Abstract
Purpose
Contemporary tools estimating increased risk of prostate cancer (PCa) relapse after radical prostatectomy (RP) are far from perfect and there has been an intensive search for additional predictive variables. We aimed to explore whether the parameters of postoperative ultrasensitive prostate-specific antigen (PSA) decline provide additional information for predicting PCa progression.
Methods
A total of 319 consecutive men, with at least 2 years of follow-up after RP for clinically localized PCa were subjected to this study. Intensive postoperative measurements of ultrasensitive PSA resulted in total of 4028 PSA values available for statistical evaluation. Biochemical recurrence (BCR) was defined as PSA ≥0.2 ng/ml. The accuracy of predictive models was quantified with the area under the curve.
Results
Over a median follow-up of 43 months (24–99 months), 107 patients (34 %) experienced BCR after RP. In patients with BCR, significantly higher values of PSA nadir (p < 0.001) and a decreased time interval from surgery to reach PSA nadir (p < 0.001) were observed. A multivariable Cox regression model confirmed that PSA nadir >0.01 ng/ml (HR 6.01, 95 % CI: 3.89–9.52) and time to PSA nadir <3 months (HR 2.86, 95 % CI: 1.74–5.01) were independent predictors of BCR. The inclusion of PSA nadir and the time to PSA nadir into the model resulted in improvement of predictive accuracy by 16 % over the model designed on the basis of established parameters.
Conclusions
Our results demonstrate that the level of PSA nadir and the time to PSA nadir determined by ultrasensitive assay significantly improve the identification of patients who are at high risk of disease recurrence after RP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serum prostate-specific antigen (PSA) declines to undetectable levels after radical prostatectomy (RP) for localized prostate cancer. The significant postoperative rise in the serum PSA is termed as the biochemical recurrence (BCR). Although controversy continues to surround the proper definition of BCR, it has been shown that BCR can signal cancer activity well before any clinical signs of disease recurrence [1]. Despite early detection and aggressive intervention, up to 40 % of patients are likely to experience BCR after RP at some time during their lifetime [2]. Accurate identification of patients at high risk of cancer relapse is of obvious importance, as treatment decisions are largely based on this information [3]. Several second line treatment options, including life-long hormonal therapy are suggested for patients at increased risk of cancer relapse after RP. Some of these strategies even form the basis for a new standard of care [4, 5]. However, the degree of accuracy with which we are able to identify men at high risk of cancer relapse is considerably limited.
Several studies have attempted to identify pre- and post-treatment variables that could have prognostic value with regard to the likelihood of disease progression after surgery [6]. The most widely recognized parameters include preoperative PSA value and pathological features of the specimen after RP such as the Gleason sum, tumor staging, seminal or lymph node invasion, and surgical margin status. Numerous reports have described different predictive nomograms, multivariate analyses and artificial neural networks incorporating these conventional variables in efforts to improve patient counseling and management in daily clinical practice [7–9]. However, these predictive tools remain imperfect in their discrimination properties. Despite better understanding of the biological behavior of prostate cancer, further improvement in the accuracy of existing models is limited by the lack of additional readily available predictive variables [10, 11].
PSA kinetics has been extensively investigated for their usefulness as predictors of BCR. Although many studies have evaluated the importance of postoperative rise of PSA in terms of doubling time, little is known of postoperative PSA decline to conventionally undetectable levels [12–14]. In this study, we aimed to investigate in detail the dynamic of postoperative decrease in serum PSA with the use of ultrasensitive PSA assays in order to define potential new parameters that could improve prediction of BCR after RP.
