Abstract
Arsenic, a toxic element has been infiltrating the food chain, raising the food safety and security issues. Due to phytotoxicity, mobility and availability of specific transporters for arsenic translocation, it is considered as serious agronomic threat to rice crop. To limit the detrimental effect of this arsenic stress, soil amendment with beneficial substances such as silicon can be helpful in acquiring arsenic tolerance by activating defense mechanism and subsequently improve seedlings vigor. However, its exact mechanism through which it enhances arsenic tolerance is not fully understood. The present study aims to evaluate the potential of exogenous application of silicon to alleviate toxic effects of arsenic stress and the associated morphological, biochemical and physiological mechanism in rice seedlings. In this study, rice seedlings of non-aromatic variety (IR-6) and aromatic variety (Super basmati) were grown in hydroponic system for 28 days. For arsenic stress, 25 µM and 50 µM sodium arsenite were applied along with 1 mM silicic acid (Treatment groups: As0, As25, As50, As25 + Si and As50 + Si). Results indicated that silicon application significantly decreased oxidative stress markers including hydrogen peroxide levels by 34.78%, lipid peroxidation by 20.06% consequently, decrease in relative membrane permeability by 37.79% at 25 µM arsenic stress in Super basmati as compared to IR-6. Furthermore, silicon application under 25 µM arsenic stress also activated enzymatic antioxidant defense system by elevating the activity of superoxide dismutase by 68.89%, guaiacol peroxidase by 53.59% and ascorbate peroxidase by 66.77% in Super basmati except catalase. Additionally, SEM images showed the anatomical adaptation as cuticle thickenings along with deposition of silica in silicon-treated plants. Silicon deposition was also evident by EDX spectra while arsenic was not detected in silicon supplemented rice seedlings under arsenic stress. Hence, these findings suggested that silicon application hinders arsenic translocation and arsenic toxicity. This study would serve as a step towards developing the approach for the rice grains with reduces arsenic content. This mitigation strategy will be helpful in environmental sustainability along with ensuring food security.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is not only staple food and export commodity but also source of arsenic exposure (Rehman et al. 2021). As it is hyper accumulator of arsenic as compared to other cereals (Su et al. 2010). The uptake of toxic metalloid (arsenic) not only causes phytotoxicity in rice plants but also threatens food safety (Upadhyay et al. 2020). Rice is traditionally cultivated in anaerobic soil where arsenite is predominately present (Singh et al. 2022). As an analog of silicic acid, arsenite enters rice roots through a silicon transporter, classified as a nodulin 26-like intrinsic protein (Ma et al. 2008). It potentially inactivates enzymes and co-factors by reacting with closely spaced cysteine residues and impairs the aquaporin function, consequently, reducing water and nutrient uptake thus, causing detrimental effects on plant development (Martínez-Castillo et al. 2022). Even low arsenite concentration hinders root elongation, reduces photosynthesis and decreases phosphorous, copper, potassium, zinc, manganese and iron contents (Murugaiyan et al. 2021). Its exposure is also responsible for excessive production of reactive oxygen species (ROS) resulting in oxidative stress consequently, causes damages in macromolecules thus, hampers normal cellular functions (Shri et al. 2009). Malondialdehyde (MDA) and H2O2 content are oxidative stress markers and represent the level of injury in plant cells (Nahar et al. 2022). ROS-induced oxidative stress is coped by enzymatic antioxidants, such as highly reactive superoxide radicals converted into hydrogen peroxide by activity of superoxide dismutase and afterwards decomposed into oxygen and water by activity of catalase and peroxidase (Zulfiqar and Ashraf 2022). Although, plant cells are well equipped with an antioxidant defense system that comprised non-enzymatic and enzymatic components but the response of antioxidant defense machinery is influenced by stress intensity (Shri et al. 2009).
To combat arsenic toxicity, several agronomic strategies can be employed, including water management, selection of cultivars and soil amendments (Farooq et al. 2016). Among all these, soil amendment is the most promising eco-friendly, cost-effective and practical approach. Soil amendment can be achieved by application of bio-stimulants like silicon (Zia et al. 2017).
