Abstract
Purpose
To evaluate predictive factors associated with detectable prostate-specific antigen (PSA) and describe clinical recurrence (CR) and cancer-specific mortality (CSM) after robot-assisted radical prostatectomy (RARP).
Methods
The study included 2500 patients who were treated with RARP at a single institution between 2000 and 2016. All patients had clinically localized PCa. Patients were divided into two groups according to PSA value at 6 weeks after surgery: undetectable (n = 2271; PSA < 0.1 ng/dl) and persistently elevated (n = 229; PSA ≥ 0.1 ng/dl). The association between various covariates and: (1) detectable PSA and (2) CR was evaluated. Kaplan–Meier analyses estimated CR and CSM rates according to PSA persistence.
Results
Inside the group of detectable PSA, 146 men (63.75%) received adjuvant treatments, 44 patients (19.21%) salvages therapies and 38 men (16.5%) experienced CR. Factors associated with aggressive disease predicted PSA persistence. Within patients with detectable PSA, pathologic stage ≥ pT3a (HR 2.71; p < 0.029) and to received adjuvant androgen deprivation therapy (ADT) due to bad prognosis tumors (HR 13.36; p < 0.001) were associated with CR. Overall 14 (0.56%) died of PCa. 5 and 10-year CSM rates were higher for patients with CR (9.6 and 23.7%, p < 0.001), and Gleason ≥ 8 (5.7 and 6.9%, p = 0.003).
Conclusions
A detectable PSA is affected by factors associated with aggressive prostate cancer. Within men with persistent PSA, those with higher pathologic stage and who received adjuvant ADT are more likely to have CR. Patients with CR, Gleason ≥ 8, and those who received adjuvant ADT must have a close monitoring due to the high rate of mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is one of the most frequently diagnosed tumors in men, with an increasing incidence due to the widespread use of prostate-specific antigen (PSA) [1]. PCa is being diagnosed earlier at lower clinical stage, lower grade and smaller volumes, with an annual percentage increase of 9.5% as reported by data from the Surveillance, Epidemiology, and End Results [2, 3]. Approximately, 90% of patients newly diagnosed have localized PCa [3]. Radical prostatectomy (RP) represents a treatment modality for patients with clinically localized PCa that provides effective oncological control [4]. About 15–35% of men undergoing RP will demonstrate biochemical recurrence (BCR) [5] and the majority occur during the 1st years after RP [6]. In the last years, there have been many advances in robot-assisted radical prostatectomy (RARP). Some studies have recently reported better functional outcomes and comparable oncological results for RARP, compared to open and laparoscopic radical prostatectomy (LARP) [7, 8]. BCR-free survival after RARP has been reported in 84, 86 and 84.9% at 5 years, respectively [9,10,11]. However, long-term oncologic outcomes are still not available for RARP.
After RP, the measurement of any detectable PSA at 6 weeks has been considered as an adverse oncologic surrogate marker because could occult residual disease or distant systemic disease [12, 13]. The most used definitions of persistent PSA reported in many series was PSA ≥ 0.1 ng/dl [13, 14]. There have been reported predictors of detectable PSA after RP such as preoperative PSA, positive surgical margin (PSM), pathologic stage, nodal status or pathologic Gleason [13,14,15,16]. Kumar et al. reported that patients with persistent PSA were more likely to have BCR, but still a significant proportion of patients with persistent PSA who remained free of BCR [16]. Recently, it has been reported that in node-positive patients the assessment of early detectable PSA after surgery has an important prognostic role in the prediction of clinical recurrence (CR) and cancer-specific mortality (CSM) [17]. This might have important implications in planning an optimal follow-up, in order to use unnecessary adjuvant treatments in case of undetectable PSA. However, data regarding an association between detectable PSA and CR, distant metastases or CSM are currently limited. Therefore, we conducted a study with the aim of evaluating predictive factors associated with detectable PSA and assessing CR and CSM according to PSA levels after RARP.