Methods
Patient population
The study has received ethical approval by institutional review board. Data from 690 consecutive patients who underwent RP for clinically localized prostate cancer between May 2001 and February 2010 at our institution were evaluated. Of those, 467 patients had at least 2 years of postoperative follow-up data. Patients with PSA nadir greater than 0.1 ng/ml were classified as radical prostatectomy failure and were excluded from the study (n = 119). In accordance with the management policy of our institution, immediate secondary therapy in terms of radiation or hormonal treatment was recommended to these patients. In order to provide the most accurate calculation of postoperative PSA dynamic, patients with the evidence of hormonal therapy and/or radiotherapy prior to the surgery were excluded from the study as well (n = 10). Individuals with incomplete follow-up data for proper calculation of PSA nadir and time to PSA nadir (n = 15) were not included in the analysis. Additionally, since pelvic lymphadenectomy was not routinely performed in all of the patients, nodal involvement was not included in the statistical analysis and these patients (n = 4) were excluded from the study. This resulted in a final cohort of 319 patients available for statistical evaluation. Statistical comparison of clinico-pathological characteristics of patients excluded from the study did not differ significantly from the studied cohort (Chi-square test, Mann–Whitney test). Tumors were staged according to the 2002 TNM staging system. Prostate cancer Gleason grading was performed by a dedicated genitourinary pathologist [15].
Follow-up
The patients’ follow-up consisted of ultrasensitive serum PSA level measurements, physical examination, and quality of life assessment at an interval of 2 weeks, 1, 2, and 3 months after surgery, and then subsequently every 3 months thereafter. All the PSA tests were performed in a single hospital laboratory under standardized settings using the Immulite third-generation PSA assay (Diagnostic Products Corp, Los Angeles, California; lower detection limit 0.003 ng/ml). PSA nadir was determined as the lowest postoperative value of PSA. To accurately evaluate the potential of PSA nadir and the time interval taken to reach PSA nadir, as a reliable predictor of BCR, only patients with PSA measurements 3 months before the PSA nadir and 3 months after this were included in the study. This was done in order to ensure that PSA nadir had been reached during that specific time interval. A total of 3659 ultrasensitive PSA measurements were evaluated in the final analysis. Changes of PSA not exceeding the analytical sensitivity of the assay (0.01 ng/ml) were noted as insignificant. Biochemical recurrence was defined as a single postnadir PSA level of 0.2 ng/ml or greater, and the recurrence date was assigned to the first PSA value ≥0.2 ng/ml.
Statistical methods
Statistical analysis was performed using the SAS statistical software program JMP 6 (SAS Institute, Cary, NC, USA). Mann–Whitney test and Chi-square test were used to compare several variables between groups of patients. Biochemical recurrence-free survival curves comparing different groups of patients were calculated using the Kaplan–Meier product-limit method, with significance evaluated by two-sided log-rank statistics. Patients were censored at the time of their last tumor-free clinical follow-up appointment. The cutoff values of continuous variables (preoperative PSA, PSA nadir, time to PSA nadir) that best predicted the biochemical progression were determined by using the Partition platform of the software. Pearson’s correlation coefficient was used to examine the relationship between continuous variables. Multivariate analysis was performed using the Cox proportional hazard model. The receiver operating characteristic curve (ROC) and area under the curve (AUC) were determined to describe the accuracy in predicting BCR postsurgically. The significance of the difference between areas under ROC curves of different predictive models was assessed using the method of DeLong et al. [16].
Results
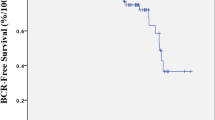
During the median follow-up interval of 43 months (24–99 months), a total of 107 patients (34 %) experienced BCR. Table 1 lists the clinico-pathological details for the entire evaluated population. The median time to BCR was 21 months (3–90 months). Table 2 demonstrates the differences in several parameters between the groups of patients divided according to the appearance of BCR during follow-up. Regression analyses showed a significant relationship between preoperative PSA and PSA nadir (r = 0.27, p < 0.001). There was no correlation between time to PSA nadir and preoperative PSA (r = −0.91, p = 0.105) or between time to PSA nadir and PSA nadir (r = −0.12, p = 0.129). Figure 1 contains the Kaplan–Meier survival curves showing an apparent difference in the probability of BCR in patients stratified according to the value of PSA nadir and time to PSA nadir. The results of univariate and multivariate analysis of factors of possible prognostic value for BCR after radical prostatectomy are depicted in Table 3. Figure 2 shows the ROC curves of different predictive models. The AUC for the base model designed on the basis of established parameters (preoperative PSA value, pathological Gleason sum, extracapsular extension, and surgical margin status) was 0.70 (CI: 0.65–0.75). When the base predictive model was complemented with PSA nadir, the AUC increased significantly to 0.83 (CI: 0.80–0.88, p < 0.0001). Addition of both parameters of postoperative ultrasensitive PSA decline (PSA nadir and time to PSA nadir) resulted in further significant improvement in prediction accuracy (AUR 0.86, CI: 0.81–0.89, p = 0.032).