Silicon is one of most prevalent element in soil and its bioavailable form is silicic acid (Epstein 1999). Rice, as hyper silicon accumulator, can absorb silicon more than 10% of shoot dry weight and deposited as amorphous silica in cell wall of epidermis and intracellular spaces, which provides structural support and enhances biomass (Chen et al. 2011; Ranganathan et al. 2006). It contributes in higher yield bearing capacity and helps in acquiring resistance against several biotic and abiotic stresses like toxicity of heavy metals (Coskun et al. 2019). It is reported that silicon inhibits arsenic uptake through influx transporter (Lsi1) by competitive inhibition and reduces arsenic-induced phytotoxicity by extrusion from cells or sequestration of arsenic with phytochelatins complexes in intracellular compartments and strengthens ROS scavenging capacity by activation of antioxidant enzymes to cope oxidative stress under stress conditions (Khan et al. 2021). Moreover, silicon is deposited in “cuticle-double layer” which in turn minimizes water lost through transpiration and pest infestation (Ma and Yamaji 2006). It is also reported that silicon inhibited heavy metal accumulation such as arsenic in rice grains in different soil types (Zhang et al. 2020). Previous studies indicated that substantial varietal differences are also present in term of arsenic and silicon uptake and accumulation potential (Niazi et al. 2022; Talukdar et al. 2019). However, the research based on the effect of silicon supplementation on arsenic toxicity is limited to geographical regions (Meharg 2004). Therefore, the present investigation was based on two commercially important aromatic and non-aromatic rice varieties to elucidate the mechanisms of silicon induced arsenic tolerance in terms of oxidative stress markers, antioxidant enzyme activities and silica deposition. The objectives of present research were (a) to evaluate the morphological, physiological, and biochemical markers for alleviating arsenic toxicity in rice seedlings through silicon application (b) to assess silicon and arsenic accumulation and anatomical alterations in rice leaves in response to silicon application under arsenic stress. This study will provide insight information about the role of silicon application in mitigation of arsenic toxicity in rice seedlings.
Material and Methods
Plant Growth Conditions
The seeds of aromatic rice variety (Super Basmati) and non-aromatic rice variety (IR-6) provided by National Agriculture Research Council, Islamabad, Pakistan, soaked in deionized water for 2 days and placed on petri plates layered in filter paper after surface sterilization with 5% sodium hypochlorite solution. Following treatment groups: As0 (control), As25 (25 µM NaAsO2), As50 (50 µM NaAsO2), As25 + Si (25 µM NaAsO2 + 1 mM silicic acid) and As50+Si (50 µM NaAsO2 + 1 mM silicic acid) were used. Healthy and uniform seedlings were placed in seedling trays having Hoagland’s solution (pH 5.5). Seedlings were established in growth chambers at 30 °C with 80% humidity for 28 days.
Germination Analyses
For germination analysis, ten sterilized rice seeds (Super Basmati and IR-6) were placed in each petri plate for control (As0), arsenic stress (As25, As50), and silicon application (As25 + Si and As50 + Si). The experiment was performed in five replicates. Germination data was recorded daily. Fresh weight (FW), dry weight (DW), shoot length (SL) and root length (RL) of the seedlings were assessed at the end of the experiment. Germination parameters were evaluated using formulas (Supplementary file).
Oxidative Stress Indicators
Relative membrane permeability (RMP) was measured using the protocol of Thind et al. (2021b). Fresh leaves (0.5g) were placed in distilled water (20 mL). Electrical conductivity (EC) was measured as EC0. Samples were stored at 4 °C overnight and EC1 was recorded. EC2 was measured after autoclaving. The relative membrane permeability was estimated by using the following formula (Yang et al. 1996).
Lipid peroxidation was assessed through TBA-reactive compound (MDA) quantification as described by Heath and Packer (2022). Rice leaves (0.1g) were homogenized in 5% trichloroacetic acid (TCA). After centrifugation, supernatant was allowed to react with equal volume of 0.5% thiobarbituric acid (TBA). Samples were heated for 30 min at 95 °C then allowed to cool and absorbance was taken at 532 and 600 nm.
Rice leaves (0.1g) were homogenized in 0.1% TCA and supernatant was collected. The supernatant mixed with Sodium phosphate buffer (pH 7.0) and 1 M potassium iodide solution. Absorbance was recorded at 390 nm to determine the H2O2 content (Hussain et al. 2023).
Antioxidant Enzyme Activities
Rice leaves (0.1g) were homogenized 0.2M potassium phosphate buffer (pH 7.8) having 1% Polyvinylpyrrolidone (PVP), 1 mM Ethylene diamine tetra acetic acid (EDTA) and 1 mM phenylmethylsulfonyl fluoride (PMSF). The supernatant was collected and used for antioxidant enzyme quantification.
