Abstract
Purpose
Clinical outcomes prognostic markers are awaited in clear-cell renal carcinoma (ccRCC) to improve patient-tailored management and to assess six different markers’ influence on clinical outcomes from ccRCC specimen and their incremental value combined with TNM staging.
Materials and methods
This is a retrospective, multicenter study. One hundred and forty-three patients with pT1b-pT3N0M0 ccRCC were included. Pathology specimens from surgeries were centrally reviewed, mounted on a tissue micro-array and stained with six markers: CAIX, c-MYC, Ki67, p53, vimentin and PTEN. Images were captured through an Ultra Fast Scanner. Tumor expression was measured with Image Pro Plus. Cytoplasmic markers (PTEN, CAIX, vimentin, c-MYC) were expressed as surface percentage of expression. Nuclear markers (Ki67, p53) were expressed as number of cells/mm2. Clinical data and markers expression were compared with clinical outcomes. Each variable was included in the Cox proportional multivariate analyses if p < 0.10 on univariate analyses. Discrimination of the new marker was calculated with Harrell’s concordance index.
Results
At median follow-up of 63 months (IQR 35.0–91.8), on multivariate analysis, CAIX under-expression and vimentin over-expression were associated with worse survival (recurrence, specific and overall survival). A categorical marker CAIX-/Vimentin+ with cutoff points for CAIX and vimentin of 30 and 50 %, respectively, was designed. The new CAIX-/Vimentin+ marker presented a good concordance and comparable calibration to the reference model. Limitations are the retrospective design, the need for external validation and the large study period.
Conclusion
Using an automated technique of measurement, CAIX and vimentin are independent predictors of clinical outcomes in ccRCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) management has tremendously changed during the last decade. From mostly surgical management in localized tumors, a broad panel of systemic therapies can now be administered when metastatic progression occurs [1]. Reliable prediction of clinical outcomes at time of surgery is paramount to tailor the follow-up and for potential treatment adjustment for high-risk patients (to predict which patient might fit a particular systemic treatment). Although many markers had been reported to be associated with clinical outcomes in RCC, their clinical applicability is often limited by subjective interpretation of immunohistochemistry (IHC) or limited impact of the new marker on prediction models [2]. At the moment, clinical use of biomarkers to predict RCC outcome is not validated [1]. The aim of this study is to assess the ability of a set of IHC markers reported several times as associated with outcomes to predict oncological outcomes in clear-cell renal carcinoma (ccRCC).
Patients and methods
Patient selection
From January 1998 to December 2009, 184 patients who underwent surgical treatment (partial or radical nephrectomy) for non-metastatic pT1b-pT3 ccRCC (144 radical, 40 partial nephrectomies) according to 2002 TNM [3] were selected, and non-cN0MO were excluded. Pathological T1a tumors were excluded because of its relative benign course and rare metastatic or local progression events [4]. For descriptive purposes, cases were also converted to 2009 TNM [5]. Central pathology re-review was performed by a dedicated uro-pathologist.
Patients were excluded if central review showed other subtype than ccRCC, if there were concomitant cancers with doubts on origin of the metastatic occurrence, if patient received neo-adjuvant treatment or if patients died within 1 month of surgery for other cause that RCC cancer (Fig. 1).
Immunohistochemistry
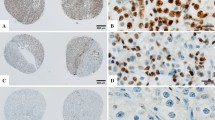
For every patient, the clear-cell subtype and the Fuhrman tumor grade were confirmed by one uro-pathologist with hematoxylin and eosin (HE)-stained slides. In case of discrepancies in tumor grading, our uro-pathologist estimation was finally retained to prevent inter-observer variability. For each tumor, representative regions of the tumor were defined from the corresponding HE slide and the block was punched in the corresponding area to extract the tissue micro-array (TMA) core. One to six cores per patient were extracted and mounted on paraffin-embedded TMA blocks. The TMA was stained with HE for architectural analyses and 6 immunomarkers previously reported to be associated with ccRCC clinical outcomes: Ki67 (DAKO clone MIB1) [6, 7], C-myc (Abcam, Agilent Technologies) [8], p53 (DAKO clone DO7) [9], carbonic anhydrase 9 (CAIX) (Abcam, clone ab15086) [10–12], vimentin (DAKO clone V9) [13–16] and PTEN (DAKO clone 6H2.1) [17].