Plot of ROC (receiver operating characteristics) curves showing predicted probabilities of biochemical recurrence on the basis of established parameters (preoperative PSA, pathological Gleason sum, extracapsular extension, and surgical margin status), established parameters plus PSA nadir and established parameters plus PSA nadir and plus time to PSA nadir
Discussion
Comparing studies on PSA kinetics after RP, some have used PSA assays with lower detection limit of 0.1 ng/ml and higher [12]. Other studies have combined PSA values from several laboratories using different assay methods [14, 17]. The calculation of the PSA nadir has been often based only on a limited amount of PSA measurements obtained during relatively long time period [13, 18]. Further, a number of authors have included patients with very short follow-up after RP [13, 19]. To overcome these limitations, we present a single institution and single laboratory study, with all the PSA measurements performed by equal PSA assay. The ultrasensitive PSA test used in the present analysis had a functional sensitivity of 0.01 ng/ml, which is in the order of magnitude greater than that of other conventional assays. A total of 4028 ultrasensitive measurements were analyzed. Only patients with a complete set of PSA measurements needed for the accurate calculation of PSA nadir were included. It has been stated that the majority of BCR (80 %) appears in the first 2 years after the operation [7]. Thus, only men with minimum follow-up of 2 years after RP were included in our study.
During the median follow-up of 43 months (range 24–99 months), a total of 107 patients (34 %) experienced BCR. The risk of BCR was strongly associated with the level of PSA decline. Multivariate models showed that patients with a postoperative PSA nadir of more than 0.01 ng/ml had an 85 % probability of experiencing BCR (HR 6.01, 95 % CI: 3.89–9.52). These results are compatible with several reports on prognostic significance of ultrasensitive PSA nadir [13, 14, 17]. Moreover, our analysis showed that those patients who reached the PSA nadir within the first 3 months after surgery had an increased risk of BCR by 186 % (HR 2.86, 95 % CI: 1.74–5.01). A possible explanation for this finding may be that the PSA level drops only as far as the baseline level of PSA being produced by residual cancer tissue in the pelvis or by occult micro-metastases already present at the time of surgery. According to this hypothesis, in patients who are fully cured after RP and have no residual cancer tissue producing any significant amounts of PSA, the decline of PSA measurements to the ultrasensitive PSA nadir value is longer.
The predictive accuracy of the most widely used preoperative model for the prediction of biochemical recurrence after radical prostatectomy ranges from 79 to 86 % [6, 10, 20]. The factors that predicted the outcome of RP in terms of BCR were preoperative PSA, Gleason score, lymph node status, seminal vesicle invasion, extracapsular extension, and positive surgical margin status. Same factors were tested in Cox regression analysis of our patients after radical prostatectomy. However, only extracapsular extension and a pathological Gleason score were significantly associated with the predicted outcome of surgery. Concordance index of our predicting model achieved the value of 70 %. Additional variables derived from postoperative PSA decline like PSA nadir and time to PSA nadir demonstrated a powerful ability to improve the models accuracy by 16 %.
This study has several potential limitations. These include limitations inherent to any retrospective study. Results may be influenced by selection bias. In this study group, restricted amount of patients with locally advanced prostate cancer was admitted for surgery treatment, which could partly explain low-risk characteristics of analyzed cohort. Thus, true external validation with an independent data set is needed to confirm the conclusions of presented paper. Another limitation is an unclear prognostic and therapeutic implication of BCR. Elevation of serum PSA value after RP can signal that prostate cancer has reappeared in some form and most experts would agree that postoperative serum PSA concentration of 0.2 ng/ml constitutes a true BCR. It has been shown that during 8 years of follow-up approximately 37 % of patients with BCR will develop clinical metastasis and 57 % of these patients will die of prostate cancer within the next 5 years [1]. However, although BCR is a surrogate end point for clinical progression, not all patients with BCR will progress to clinical relapse. Thus, the clinical usefulness of these new predictive parameters must be confirmed by their ability to add unique prognostic information with regard to prostate cancer metastases and survival.