Superoxide dismutase (SOD) activity was analyzed by inhibition of the photochemical reduction of nitro blue tetrazolium (NBT) by SOD (Thind et al. 2020). Reaction mixture containing 50 mM potassium phosphate buffer (pH 7.8) with EDTA, NBT (50 µM), Triton X-100 (0.0025%) and l-Methionine (10mM) was mixed with riboflavin (0.00037%) and enzyme extract. The tubes were placed in light for 15 min. One unit of SOD represented the amount of enzyme required for 50% inhibition of the reduction of NBT and exhibited as units per gram of fresh weight.
Catalase activity was analyzed by decomposition of H2O2 as described by Chance and Maehly (1955) in the reaction mixture containing 50 mM potassium phosphate (pH 7.0) and 2 mM H2O2. Change in absorbance was observed at 240 nm.
Ascorbate peroxidase coverts H2O2 into H2O using ascorbate as the electron donor, and its activity was measured in the reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 1 mM ascorbate and 0.5 mM H2O2 (Thind et al. 2021a). The oxidation of ascorbate was initiated by adding H2O2and change in absorbance was recorded up to 1 min at 290 nm (extinction coefficient, 2.8 mM−1 cm−1).
The activity of guaiacol peroxidase was assayed by Polle et al. (1994). The oxidation of guaiacol (extinction coefficient, 26.6 mM−1 cm−1) in the reaction mixture comprising 50 mM potassium phosphate buffer (pH 7.0), guaiacol (20 mM), and H2O2 (10 mM). Change in absorbance was measured for 1 min at 470 nm.
Elemental Analysis and Scanning Electron Microscopy
Scanning electron microscopy (SEM) with Energy dispersive X-ray spectroscopy (EDX) was performed as previously reported by Ranganathan et al. (2006). Rice leaves were cut into the pieces of 2 cm. To remove moisture, samples were kept at 80 °C in dry oven, sputter coated with gold, and placed onto the apparatus. SEM and EDX were performed with an accelerating voltage of 20 kV. The surfaces rice samples were scanned with integrated analytical SEM (Philips, The Netherlands) and attached EDX.
Data Analysis
ANOVA was performed by using SPSS version 17.0 for statistical analysis. Data are presented as mean ± standard error. Differences among treatments within a variety were determined by Tukey’s test (p value < 0.05). Oxidative stress markers and activities of antioxidant enzymes were subjected to Principle Component Analysis (PCA) for validation of the interaction of arsenic and silicon application in rice seedlings using OriginPro (version 8.5).
Results
Analysis of Seed Germination Parameters
Germination percentage, germination index and germination rate were significantly decreased in comparison with control in Super Basmati at 50 µM arsenic stress, although no significant difference was observed with the silicon application. Germination percentage and germination rate remained unchanged in all treatments as compared to control in IR-6. Whereas the germination index decreased at 50 µM arsenic stress, but no significant difference was observed with silicon supplementation in IR-6. The vigor index was significantly decreased with arsenic exposure in both varieties in comparison with the control, but silicon significantly improved the vigor index by 8.75% and 82.89% at 25 µM and 50 µM arsenic stresses respectively in IR-6 and 176.53% at 50 µM arsenic stress in Super Basmati (Table S1).
Analysis of Seedling Growth Attributes
In comparison with control, arsenic stress of 25 and 50 µM considerably decreased the relative water content, shoot length, root length and fresh weight in both varieties. While silicon supplementation significantly promoted the shoot length elongation (194.26% and 89.44%), helped in maintaining the relative water content (87.71% and 130.1%), improved the fresh weight (67.85% and 93.1%) and dry weight (43.93% and 42.27%) in 50 µM arsenic-stressed seedlings of Super Basmati and IR-6 respectively in comparison with arsenic stress of 50 µM without silicon (Table S2).
Analysis of Stress Indices
To further validate the influence of silicon application on rice seedlings under arsenic stress, stress indices were calculated by considering various parameters like root and shoot length, dry and fresh weight and germination index (Table S3). Silicon application significantly increased the dry weight stress index (46.31% and 41.86%), fresh weight stress index (66.57% and 92.6%) and shoot length stress index (193.91% and 78.03%) at 50 µM arsenic stress in comparison with arsenic stress of 50 µM alone in Super Basmati and IR-6 respectively. The root length stress index was significantly increased by 204.25% with silicon application at 25 µM arsenic stress in IR-6 as compared to 25 µM arsenic-stressed without silicon.