Image analysis
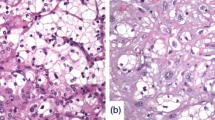
The TMA slides were digitized using the Philips IntelliSite Ultra Fast Scanner (Philips Digital Pathology Solutions, Best, the Netherlands). Individual cores were manually outlined in the user interface and saved at 20× magnification (2 px/mu). Digital images were analyzed with Image Pro Premier (IPP) software (vs 9.1, Media Cybernetics, Rockville, MD), previously used for onco-pathology evaluations [18–22]. If necessary, regions of interest were delineated to exclude zones of normal parenchyma, fibrosis, necrosis, cysts or folded tissue that might jeopardize analyses (834 ROI (39 %) for 2105 spots). Using the smart segmentation option of IPP, one segmentation setting was designed to detect 3,3′-diaminobenzidine (DAB) signal and a second one for determining total tissue area. The same segmentation settings were used to analyze all spots and markers. Cytoplasmic markers outcomes (CAIX, C-Myc, vimentin and PTEN) were expressed as a percentage of the immune-positive area (100*number of DAB+ pixels/total tissue area in pixels). Nuclear markers (Ki67 and p53) were expressed as number of positive cells/mm2. DAB+ particles smaller than 9 pixels2 were considered as noise. The relative size option of IPP was used for counting clustered nuclei (Fig. 2). For each patient, the value used for statistical analysis was the median of the several spots measurements.
Automatic image analyses. A–D: same tissue sample (original scanning magnification ×10, 1 px/µm) with HE staining (a), CAIX staining (b), automatic detection of DAB signal (c) and detection of the total surface area (d). Results are expressed as the percentage immunopositive surface area (number of segmented pixels in c/number of pixels segmented in d). e, f Representative example of automatic counting of Ki67 + cells. Detail of Ki 67 DAB staining (e) and automatic particle counting based on DAB signal (f). Results are expressed as number of Ki67 + cells/µm2
Statistics
Univariate and multivariate Cox regression analyses were performed to identify independent predictors (age, gender, body mass index, pathological stage, grade, physical status score, CAIX, vimentin, PTEN, c-MYC, p53, Ki67) of RFS, CSS and OS. Stages were split in two groups pT1b versus pT2-3 since no significant difference in survival outcomes appeared between the pT2 and pT3 groups in our cohort. Variables were analyzed in Cox multivariate analysis if p < 0.10 on univariate analysis. Association was considered significant when p < 0.05. We defined the clinical and pathological parameters that were prognostic of the disease recurrence-free survival (time from surgery to distant or local recurrence or censor), the CSS (time from surgery to RCC-related death or censor) and the OS (time from surgery to any kind of death or censor) within our cohort. For a better clinical relevance, we designed a categorical variable based on IHC markers independently associated (p < 0.05) with primary outcome on multivariate analyses. To the best, the cutoff point to transform a continuous variable into categorical was found with a lower p value approach based on a log-rank test [23]. Secondly, we tested these variables in the multivariate analyses of the recurrence-free, cancer-specific and overall survival. Pathological stage and grade’s correlations with markers were tested with a binary logistic regression model treating pathological features as two values variables (grade: I–II or III–IV; stage: 1b or 2–3). The correlation between significant markers was tested with a nonparametric Spearman test (bilateral hypothesis). Two sensitivity analyses were performed categorizing stages as pT1b, pT2 or pT3 and assessing markers as continuous.
Discrimination of the new marker was calculated by Harrell’s concordance index. Bootstrap correction (150 samples) for over-fitting biases was conducted. Harrell’s concordance index was calculated for models including significant clinical and pathological variables in multivariate analysis (reference) and for the model including the new markers. An increase in the C-index superior to 0.005 was considered as the criteria to not discard the model [24]. We explored calibration plots by comparing the models including the new marker with the rest of the models.
Results
Clinical outcomes
Forty-one patients were excluded of analyses for diverse reasons (Fig. 1). One hundred forty-three patients were included in analyses. Characteristics of the population are resumed in Table 1. Median follow-up was of 62.9 months (IQR 35.0–91.8). Forty-six patients (32.2 %) recurred (2 local, 44 metastatic), 24 (16.8 %) died specifically of ccRCC, and 46 (32.2 %) died of any cause. Stage pT1b exhibited 90 % 5-year RFS, 98 % CSS and 91 % OS; the respective figures for pT2 and pT3 were 51 and 62 % for RFS, 81 and 78 % for CSS and 78 and 67 % for OS.