Materials and methods
Between January 2000 and July 2016, 2500 patients were treated with RARP at a single institution. All patients in our cohort had clinically localized PCa. Exclusion criteria were patients with prior radiation, focal therapy, androgen deprivation therapy (ADT) or evidence of distant metastases, and patients with missing data. None of the patients included in the study received postoperative treatments before the first PSA assessment. Our institutional review board (CEPAR: comité d’evaluation des protocoles et d’aide á la recherche) approved the study, and patients provided informed consent. All patients had completed data, including pathologic stage, pathologic Gleason score, surgical margin status, extracapsular extension, pathologic node status, percentage of positive biopsy, status of adjuvant and salvage therapies, and PSA values at 6 weeks after RARP. Extended pelvic lymph node dissection (ePLND) was performed if the estimated risk of lymph node involvement (LNI) exceeded 5% based on the Briganti nomogram. Before 2012, we used the Partin tables to predict LNI. Neurovascular bundle sparing (NVB) was performed in 80.9% of the RARPs (unilateral in 36.6% and bilateral in 44.3%). PSM was defined as PCa at the inked margin. Patients were divided into two groups according to PSA value at 6 weeks after surgery: 2271 patients had undetectable PSA (PSA < 0.1 ng/dl) and 229 (9.16%) patients had PSA persistently elevated (PSA ≥ 0.1 ng/dl).
Follow-up at our institution was done with clinical visits and PSA determinations every 3–6 months in the 1st year after surgery, every 6 months in the following 2nd and 3rd year, and then annually.
BCR was defined as a confirmed serum PSA level of ≥ 0.2 ng/dl. CR was defined as positive imaging during follow-up after the onset of BCR. PCa death was defined as patients who died with metastasis in an androgen-independent setting, and was identified by the attending urologist or oncologist who followed the patients and or death certificated. All patients with CR underwent imaging consisted in bone scan and/or computed tomography (CT) and/or abdominal magnetic resonance imaging and/or 11C-choline positron emission tomography/CT scan. Patients were stratified in function of CR sites: prostate bed, pelvis lymph nodes, retroperitoneal lymph nodes and systemic recurrence (skeletal or visceral).
Adjuvant therapies were initiated within 3 months after RARP to patients with high-risk pathologic features (pathologic Gleason ≥ 8, pathologic stage ≥ pT3a, PSM, or LNI) and consisted in ADT when ≥ 2 positive lymph nodes were involved, and adjuvant external-beam radiation therapy (EBRT) defined as local radiation directed to the prostatic and seminal vesicle bed, including the pelvic region. EBRT was given when PSM was detected. Salvage radiotherapy was administered when there was not systemic recurrence and the recurrence was affecting pelvic site, and salvage ADT when systemic recurrence occurred.
Comparison of features by PSA level and type of additional treatment after surgery were evaluated using Chi square or ANOVA in categorical variables. Two-sample T test was used to test for equality of means in continuos variables. A univariate and multivariable logistic regression analysis was used to evaluate the significant association between detectable PSA and covariates, and CR and covariates in detectable PSA group. Covariates consisted of pathologic tumor characteristics, surgical node status, PSM status and age at surgery. Multivariable analyses were performed analyzing all variables that reached statistical significance on single variable analysis.
Kaplan–Meier analyses were used to assess CR and CSM rates according to PSA persistence after surgery. Statistical analyses were performed using SPSS v. 17 Software (Inc., Chicago, IL, USA) with a two-sided significance level set at p < 0.05.
Results
Clinical and demographic characteristics for the 2500 patients are shown in Table 1. The median follow-up was 4.85 years (IQR 0.15–15.4 years). Detectable PSA patients were significantly older, with higher preoperative PSA. In this group of men, the proportion of patients with high D’Amico risk score was significantly higher, as well as the number of bilateral tumors, and the biopsies with more than 33% of tumor involvement. Patients with PSA ≥ 0.1 ng/dl had a higher proportion of pathological Gleason ≥ 8 and pathological tumor stage ≥ pT3a, as well as higher proportion of PSM and LNI (all p < 0.001).
In the subgroup of patients with PSA ≥ 0.1 ng/dl, 146 men (63.75%) received adjuvant treatments (10.91% ADT vs 52.83% EBRT/EBRT + ADT), and 44 patients (19.21%) salvages therapies (8.73% ADT vs 10.48% EBRT/EBRT + ADT). As expected, the majority of patients with low-risk PCa, pT2 stage, and the lack of extracapsular extension and PSM did not receive any adjuvant therapy. Conversely, high-risk PCa patients with PSA > 10, ≥ pT3a stage, LNI, extracapsular extension and PSM were treated mostly with EBRT alone or with ADT (all p < 0.001) (Table 2). Regarding salvage treatments, there were no differences between therapies and patient characteristics (Online Resource Table 1).