Analysis of Oxidative Stress Markers
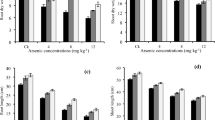
In both rice varieties, hydrogen peroxide level was elevated in dose-dependent manner under arsenic treatments in comparison with control. Silicon application significantly decreased hydrogen peroxide content by 34.78% and 41.18% at 25 and 50 µM arsenic stresses in Super Basmati and 24.78% and 29.93% at 25 and 50 µM arsenic-treated seedlings of IR-6 respectively (Fig. 1A). MDA is considered as an indicator of lipid peroxidation. Its content was increased significantly with arsenic stress of 25 and 50 µM in both rice varieties. In contrast, silicon application decreased the lipid peroxidation by 20.06% at 25 µM arsenic in seedlings of Super Basmati and 33.58% in IR-6 at 50 µM of arsenic stress (Fig. 1B). Electrolyte leakage was considerably increased in arsenic-stressed seedlings of both varieties in comparison with control. Silicon supplementation significantly improved membrane integrity, which was indicated by lower electrical conductivity (37.79% and 28.97%) in 25 and 50 µM arsenic-exposed seedlings of Super Basmati while (32.92% and 37.3%) at 25 and 50 µM arsenic stress in IR-6, respectively. (Fig. 1C).
Oxidative stress markers A hydrogen peroxide content, B malondialdehyde content, C relative membrane permeability in Super basmati and IR-6 under arsenic stress (25 and 50 µM) with and without silicon application. Significant differences (p < 0.05) among the treatments within a variety are indicated by different letters (a, b, c)
Analysis of Antioxidant Enzyme Activities
Antioxidant enzyme activities were altered under arsenic stress to cope with the oxidative stress due to the excessive ROS production. SOD is considered as a key antioxidant enzyme. Silicon application significantly increased SOD activity 68.89% and 36.89% in Super Basmati and IR-6 at 25 µM arsenic stress (Fig. 2A). Whereas CAT activity was increased (135.58% and 51.72%) at 25 and 50 µM of arsenic stress with silicon application in IR-6 seedlings respectively (Fig 2B). The POD and APX activities were significantly higher in both varieties upon arsenic exposure, but the increased POD activity (53.59% and 48.69%) and APX activity (66.77% and 59.36%) were observed in arsenic stress of 25 µM with silicon supplementation in Super Basmati and IR-6, respectively (Fig. 2C, D).
Quantification of antioxidant enzyme activities A superoxide dismutase, B catalase, C ascorbate peroxidase, D guaiacol peroxidase in Super basmati and IR-6 under arsenic stress (25 and 50 µM) with and without silicon application. Significant differences (p < 0.05) among the treatments with in a variety are indicated by different letters (a, b, c)
Principle Component Analysis
Principle component analysis was executed to analyze the interactions of important attributes including oxidative stress markers and activities of antioxidant enzymes under arsenic stress in the presence and absence of silicon application in Super Basmati and IR-6. Oxidative stress markers (H2O2 content, MDA content, and electrolyte leakage) formed a separate cluster on the positive coordinate of the biplot of PCA, indicating its positive correlation with arsenic stress susceptibility, specifically at 50 µM arsenic stress without silicon in Super Basmati and IR-6. Whereas, antioxidant enzymes (SOD, CAT, APX, POD) activities made another cluster on negative side on biplot, suggesting a negative correlation with arsenic stress susceptibility, particularity at 25 µM arsenic stress with silicon in both varieties (Fig. 3).
Analysis of Silica Deposition
The EDX spectra of Super Basmati and IR-6 rice leaves, having arsenic stress of 25 and 50 µM alone and with 1 mM silicic acid are displayed respectively in Figs. 4 and 5. The needle-like structures, continuous to the epidermal cell walls were detected in rice leaves of Super Basmati and IR-6 supplemented with silicon under arsenic stress (Figs. 6, 7). In Super Basmati, silicon content (7.64 atomic wt%) was higher in the treatment of 50 µM arsenic stress supplemented with 1 mM silicic acid as compared to 25 µM arsenic stress along with 1 mM silicic acid (Table 1). While, in IR-6 silicon contents (28.99 and 23.62 atomic wt%) were found in 25 and 50 µM arsenic stresses supplemented with 1 mM silicic acid, respectively (Table 2). Arsenic was detected under 50 µM arsenic stress without silicon (15.82 and 11.20 atomic weight %) in Super Basmati and IR-6, respectively (Tables 1, 2). Whereas arsenic was not detected in all the treatments of silicon application under arsenic stress in both rice varieties.