Clinical outcomes’ predictors
Univariate analysis for RFS is presented in Table 2. BMI (p 0.002), stage (p < 0.001), grade (p 0.003) and vimentin (p 0.03) were predictors of recurrence. Because of 15 missing data, we decided to perform analyses without the BMI; results were consistent while implementing this variable (better survival among over-weighted population, supplement, Table 5). Cox multivariate analyses for RFS are reported in Table 2. In the first set of analyses, marker expressions were treated as continuous variables. Recurrence-free survival was significantly associated with lower body mass index (BMI), higher pathological T stage and Fuhrman grade, lower CAIX and higher vimentin expression. Cancer-specific survival was significantly associated with higher age, higher Fuhrman grade, lower CAIX and higher vimentin expression. Overall survival was significantly associated with higher age, Fuhrman grade and vimentin expression.
Stage and grade were neither correlated with vimentin (stage p = 0.239, grade p = 0.660) nor with CAIX (stage p = 0.608, grade = 0.963). Vimentin and CAIX were not correlated with each other (p = 0.443). Therefore, they could be included in a common variable CAIX-/Vimentin+ leading to a more discriminative test. The best cutoff points to discriminate CAIX under-expression and vimentin over-expression were, respectively, of 30 and 50 %.
We built-up the categorical variable CAIX-/Vimentin+: patients were considered positive when the tumor harbored less than 30 % of CAIX expression and more than 50 % of vimentin expression together (group 1) and negative in any other situation (group 2). Thirty-nine (27.3 %) patients belonged to group 1, 103 (72.0 %) belonged to group 2, and 1 (0.7 %) patient had missing data (vimentin). On multivariate analysis, group 1 presented a higher risk of recurrence (p < 0.001, HR = 3.6) (Table 2), specific RCC death (p < 0.001, HR = 4.6) and overall death (p = 0.010, HR = 2.4).
Correlation and calibration
Differences between the reference models and models including the new markers’ C-indexes were always superior to 0.005. This statement was confirmed for the three clinical outcomes: recurrence-free (+0.03), cancer-specific (+0.02) and overall survival (+0.01). The comparable calibration plots between the reference models and the models including the CAIX-/Vimentin+ marker demonstrated that the new model did not lose too much calibration while increasing the discrimination.
Discussion
In this study, we demonstrated with a long-term follow-up cohort, the negative effect on clinical outcomes of an under-expression of CAIX and over-expression of vimentin in the resected tumor tissue of non-metastatic ccRCC. These associations were robust enough to be considered as independent predictors of recurrence, cancer-specific and overall death with higher accuracy than the clinicopathological models.
CAIX protein is a member of the carbonic anhydrase family, and it has been reported to interfere in cell proliferation regulation in response to hypoxia, in oncogenesis and in tumor progression [25]. CAIX is expressed in about 85 % of ccRCC but not in non-ccRCC. The negative association between CAIX under-expression and clinical outcomes in RCC had been reported in other studies [10, 11]. This association concerns other malignancies such as cervical [26], esophageal [27] and colorectal carcinoma [28]. The interest of CAIX in the diagnosis, prognosis and treatment had been reported in various studies [29].
Vimentin is an intermediate filament protein; it is physiologically expressed in mesenchymal cells. In oncogenesis, vimentin plays a role in epithelial-to-mesenchymal transition conferring migratory and invasive properties to carcinoma [30]. Vimentin is expressed in up to 87 % of ccRCC [16]. The deleterious impact of vimentin expression on RCC clinical outcomes had also been reported in several studies before [13–15].
Previous studies reported the potential of IHC markers to predict ccRCC clinical outcomes. Kim et al. [13] designed a nomogram based on a cohort of 318 patients harboring ccRCC at any stage, including metastatic. The IHC prognostic markers included in their model were p53, CAIX and vimentin. By including CAIX under-expression and vimentin over-expression role in ccRCC outcomes, their model is fairly close to ours despite the additional parameters p53. Other studies support negative outcomes associated with CAIX under-expression [31, 32]. Klatte et al., assessing different markers in their original hypothesis, eventually presented a model with different markers: Ki-67, p53, endothelial VEGFR-1, epithelial VEGFR-1 and epithelial VEGF-D. In their model, CAIX was not predictive of clinical outcomes. However, their cohort was underpowered with 170 patients included and 20 different markers tested, which presents a risk of bias in their statistical analyses [33]. For this reason, we designed our marker inclusion in a stepped way.
Immunohistochemistry is a powerful strategy for protein expression analysis. The problem is the subjectivity [2]. Previous IHC markers evaluations in RCC cited above are based on manual readings with semiquantitative measurements. The method is changing from one study to another and authors often base their quantification on DAB signal intensity, which is wrong since IHC is not a stoichiometric procedure [34]. Automatic image analysis aims to palliate this limitation. For this study, we performed our analysis with the software IPP. The setting we defined could be used on other cohorts for potential external validation without interpretation bias. This point makes our methodology more reproducible than the previous reports. The reliability of this software had been compared with the visual assessment for survivin expression in rectal cancers [18]. They concluded in a substantial agreement between the two methods and an advantage of IPP automatic analysis in terms of increased sensitivity in scoring, reliability and reproducibility for protein expression measurements. The software had been tested in oncology to evaluate the association between IHC markers, clinicopathological features and clinical outcomes in endometrioid [19], non-small cell lung [20], laryngeal [21] and colorectal [22] cancers. To our knowledge, this study is the first application in ccRCC.