At univariable logistic regression, preoperative PSA, D’Amico risk score, percentage of positive biopsy, pathologic Gleason, pathologic stage, extension extracapsular, PSM and LNI were significantly associated with an increased risk of detectable PSA (all p < 0.001). The variables that remained after backward elimination in multivariable analysis were preoperative PSA (p = 0.002), pathologic Gleason (p < 0.001), pathologic stage (p = 0.008), PSM (p = 0.006) and LNI (p = 0.005). Predictors of CR in patients with detectable PSA are high D’Amico score (HR 3.41; 95% CI 1.02–11.9), pathologic Gleason 4 + 3 (HR 2.1; 95% CI 1.04–4.46), pathological stage (HR 3.1; 95% CI 1.35–7.11), LNI (HR 3.2; 95% CI 1.07–9.51) and to receive ADT in bad prognosis tumors (HR 14.7; 95% CI 5.8–57.2); (Online Resource Table 2). Multivariable analysis showed only pathologic stage (p = 0.02) and to receive adjuvant ADT (p < 0.001) to be associated with CR.
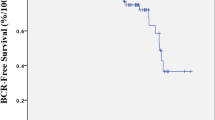
Inside the group of patients with detectable PSA, 38 men (16.5%) experienced CR. After stratifying patients according to the clinical characteristics, CR-free survival (CRFS) rates at 5–7 and 10 years follow-up were significantly higher in men with PSA ≤ 10 ng/dl, low-risk D’Amico score, pT2 stage, pathologic Gleason ≤ 3 + 4, and lack of extracapsular extension (Online Resource Fig. 1A, 1B, 1C). Men with PSA ≤ 10 ng/dl had at 5- and 10-year follow-up CRFS rates of 84.3 and 84.8% vs 70.4 and 60.2% for PSA > 10 ng/dl (p = 0.015). Patients with pathologic stage ≥ T3 showed at 10-year follow-up CRFS rates of 61.2 vs 90.8% for pT2 patients (p = 0.001). Those men with extracapsular extension had at 5- and 10-year after surgery lower CRFS rates regarding negative extracapsular extension (72.3 and 64.1% vs 84.2 and 82.1% p = 0.023). Among patients with detectable PSA, those who have LNI showed lower CRFS rates at 5 year (41.2 vs 72.3% for negative LNI; p = 0.003), and those who received adjuvant ADT showed at 5-year follow-up CRFS rates of 34.2% (81.2% EBRT/EBRT + ADT vs 92.1 surveillance; p < 0.001).
Regarding the relationship between D’Amico categories stratified by the type of adjuvant treatment with clinical recurrence, we can say that clinical recurrence-free survival is lower in patients receiving adjuvant therapies than those who do not receive subsequent treatments, in all D’Amico risk groups (Online Resource Fig. 3).
Overall 14 (0.56%) died of PCa. CSM-free survival (CSMFS) rates at 5–8 and 10 years follow-up were significantly lower in patients with pathologic Gleason ≥ 8 and those who received adjuvant ADT (p = 0.003 and p = 0.03, respectively) (Online Resource Fig. 2A, 2B). No differences were found regarding PSA levels after surgery (p = 0.23). The 5-and 10 years CSMFS rates were significantly lower in men who experienced CR compared with those who did not develop CR (90.4 vs 100% and 76.5 vs 99.6%, respectively; p = 0.001) (Online Resource Fig. 2C). No differences were found in terms of salvage therapies (p = 0.75).
The first site of relapse was local, pelvis lymph nodes, retroperitoneal lymph nodes and skeletal and/or visceral in 3.7, 37.03, 18.51 and 40.7%, respectively, of patients with CR.
Discussion
Serum PSA is expected to reach an undetectable level within 21-30 days after RP, due to its half-life of 3.15 days [18]. Therefore, detectable PSA at 6 weeks after surgery has been considered as an adverse oncologic outcome [12, 13, 19]. Many definitions for clinically significant persistently elevated PSA have been reported (from ≥ 0.03 to ≥ 0.1) [5, 13, 14, 20]. In the present study, 9.16% of the population-based cohort had detectable PSA after surgery that is similar than previous studies. Rogers et al. reported that 8.36% patients developed PSA ≥ 0.1 ng/dl after RP, with 47% of these patients developed distant metastasis, at a mean follow-up of 5.3 years [21]. Another study reported by Naselli et al. that persistent PSA was found in 10.3% patients, having 72.7% of these men BCR at a median follow-up of 6 months [22]. The study of Kumar et al. reported lower rates of PSA ≥ 0.1 (3.07% patients), while Audenet et al. showed 34.58% men with detectable PSA after RP [13, 16].