Discussion
Heavy metal stress due to the rhizospheric coexistence and uptake of toxic metals or metalloids like arsenic causes phytotoxicity by reacting with the biomolecules subsequently hindering several metabolic processes and affects overall plant growth (Rahman et al. 2008; Shri et al. 2009). It is reported that silicon exposure enhances plant growth and development under various biotic and abiotic stress including heavy metal toxicity (Khan et al. 2021). The current study examines the role of silicon application in mitigation of arsenic toxicity in two rice cultivars (Super Basmati and IR-6) and investigates the morphological, biochemical and physiological modifications.
Healthy seed germination is considered as criterion of successful plant development under stressed conditions like arsenic stress. The seed germination process which is affected by arsenic stress could be improved through silicon supplementation, as previously reported (Ramírez-Olvera et al. 2019). In the current study, the vigor index was enhanced by silicon supplementation significantly which demonstrates the capability of plant to survive in arsenic stress. Silicon up regulates the two α- amylases during seed germination, involved in starch degradation to generate glucose for glycolysis, which in turn provides energy and consequently improves the stress tolerance (Sheng et al. 2018).
Rice plants exhibited alterations in morpho-physiological traits including relative water content, shoot length, fresh weight, dry weight and root length under arsenic stress. These alterations hinder the growth and development of plants (Rahman et al. 2008). In the current study, at 50 µM arsenic stress, shoot length, fresh and dry weight and relative water content were significantly increased with silicon application. These results are in accordance with previous studies (Khan and Gupta 2018; Raza et al. 2016) which demonstrated the role of silicon as growth regulator like compound that involved in cell division, cell expansion and inter-nodal elongation, suggesting better reason for improved plant growth (Hussain et al. 2019). In present study, Silicon application increases the fresh and dry weight of rice seedlings under arsenic stress indicating the enhance biomass production which might be due to the improved nutrients uptake and immobilized arsenic translocation. Furthermore, silicon content shares in shoot dry weight approximately up to 10% (Ma and Takahashi 2002) and provides better structural support and rigidity to plant leaves, thus promoting net photosynthesis (Chen et al. 2011). However, as first contact point of toxic arsenic exposure, significant retardation in root length was observed under arsenic stress. The reduction in root length in arsenic-exposed sprouts also has been observed in previous studies (Shri et al. 2009).
Arsenic induces oxidative stress by generating various reactive free radicals like superoxide, singlet oxygen, hydroxyl and hydroperoxyl radicals (Tripathi et al. 2007) which are responsible for damages in biomolecules, including DNA, proteins, and lipids. Reactive oxygen radicals induce electrolyte leakage by initiating chain like peroxidation of unsaturated fatty acid eventually leads to production of MDA. In the present study, oxidative stress was elevated in dose-dependent manner due to arsenic exposure. Production of ROS (e.g., H2O2), noticeably increased the MDA content and electrolyte leakage were observed at 50 µM arsenic stress in both varieties. Earlier studies revealed that arsenic toxicity induces the generation of H2O2 and MDA content (Nahar et al. 2022; Shri et al. 2009). However, silicon supplementation reduced the oxidative stress by lowering H2O2 content, MDA accumulation and electrolyte leakage. Arsenic stress induces oxidative damage in cells which promotes the activation of enzymatic and non-enzymatic antioxidant defense mechanisms (Khan and Gupta, 2018; Nahar et al. 2022). Enzymatic antioxidants like SOD, GPX, CAT and APX play an important role in scavenging reactive oxygen species (ROS) that are generated under arsenic stress. Initially SOD converts the highly toxic radical (O2˙−) into the less toxic form (H2O2) which further breaks down into H2O and O2 through CAT activity. APX and GPX are also considered major H2O2 scavenging enzymes in plant cells which reduce H2O2 into H2O (Zulfiqar and Ashraf 2022). In the current study, significant increase in SOD, APX and GPX activity was observed under arsenic stress with silicon application in both varieties whereas CAT activity was increased in IR-6 variety. As a result of increased activities of antioxidant enzymes, the levels of H2O2 and lipid peroxidation were decreased. The reduced H2O2, levels and lipid peroxidation indicated that silicon is helpful in alleviating oxidative stress by enhancing the arsenic-induced ROS scavenging ability of plant cells and antioxidant capacity which is associated with superoxide dismutase, catalase and the enzymes of ascorbate–glutathione cycle (Khan and Gupta 2018; Zulfiqar and Ashraf 2022). As silicon application increased the antioxidant enzymes activities therefore, it can be suggested that silicon may be involved in up-regulation of genes encoding these antioxidant enzymes that are associated with improved stress tolerance in rice plants.