Another limitation of IHC is to define a good threshold to define risk groups based on the level of marker expression. We aimed to define a rationale threshold by using automatic image analysis instead of manual reading, providing a precise percentage instead of semiquantitative data. Secondly, we used the lower p value approach to define the best cutoff point. In our series, 50 % for vimentin and 30 % for CAIX seemed to be the right thresholds to separate patients at risk. Indeed, other cutoffs have been used in previous reports, mostly arbitrarily chosen. The aim of our model was to distinguish risk groups for recurrence, independent from the TNM groups. In order to simplify the number of groups (high- vs low-risk patients), we have decided to combine the CAIX-/Vimentin+ group.
Despite a long follow-up and robust results confirmed on three different survival analysis plus the sensitivity analysis, our study presents some limitations. As in any retrospective study, there is a risk of selection bias. In the 11-year study period, follow-up varied and even a slight variation was detected among participating centres. Meantime, the emergency of targeted therapies during the same period shifted the specific and overall survival of metastatic ccRCC. Exclusion of pT1a tumors was considered based on the low prevalence of metastatic events. While this exclusion supports our statistical analysis, we cannot rule out the possible interest of markers determination in the pT1a population although a longer follow-up and a larger cohort is needed. This study is based on a TMA with sometimes, delineation of region of interest instead of a whole tumor surface analysis. This might lead to inter-/intra-observer variation. Some variables such as lymphovascular invasion or necrosis were not evaluated because not systematically reported in our database. However, TNM classification remains the only universal prognostic system used in clinics. The size of our cohort was too small to split a validation subcohort. Consequently, our encouraging results remain pending on external validation and the present report has to be considered as exploratory.
Conclusion
In this study, we report the potential role of two IHC markers (CAIX and vimentin) to predict clinical outcomes for localized (pT1b-pT3) ccRCC treated surgically. We report a reliable and reproducible technique to evaluate these markers’ expression that should allow easy external validation and potential applicability in daily clinical practices.
References
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67(5):913–924
Rüdiger T, Höfler H, Kreipe H-H, Nizze H, Pfeifer U, Stein H et al (2002) Quality assurance in immunohistochemistry: results of an interlaboratory trial involving 172 pathologists. Am J Surg Pathol 26(7):873–882
Sobin LH, Wittekind Ch (eds) (2002) TNM classification of malignant tumors, UICC International Union Against Cancer, 6th edn. Wiley, NY, pp 193–195
Karakiewicz PI, Briganti A, Chun FK-H, Trinh Q-D, Perrotte P, Ficarra V et al (2007) Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol Off J Am Soc Clin Oncol 25(11):1316–1322
Sobin L, Gospodarowitcz M, Wittekind Ch (eds) (2009) TNM classification of malignant tumors, UICC International Union Against Cancer, 7th edn. Wiley, NY, pp 255–257
Sakai I, Miyake H, Takenaka A, Fujisawa M (2009) Expression of potential molecular markers in renal cell carcinoma: impact on clinicopathological outcomes in patients undergoing radical nephrectomy. BJU Int 104(7):942–946
Gayed BA, Youssef RF, Bagrodia A, Darwish OM, Kapur P, Sagalowsky A et al (2014) Ki67 is an independent predictor of oncological outcomes in patients with localized clear-cell renal cell carcinoma. BJU Int 113(4):668–673
Tang S-W, Chang W-H, Su Y-C, Chen Y-C, Lai Y-H, Wu P-T et al (2009) MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett 273(1):35–43
Shvarts O, Seligson D, Lam J, Shi T, Horvath S, Figlin R et al (2005) p53 is an independent predictor of tumor recurrence and progression after nephrectomy in patients with localized renal cell carcinoma. J Urol 173(3):725–728
Li G, Feng G, Gentil-Perret A, Genin C, Tostain J (2007) CA9 gene expression in conventional renal cell carcinoma: a potential marker for prediction of early metastasis after nephrectomy. Clin Exp Metastasis 24(3):149–155
Bui MHT, Seligson D, Han K, Pantuck AJ, Dorey FJ, Huang Y et al (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res Off J Am Assoc Cancer Res 9(2):802–811
Luong-Player A, Liu H, Wang HL, Lin F (2014) Immunohistochemical reevaluation of carbonic anhydrase IX (CA IX) expression in tumors and normal tissues. Am J Clin Pathol 141(2):219–225
Kim HL, Seligson D, Liu X, Janzen N, Bui MHT, Yu H et al (2004) Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 10(16):5464–5471