Conclusions
Our analysis suggests that variables derived from the dynamic of postoperative PSA decline to very low levels appear to further improve the accuracy of existing predictive tools. More accurate nomograms could improve patients’ selection and identification of high-risk individuals, thus allowing for better judgments about postoperative surveillance strategy and the need for secondary therapy.
References
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281:1591–1597
Chun FK, Graefen M, Zacharias M, Haese A, Steuber T, Schlomm T, Walz J, Karakiewicz PI, Huland H (2006) Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol 24:273–280
Simmons MN, Stephenson AJ, Klein EA (2007) Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol 51(5):1175–1184
Siddiqui SA, Boorjian SA, Inman B, Bagniewski S, Bergstralh EJ, Blute ML (2008) Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: a matched cohort study. J Urol 179(5):1830–1837
Gomella LG, Zeltser I, Valicenti RK (2003) Use of neoadjuvant and adjuvant therapy to prevent or delay recurrence of prostate cancer in patients undergoing surgical treatment for prostate cancer. Urology 62(1):46–54
Lugghezani G, Briganti A, Karakiewicz PI, Kattan MW, Montorsi F, Shariat SF, Vickers AJ (2010) Predictive and prognostic models in radical prostatectomy candidates: a critical analysis of the literature. Eur Urol 58(5):687–700
Walz J, Chun FKH, Klein EA, Reuther A, Graefen M, Huland H, Karakiewicz PI (2009) Risk-adjusted hazard rates of biochemical recurrence for prostate cancer patients after radical prostatectomy. Eur Urol 55(2):412–419
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW (2005) Risk of prostate cancer mortality following biochemical recurrence after radical prostatectomy. JAMA 294:433–439
Cowen ME, Halasyamani LK, Kattan MV (2006) Predicting life expectancy in men with clinically localized prostate cancer. J Urol 175:99–103
Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Dotan ZA, DiBlasio CJ, Reuther A, Klein EA, Kattan MW (2005) Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol 23:7005–7012
Shariat SF, Karam JA, Walz J, Roehrborn CG, Montorsi F, Margulis V, Saad F, Slawin KM, Karakiewicz PI (2008) Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res 14(12):3785–3791
Semjonow A, Hamm M, Rathert P (1993) Prediction of tumor recurrence after radical prostatectomy using elimination kinetics of prostate-specific antigen. World J Urol 11(4):218–220
Shen S, Lepor H, Yaffee R, Taneja SS (2005) Ultrasensitive serum prostate specific antigen nadir accurately predicts the risk of early relapse after radical prostatectomy. J Urol 173(3):777–780
Eisenberg ML, Davies BJ, Cooperberg MR, Cowan JE, Carroll PR (2010) Prognostic implications of an undetectable ultrasensitive prostate-specific antigen level after radical prostatectomy. Eur Urol 57(4):622–629
Epstein JI, Allsbrook WC Jr, Amin MB, Egevald LL, ISUP grading committee (2005) The 2005 international society of urologic pathology (ISUP) Consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 29:1228–1242
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Doherty AP, Bower M, Smith GL, Miano R, Mannion EM, Mitchell H, Christmas TJ (2000) Undetectable ultrasensitive PSA after radical prostatectomy for prostate cancer predicts relapse-free survival. Br J Cancer 83(11):1432–1436
Hong SK, Park HZ, Lee WK, Kim DS, Lee JS, Doo SH, Jeong SJ, Yoon CY, Byun SS, Lee SE (2010) Prognostic significance of undetectable ultrasensitive prostate-specific antigen nadir after radical prostatectomy. Urology 76(3):723–727
Haese A, Huland E, Graefen M, Hammerer P, Noldus J, Huland H (1999) Ultrasensitive detection of prostate specific antigen in the followup of 422 patients after radical prostatectomy. J Urol 161(4):1206–1211
Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H (2000) Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol 164:101–105
Acknowledgments
This study was supported by a research grant IGA MZ CR NT13472-4.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vesely, S., Jarolim, L., Schmidt, M. et al. Parameters derived from the postoperative decline in ultrasensitive PSA improve the prediction of radical prostatectomy outcome. World J Urol 31, 299–304 (2013). https://doi.org/10.1007/s00345-012-0892-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-012-0892-3