Arsenic accumulation in leaves of both rice varieties was observed under arsenic stress (50 µM) in the absence of silicon. However, arsenic was not detected in arsenic-stressed rice leaves supplemented with silicon in both varieties, which indicated that silicon application hinders the arsenic uptake and translocation into rice shoots. The reduction in arsenic contents in silicon supplemented plants were previously reported (Gao et al. 2021). In the previous studies, higher silicon content was also observed in the epidermal cell walls of rice leaves supplemented with silicon as compared to rice leaves not supplemented with silicon (Zhang et al. 2013; Zia et al. 2017). These results suggested the protective role of silicon in coping with arsenic toxicity (Gang et al. 2018; Gao et al. 2021). The findings of current investigation should be further validated by high throughput technologies like ICP-MS and ICP-OES to elucidate the role of silicon in restricting arsenic translocation from roots to above ground parts of the plant. Furthermore, the studies based on transcriptional regulation of transporters might be helpful to increase the understanding of mechanistic association of silicon in reducing arsenic content in plants.
Conclusion
The present study revealed that arsenic-induced phytotoxicity was responsible for reduced biomass production and decreased relative water content which consequently arrested the growth of rice seedlings. Silicon application improved the arsenic tolerance in rice seedlings by modulating plant growth, decreasing oxidative stress and activating the antioxidant defense system. In conclusion, silicon application is not only helpful in preventing oxidative stress by improving the ROS scavenging capacity through the modulation of antioxidant defense mechanism but also helpful in reducing the arsenic content in above ground parts of plants. In this context, the current study could provide an additional piece of evidence in terms of phenotypical, physiological and biochemical attributes that must be considered under arsenic stress for successful cultivation of rice crop with silicon supplementation. Silicon application may provide an attractive mitigation strategy for reducing arsenic toxicity in rice plant.
References
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Meth Enzymol 2:764–775. https://doi.org/10.1016/s0076-6879(55)02300-8
Chen W, Yao X, Cai K, Chen J (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res 142:67–76. https://doi.org/10.1007/s12011-010-8742-x
Coskun D, Deshmukh R, Sonah H, Menzies JG, Reynolds OL, Feng J, Kronzucker HJ, Bélanger RR (2019) The controversies of silicon’s role in plant biology. New Phytol 221:67–85. https://doi.org/10.1111/nph.15343
Epstein E (1999) Silicon. Annu Rev Plant Physio Plant Mol Biol 50:641–664. https://doi.org/10.1146/annurev.arplant.50.1.641
Farooq MA, Islam F, Ali B, Ullah N, Mao B, Gill RA, Yan G, Siddique KHM, Zhou W (2016) Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot 132:42–52. https://doi.org/10.1016/j.envexpbot.2016.08.004
Gang LI, Zheng M, Jianfeng TANG, Hojae SHIM, Chao CAI (2018) Effect of silicon on arsenic concentration and speciation in different rice tissues. Pedosphere 28:511–520. https://doi.org/10.1016/S1002-0160(17)60409-0
Gao Z, Tang X, Ye M, Gul I, Chen H, Yan G, Chang SX, Liang Y (2021) Effects of silicon on the uptake and accumulation of arsenite and dimethylarsinic acid in rice (Oryza sativa L.). J Hazard Mater 409:124442. https://doi.org/10.1016/j.jhazmat.2020.124442
Heath RL, Packer L (2022) Reprint of: Photo peroxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 726:109248. https://doi.org/10.1016/j.abb.2022.109248
Hussain I, Parveen A, Rasheed R, Ashraf MA, Ibrahim M, Riaz S, Afzaal Z, Iqbal M (2019) Exogenous silicon modulates growth, physio-chemicals and antioxidants in barley (Hordeum vulgare L.) exposed to different temperature regimes. Silicon 11:2753–2762. https://doi.org/10.1007/s12633-019-0067-6