Sabo E, Miselevich I, Bejar J, Segenreich M, Wald M, Moskovitz B et al (1997) The role of vimentin expression in predicting the long-term outcome of patients with localized renal cell carcinoma. Br J Urol 80(6):864–868
Moch H, Schraml P, Bubendorf L, Mirlacher M, Kononen J, Gasser T et al (1999) High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am J Pathol 154(4):981–986
Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P et al (2005) Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 29(6):747–754
Shin Lee J, Seok Kim H, Bok Kim Y, Cheol Lee M, Soo Park C (2003) Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol 84(3):166–172
Wang C-J, Zhou Z-G, Holmqvist A, Zhang H, Li Y, Adell G et al (2009) Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol AIMM Off Publ Soc Appl Immunohistochem 17(6):530–535
Gu J, Liang Y, Qiao L, Li X, Li X, Lu Y et al (2013) Expression analysis of URI/RMP gene in endometrioid adenocarcinoma by tissue microarray immunohistochemistry. Int J Clin Exp Pathol 6(11):2396–2403
Zhang X, Wang H, Wang J (2013) Expression of HMGB1 and NF-κB p65 and its significance in non-small cell lung cancer. Contemp Oncol Pozn Pol 17(4):350–355
Lu JG, Li Y, Li L, Kan X (2011) Overexpression of osteopontin and integrin αv in laryngeal and hypopharyngeal carcinomas associated with differentiation and metastasis. J Cancer Res Clin Oncol 137(11):1613–1618
Shi H, Hayes M, Kirana C, Miller R, Keating J, Macartney-Coxson D et al (2012) TUFM is a potential new prognostic indicator for colorectal carcinoma. Pathology (Philadelphia) 44(6):506–512
Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S et al (2012) Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 7(12):e51862
Nguyen CT, Kattan MW (2011) How to tell if a new marker improves prediction. Eur Urol 60(2):226–228 (discussion 228–230)
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G et al (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158(3):905–919
Brewer CA, Liao SY, Wilczynski SP, Pastorekova S, Pastorek J, Zavada J et al (1996) A study of biomarkers in cervical carcinoma and clinical correlation of the novel biomarker MN. Gynecol Oncol 63(3):337–344
Turner JR, Odze RD, Crum CP, Resnick MB (1997) MN antigen expression in normal, preneoplastic, and neoplastic esophagus: a clinicopathological study of a new cancer-associated biomarker. Hum Pathol 28(6):740–744
Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastoreková S, Pastorek J et al (1998) Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol 153(1):279–285
Stillebroer AB, Mulders PFA, Boerman OC, Oyen WJG, Oosterwijk E (2010) Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol 58(1):75–83
Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R et al (2006) Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol 364(5):938–944
Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T (2007) Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int 100(3):556–560
Patard J-J, Fergelot P, Karakiewicz PI, Klatte T, Trinh Q-D, Rioux-Leclercq N et al (2008) Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer J Int Cancer 123(2):395–400
Klatte T, Seligson DB, LaRochelle J, Shuch B, Said JW, Riggs SB et al (2009) Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 18(3):894–900
True L, Feng Z (2005) Immunohistochemical validation of expression microarray results. J Mol Diagn 7(2):149–151
Acknowledgments
We would like to thank Nicola Macchione for his help in the data collection.
Author’s contribution
A. Ingels and M. Pilar Laguna Pes were involved in protocol/project development, data collection and management, analyzed the data, and wrote and edited the manuscript. M. Hew and J. J. M. C. H. de la Rosette were involved in protocol/project development and data collection. F. Algaba was involved in protocol/project development and data collection and management. O. J. de Boer was involved in data collection and management. R. J. A. van Moorselaar, S. Horenblas, and P. Zondervan were involved in data collection..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All the patients included in the analyses signed an informed consent to use the material from surgery for research purposes.
Additional information
A. Ingels and M. Hew are equally contributed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ingels, A., Hew, M., Algaba, F. et al. Vimentin over-expression and carbonic anhydrase IX under-expression are independent predictors of recurrence, specific and overall survival in non-metastatic clear-cell renal carcinoma: a validation study. World J Urol 35, 81–87 (2017). https://doi.org/10.1007/s00345-016-1854-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1854-y