, myocardial infarction, and inflammatory lung diseases. The binding of these cytokines to their respective receptors set the stage for further inflammatory events leading to tissue damage [24]. As a therapeutic mechanism, MSCs are known to express IL-1 receptor agonist (1L-1Ra), a cytokine which binds to the IL-1 receptor thereby, competitively inhibiting IL-1β [25]. Interestingly, in our study, both cytokines showed the same pattern of expression in culture where levels were only reduced in the direct co-cultures and not in the indirect method of culture. This strongly suggests that the MSC-specific paracrine action alone was not sufficient to immunomodulate these two cytokines but the physical MSCs to bSMCs contact was also required. Thus, providing evidence to support the report that the paracrine-mediated immunosuppressive effects of stem cells are further enhanced if direct cell–cell contact between stem cells and other cells are allowed [26].
IL-10 is a potent anti-fibrotic and immunomodulatory cytokine secreted in response to elevated levels of inflammatory cytokines such as IL-β, TNFα, and interferon (IFN) [22, 27]. In our study, the exposure of bSMCs to hypoxia substantially reduced IL-10 levels. However, its elevated levels in the MSC co-cultures are an important part of this study. With the high levels of IL-β and TNFα in the transwell co-cultures, it was not surprising that we found higher IL-10 transcript levels in the transwell co-cultures than the direct co-culture system. These data support the anti-inflammatory, anti-fibrotic, and immunomodulatory nature of MSCs. Overall, our results are consistent with the study by Choi et al. who reported that MSCs co-culture with macrophages inhibits lipopolysacharide-induced expression of IL-6 and IL-1β and increased IL-4-induced macrophage expression of IL-10 [28]. Aggarwal et al. also found increased IL-10 and decreased TNFα levels when MSCs were co-cultured with immune cells [29].
Studies by our group and others have shown that collagens 1 and 3 are important collagen subtypes commonly upregulated in the obstructed and fibrotic bladder [3, 9, 21]. Essentially, the reduction in TGFβ1 expression coupled with the upregulation of IL-10 in both the direct and indirect co-culture systems may have accounted for the reduced collagen levels in the MSCs co-cultures. Thus, despite the high levels of the inflammatory cytokines IL-β and TNFα in the indirect/transwell co-cultures, the potent anti-fibrotic effect of MSCs significantly inhibited hypoxia- induced total collagen levels. Therefore, from this outcome, we theorize that the therapeutic use of MSC-conditioned media to treat hypoxic and fibrotic conditions may be equally effective as the physical injection of mesenchymal stem cells.
The hypoxia-inducible transcriptional factor (HIF) is important in stimulating the transcription of cyto-protective genes under conditions of low oxygen tension [1]. All the three known members of the HIF family; HIF 1, 2, and 3 have been identified in bSMCs [9]. Our current finding is consistent with our previous studies that showed that the oxygen-sensitive alpha subunit, HIF 3α transcription increased in response to prolonged hypoxia (72 h). However, HIF 1α response was immediate and transient increasing only after 2 h of hypoxia. This study did not determine HIF 2α response because its activity appeared redundant in bSMCs from our previous findings. In the current study, it was interesting to observe that the HIF expression of the bSMCs was not significantly affected by co-culturing with mesenchymal stem cells under reduced oxygen tension. The unaltered VEGF expression, a proven hypoxia-response gene, provides evidence that HIF function is unaffected by the MSC co-culture. Since the HIF cellular system is protective, our data support the fact that MSCs have the ability to inhibit hypoxia-induced signaling pathways in a HIF-independent fashion.
These data add to our work whereby exposure of bladder smooth muscle cells to hypoxia incites a cascade of cellular responses of inflammation and increased extracellular matrix synthesis. However, MSCs have a profound ability to prevent this response by inhibiting pro-inflammatory and pro-fibrotic cytokine production and enhancing anti-fibrotic cytokine secretion. This work has unlocked mechanistic clues that may open avenues for therapeutic intervention.