In the present study, pathologic Gleason ≥ 3 + 4, pathologic stage ≥ T3a, preoperative PSA, PSM and LNI were significantly associated with PSA persistence and they are in agreement with studies previously published [16, 17, 22]. Nevertheless, it has to underline that not all men with detectable PSA experienced CR; patients with LNI, pathologic stage ≥ T3a and those who receive adjuvant ADT were associated with CR. In this study, only 16.5% of these patients experienced CR, as compared with the 80% of the study of Bianchi et al. [17]. The reason is that our patients are not only men with LNI and high-risk PCa. That study reported that adverse pathologic characteristics did not impact on the rates of CR and CSM after stratifying by PSA levels after surgery. Taking into account our results, we were able to identify patients at high risk of developing CR, and treat them earlier than those patients with detectable PSA without risk factors. When evaluating the first site of recurrence, up to 40.7% of men harbored systemic metastases, compared with the 50% of other investigation [17].
In this study, CSMFS rates were lower in patients with Gleason ≥ 8, those who received adjuvant ADT and men who experienced CR. Patients who were treated with EBRT/EBRT + ADT had higher CSMFS; however, no differences were found regarding early PSA levels after surgery. We observed that patients with detectable PSA who received early adjuvant EBRT/EBRT + ADT after RP had lower incidence of CR and CSM than those treated only with ADT. The reason for these results may be due to the fact that patients who received ADT had a higher proportion of high-risk tumors compared to EBRT/EBRT + ADT. It is not that the therapy contributed to a poor prognosis being a predictor itself, but an indication of ADT was more likely to be used for worse tumors. This is in the line with the findings from Stish et al. who published that pre-radiotherapy PSA level adjusting for well-validated risk factors (Gleason, pathologic stage and preoperative PSA) is independently associated with the risk of metastasis in long-term follow-up, worsening CSM [23]. In the present study, we have been able to show that CRFS is higher in detectable PSA patients with favorable pathologic features who were monitored. Subsequently, this information can be used for counseling of these patients explaining what subset of men is likely to remain free of CR and need for adjuvant therapies.
To our knowledge, this is the second study to report the rates of detectable PSA after RP, in men with LNI. In this cohort, 56.2% men from the total of 32 pN + patients experienced PSA ≥ 0.1 ng/dl, and 44.4% of those men developed CR. No patient with undetectable PSA developed CR. Bianchi et al. reported rates of detectable PSA in LNI patients of 25% that support the role of extensive local treatment in patients with LNI [17]. Men with detectable PSA after ePLND can have either residual PCa in the prostatic bed/nodal pelvic areas or occult distant metastases at the time of RP. Although there is high level of evidence supporting the role of early ADT after RP [24], we suggest that some patients with pN + PCa and favorable pathologic characteristics (low Gleason, low number of LNI) may be managed expectantly in the presence of undetectable PSA after surgery that is in the line with the findings from Schumacher et al. [25], although prospective studies are needed to validate it.
The strength of this study is the large RARP cohort being up to our knowledge the first to report detectable PSA after surgery. However, certain limitations need to be considered. First, our analyses are limited by their retrospective nature. Second, the extent of extracapsular extension and the extent and site of PSM have not been deeply assessed. And third, as the study covered a long time, diagnostic, grading, and therapeutic changes that occurred during that time might have influenced our outcomes (heterogeneity in lymphadenectomy techniques).
Conclusions
In an RARP cohort, factors associated with aggressive disease predict detectable PSA. Within patients with persistent PSA, those with higher pathologic stage and who received adjuvant ADT because of bad prognosis tumors are more likely to have CR. Patients with higher pathologic Gleason and CR showed less favorable survival rates over time. These subsets may benefit the most from more extensive and earlier multimodal treatments.
References
Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur J Cancer. 2013;8:S0959–8049.
Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: national Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102:950–8.
Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–314.
Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977.
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591.
Walz J, Chun FK, Klein EA, Reuther A, Saad F, Graefen M, et al. Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. J Urol. 2009;181:601.
Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparatives studies. Eur Urol. 2009;55:1037–63.
Coelho RF, Rocco B, Patel MB. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers. J Endourol. 2010;24:2003–15.