Hussain I, Ayub A, Nayab A, Ashraf MA, Ashraf MA, Hussain S, Manzer HS, Muhammad AS, Usman Z, Khan TH (2023) Exogenous application of silicon and zinc attenuates drought tolerance in Eruca sativa L. through increasing chlorophyll pigments, osmoprotectants, and modulating defense mechanisms. J Plant Growth Regul. https://doi.org/10.1007/s00344-023-11116-7
Khan E, Gupta M (2018) Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci Rep 8:10301. https://doi.org/10.1038/s41598-018-28712-3
Khan İ, Awan SA, Rizwan M, Ali S, Hassan MJ, Brestič M, Zhang X (2021) Effects of silicon on heavy metal uptake at the soil-plant interphase: a review. Ecotoxicol Environ Saf 222:112510. https://doi.org/10.1016/j.ecoenv.2021.112510
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier, Amsterdam, p 294
Ma FJ, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Ma FJ, Yamaji N, Mitani N, Xu X, Su Y, McGrath SP, Zhao F (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Martínez-Castillo J, Saldaña-Robles A, Ozuna C (2022) Arsenic stress in plants: a metabolomic perspective. Plant Stress 3:100055. https://doi.org/10.1016/j.stress.2022.100055
Meharg AA (2004) Arsenic in rice–understanding a new disaster for South-East Asia. Trends Plant Sci 9:415–417. https://doi.org/10.1016/j.tplants.2004.07.002
Murugaiyan V, Zeibig F, Mahender A, Siddiq SA, Frei M, Murugaiyan J, Ali J (2021) Arsenic stress responses and accumulation in rice. In: Ali J, Wani SH (eds) Rice improvement. Springer, Cham, pp 281–313. https://doi.org/10.1007/978-3-030-66530-2_9
Nahar K, Rhaman MS, Parvin K, Bardhan K, Marques DN, García-Caparrós P, Hasanuzzaman M (2022) Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2:179–209. https://doi.org/10.3390/stresses2020013
Niazi NK, Hussain MM, Bibi I, Shahid M, Ali F, Iqbal J, Shaheen SM, Abdelrahman H, Akhtar W, Wang H, Rinklebe J (2022) The significance of eighteen rice genotypes on arsenic accumulation, physiological response and potential health risk. Sci Total Environ 832:155004. https://doi.org/10.1016/j.scitotenv.2022.155004
Polle A, Otter T, Seifert F (1994) Apoplastic peroxideses and lignification in needles of Norway sprue (Picea abies L.). Plant Physiol 106:53–60. https://doi.org/10.1104/pp.106.1.53
Rahman MA, Hasegawa H, Rahman MM, Miah M, Tasmin A (2008) Straight head disease of rice (Oryza sativa L.) induced by arsenic toxicity. Environ Exp Bot 62:54–59. https://doi.org/10.1016/j.envexpbot.2007.07.016
Ramírez-Olvera SM, Trejo-Téllez LI, Pérez-Sato JA, Gómez-Meriño FC (2019) Silicon stimulates initial growth and chlorophyll a/b ratio in rice seedlings, and alters the concentrations of Ca, B, and Zn in plant tissues. J Plant Nutr 42:1928–1940. https://doi.org/10.1080/01904167.2019.1648678
Ranganathan S, Suvarchala V, Rajesh Y, Prasad MS, Padmakumari AP, Voleti SR (2006) Effects of silicon sources on its deposition, chlorophyll content, and disease and pest resistance in rice. Biol Plant 50:713–716. https://doi.org/10.1007/s10535-006-0113-2
Raza MM, Ullah S, Ahmad Z, Saqib S, Ahmad S, Bilal HM, Wali F (2016) Silicon mediated arsenic reduction in rice by limiting its uptake. Agric Sci 7:1–10. https://doi.org/10.4236/as.2016.71001
Rehman MU, Khan R, Khan A, Qamar W, Arafah A, Ahmad A, Ahmad A, Akhter R, Rinklebe J, Ahmad P (2021) Fate of arsenic in living systems: Implications for sustainable and safe food chains. J Hazard Mater 417:126050. https://doi.org/10.1016/j.jhazmat.2021.126050
Sheng H, Ma J, Pu J, Wang L (2018) Cell wall-bound silicon optimizes ammonium uptake and metabolism in rice cells. Ann Bot 122:303–313. https://doi.org/10.1093/aob/mcy068
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110. https://doi.org/10.1016/j.ecoenv.2008.09.022