References
Semenza GL (2014) Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76:39–56
Schröder A, Chichester P, Kogan BA et al (2001) Effect of chronic bladder outlet obstruction on blood flow of the rabbit bladder. J Urol 165(2):640–646
Metcalfe PD, Wang J, Jiao H et al (2010) Bladder outlet obstruction: progression from inflammation to fibrosis. Br J Urol 106(11):1686–1694
Howard PS, Kucich U, Coplen DE, He Y (2005) Transforming growth factor-beta1-induced hypertrophy and matrix expression in human bladder smooth muscle cells. Urology 66(6):1349–1353
Chen L, Wei TQ, Wang Y, Zhang J, Li H, Wang KJ (2012) Simulated bladder pressure stimulates human bladder smooth muscle cell proliferation via the PI3K/SGK1 signaling pathway. J Urol 188(2):661–667
Ghafar MA, Anastasiadis AG, Olsson LE et al (2002) Hypoxia and an angiogenic response in the partially obstructed rat bladder. Lab Investig 82(7):903–909
Koritsiadis G, Tyritzis SI, Koutalellis G, Lazaris AC, Stravodimos K (2010) The effect of alpha-blocker treatment on bladder hypoxia inducible factor-1 alpha regulation during lower urinary tract obstruction. Int Braz J Urol 36(1):86–94
Ekman M, Uvelius B, Albinsson S, Swärd K (2014) HIF-mediated metabolic switching in bladder outlet obstruction mitigates the relaxing effect of mitochondrial inhibition. Lab Investig 94(5):557–568
Wiafe B, Adesida A, Churchill T, Adewuyi EE, Li Z, Metcalfe P (2017) Hypoxia-increased expression of genes involved in inflammation, dedifferentiation, pro-fibrosis, and extracellular matrix remodeling of human bladder smooth muscle cells. In Vitro Cell Dev Biol Anim 53(1):58–66
Elnakish MT, Hassan F, Dakhlallah D, Marsh CB, Alhaider IA, Khan M (2012) Mesenchymal stem cells for cardiac regeneration: translation to bedside reality. Stem Cells Int 2012. https://doi.org/10.1155/2012/646038
Al-Saikan B, Ding J, Tredget E, Metcalfe P (2016) Benefits of mesenchymal stem cells after partial bladder outlet obstruction. Can Urol Assoc J 10(1–2):E1–E6
Woo LL, Tanaka ST, Anumanthan G et al (2011) Mesenchymal stem cell recruitment and improved bladder function after bladder outlet obstruction: preliminary data. J Urol 185(3):1132–1138
Lee HJ, Won JH, Doo SH et al (2012) Inhibition of collagen deposit in obstructed rat bladder outlet by transplantation of superparamagnetic iron oxide-labeled human mesenchymal stem cells as monitored by molecular magnetic resonance imaging (MRI). Cell Transplant 21(5):959–970
Wiafe B, Metcalfe PD, Adesida AB (2015) Stem cell therapy: current applications and potential for urology. Curr Urol Rep 16(11):77
Zheng Y, Chang S, Boopathi E et al (2012) Generation of a human urinary bladder smooth muscle cell line. In Vitro Cell Dev Biol Anim 48(2):84–96
Huber PA (1997) Caldesmon. Int J Biochem Cell Biol 29(8–9):1047–1051
Adesida AB, Mulet-Sierra A, Jomha NM (2012) Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther 3(2):9
Varga J, Pasche B (2008) Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr Opin Rheumatol 20(6):720–728
Macrae Dell K, Hoffman BB, Leonard MB, Ziyadeh FN, Schulman SL (2000) Increased urinary transforming growth factor beta1 excretion in children with posterior urethral valves. Urology 56:311–314
Sager C, Lopez JC, Duran V, Burek C, Perazzo E (2009) Transforming growth factor-β1 in congenital ureteropelvic junction obstruction: diagnosis and follow-up. Int Braz J Urol 35(3):315–325
Jiang X, Chen Y, Zhu H et al (2015) Sodium tanshinone IIA sulfonate ameliorates bladder fibrosis in a rat model of partial bladder outlet obstruction by inhibiting the TGF-β/smad pathway activation. PLoS One 10(6):e0129655
Anumanthan G, Tanaka ST, Adams CM et al (2009) Bladder stromal loss of transforming growth factor receptor ii decreases fibrosis after bladder obstruction. J Urol 182(4 Suppl):1775–1780
Elias JA, Lentz V, Cummings PJ (1991) Transforming growth factor-beta regulation of IL-6 production by unstimulated and IL-1-stimulated human fibroblasts. J Immunol 146(10):3437–3443
Saperstein S, Chen L, Oakes D, Pryhuber G, Finkelstein J (2009) IL-1beta augments TNF-alpha-mediated inflammatory responses from lung epithelial cells. J Interferon Cytokine Res 29(5):273–284
Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML (2010) Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 43(4):255–263
Ren G, Zhao X, Zhang L et al (2010) Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 184(5):2321–2328
Shi Y, Hu G, Su J et al (2010) Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res 20(5):510–518
Cho DI, Kim MR, Jeong HY et al (2014) Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 10(46):e70
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822
Acknowledgements
This research was funded by the Northern Alberta Urology Foundation, Edmonton Civic Employees Charitable Assistance Fund (ECECAF) and Women and Children’s Health Research Institute.
Author information
Authors and Affiliations
Contributions
BW: project development, data collection and analysis, manuscript writing. AA: project development, supervision, manuscript editing. TC: manuscript editing, data analysis. PM: project development, manuscript editing, supervision.
Corresponding author
Ethics declarations
Informed consent
Authors obtained a waiver of informed consent for research involving discarded human tissue.
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Wiafe, B., Adesida, A., Churchill, T. et al. Mesenchymal stem cells inhibit hypoxia-induced inflammatory and fibrotic pathways in bladder smooth muscle cells. World J Urol 36, 1157–1165 (2018). https://doi.org/10.1007/s00345-018-2247-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2247-1