Suardi N, Ficarra V, Willemsen P, De Wil P, Gallina A, De Naeyer G, Schatteman P, Montorsi F, Carpentier P, Mottrie A. Long-term biochemical recurrence rates after robot-assisted radical prostatectomy: analysis of a single-center series of patients with a minimum follow-up of 5 years. Urology. 2012;79:133–8.
Sooriakumaran P, Haendler L, Nyberg T, Gronberg H, Nilsson A, Carlsson S, Hosseini A, Adding C, Jonsson M, Ploumidis A, Egevad L, Steineck G, Wiklund P. Biochemical recurrence after robot-assisted radical prostatectomy in a European single-centre cohort with a minimum follow-up time of 5 years. Eur Urol. 2012;62:768–74.
Liss MA, Lusch A, Morales B, Beheshti N, Skarecky D, Narula N, Osann K, Ahlering TE. Robot-assisted radical prostatectomy: 5-year oncological and biochemical outcomes. J Urol. 2012;188:2205–10.
De Boo L, Pintilie M, Yip P, Baniel J, Fleshner N, Margel D. Time from first detectable PSA following radical prostatectomy to biochemical recurrence: a competing risk analysis. Can Urol Assoc J. 2015;9:E14–21.
Audenet F, Seringe E, Drouin SJ, Comperat E, Cussenot O, Bitker MO, Rouprêt M. Persistently elevated prostate-specific antigen at six weeks after radical prostatectomy helps in early identification of patients who are likely to recur. World J Urol. 2012;30:239–44.
Moreira DM, Presti JC, Aronson WJ, Terris MK, Kane CJ, Amling CL, Freedland SJ. Definition and preoperative predictors of persistently elevated prostate specific antigen after radical prostatectomy: results from the SEARCH database. BJU Int. 2009;105:1541–7.
Eisenberg ML, Davies BJ, Cooperberg MR, Cowan JE, Carroll PR. Prognostic implications of an undetectable ultrasensitive prostate specific antigen level after radical prostatectomy. Eur Urol. 2010;57:622–30.
Kumar A, Samavedi S, Mouraviev V, Bates AS, Coelho RF, Rocco B, et al. Predictive factor and oncological outcomes of persistently elevated prostate-specific antigen in patients following robot-assisted radical prostatectomy. J Robot Surg. 2017;11(1):37–45.
Bianchi L, Nini A, Bianchi M, Gandaglia G, Fossati N, Suardi N, Moschini M, Dell’Oglio P, Schiavina R, Montorsi F, Briganti A. The role of prostate-specific antigen persistence after radical prostatectomy for the prediction of clinical progression and cancer-specific mortality in node-positive prostate cancer patients. Eur Urol. 2016;69:1142–8.
Partin AW, Oesterling JE. The clinical usefulness of prostate specific antigen: update 1994. J Urol. 1994;152:1358–68.
Freedland SJ, Humphreys EB, Mangold LA, Eisenberg M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9.
D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Identifying patients at risk for significant versus clinically insignificant postoperative prostate specific antigen failure. J Clin Oncol. 2005;23:4975–9.
Rogers CG, Khan MA, Miller MC. Natural history of disease progression in patients who fail to achieve an undetectable prostate specific antigen level after undergoing radical prostatectomy. Cancer. 2004;101:2549–56.
Naselli A, Introini C, Andreatta R, Spina B, Truini M, Puppo P. Prognostic factors of persistently detectable PSA after radical prostatectomy. Int J Urol. 2009;16:82–6.
Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, Buskirk SJ. Metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016;34:3864–71.
Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Inmediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9.
Schumacher MC, Burkhard FC, Thalmann GN, Fleischmann A, Studer UE. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol. 2008;54:344–52.
Author information
Authors and Affiliations
Contributions
SG-B: project development, data collection and manuscript writing. FR: project development. IN-S: statistical analysis. VS: data collection. RS-S: data collection. EB: editing. MG: editing. XC: project development and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
CEPAR: Comité d’Evaluation des Protocoles et d’Aide à la Recherche (Protocol Evaluation Committee and for Research Assistance) committee approved the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García-Barreras, S., Rozet, F., Nunes-Silva, I. et al. Predictive factors and the important role of detectable prostate-specific antigen for detection of clinical recurrence and cancer-specific mortality following robot-assisted radical prostatectomy. Clin Transl Oncol 20, 1004–1010 (2018). https://doi.org/10.1007/s12094-017-1812-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1812-1