Singh S, Rajput VD, Upadhyay SK, Minkina T (2022) Arsenic contamination in rice agro-ecosystems: mitigation strategies for safer crop production. J Plant Growth Regul 42:6413–6424. https://doi.org/10.1007/s00344-022-10863-3
Su YH, McGrath SP, Zhao FJ (2010) Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 328:27–34. https://doi.org/10.1007/s11104-009-0074-2
Talukdar P, Hartley SE, Travis AJ, Price AH, Norton GJ (2019) Genotypic differences in shoot silicon concentration and the impact on grain arsenic concentration in rice. J Plant Nutr Soil Sci 182:265–276. https://doi.org/10.1002/jpln.201800373
Thind S, Hussain I, Ali S, Hussain S, Rasheed R, Ali B, Hussain HA (2020) Physiological and biochemical bases of foliar silicon-induced alleviation of cadmium toxicity in wheat. J Soil Sci Plant Nutr 20:2714–2730. https://doi.org/10.1007/s42729-020-00337-4
Thind S, Hussain I, Ali S, Rasheed R, Ashraf MA (2021a) Silicon application modulates growth, physio-chemicals, and antioxidants in wheat (Triticum aestivum L.) exposed to different cadmium regimes. Dose-Response 19:1–15. https://doi.org/10.1177/15593258211014646
Thind S, Hussain I, Rasheed R, Ashraf MA, Perveen A, Ditta A, Hussain S, Khalil N, Ullah Z, Mahmood Q (2021b) Alleviation of cadmium stress by silicon nanoparticles during different phenological stages of Ujala wheat variety. Arab J Geosci 14:1028. https://doi.org/10.1007/s12517-021-07384-w
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165. https://doi.org/10.1016/j.tibtech.2007.02.003
Upadhyay MK, Majumdar A, Kumar SJ, Srivastava S (2020) Arsenic in rice agro-ecosystem: solutions for safe and sustainable rice production. Front Sustain Food Syst 4:53. https://doi.org/10.3389/fsufs.2020.00053
Yang G, Rhodes D, Joly R (1996) Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine deficient and glycinebetaine containing maize lines. Funct Plant Biol 23:437–443. https://doi.org/10.1071/pp9960437
Zhang C, Wang L, Zhang W, Zhang F (2013) Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis? Plant Soil 372:137–149. https://doi.org/10.1007/s11104-013-1723-z
Zhang S, Geng L, Fan L, Zhang M, Zhao Q, Xue P, Liu W (2020) Spraying silicon to decrease inorganic arsenic accumulation in rice grain from arsenic-contaminated paddy soil. Sci Total Environ 704:135239. https://doi.org/10.1016/j.scitotenv.2019.135239
Zia Z, Bakhat HF, Saqib ZA, Shah GM, Fahad S, Ashraf M, Hammad HM, Naseem W, Shahid M (2017) Effect of water management and silicon on germination, growth, phosphorus and arsenic uptake in rice. Ecotoxicol Environ Saf 144:11–18. https://doi.org/10.1016/j.ecoenv.2017.06.004
Zulfiqar F, Ashraf M (2022) Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: an overview. J Hazard Mater 427:127891. https://doi.org/10.1016/j.jhazmat.2021.127891
Acknowledgements
The authors are indebted to National Agriculture Research Center (NARC), Islamabad, Pakistan for providing rice germplasm to execute the research and to Dr. A. Q Khan Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi, for the lab facilitation.
Author information
Authors and Affiliations
Contributions
1. Khadija Siddiqui: Conceptualization, Experimentation, data collecting, First draft of manuscript writing. 2. Maria Babar: Data analysis. 3. Dr. Ghlum Musharraf: Data interpretation. 4. Dr. Ishrat Jamil: Finalization of manuscript. 5. Dr. Saddia Galani: Experimental design, Supervision, Paper editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Handling Editor: Rupesh Deshmukh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqui, K., Babar, M., Jamail, I. et al. Silicon-Mediated Arsenic Tolerance: Restriction of Arsenic Uptake and Modulation of Antioxidant Defense System in Rice Seedlings. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11472-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11472-y