Abstract
Aims
Rice is a well-known silica-accumulating plant. The dumbbell-shaped silica bodies in the silica cells in rice leaf epidermis are formed via biosilicification, but the underlying mechanisms are largely unknown.
Methods
Leaves at different developmental stages were collected to investigate silica cell differentiation by analyzing structures and silicon localization in the silica cells.
Results
Exogenous silicon application increased both shoot and root biomass. When silicon was supplied, silica cells in the leaf epidermis developed gradually into a dumbbell-shape and became increasingly silicified as leaves aged. Silicon deposition in the silica cells was not completed until the leaf was fully expanded. Multiple lines of evidence suggest that lignification of silica cell walls precedes silicon deposition in the lumen of silica cells. The organized needle-like silica microstructures were formed by moulding the inner cell walls and filling up the lumen of the silica cell following leaf maturation.
Conclusions
Two processes were involved in silicon deposition: (1) the silica cell wall was lignified and silicified, and then (2) the silicon was deposited gradually in silica cells as leaves aged. Silica body formation was not completed until the leaf was fully mature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicon is one of the major elements in the Earth’s crust, second to oxygen in abundance (Sposito 1989). Diatoms absorb silicon from seawater to form biosilicated structures (Lopez et al. 2005) that are biologically produced under mild conditions and controlled by a set of proteins called silaffins (Kröger et al. 1997, 1999, 2000, 2001; Poulsen et al. 2003). Similarly, some higher plants, including most Poaceae (Epstein 1999; Piperno 2006) but not all monocot species (Hodson et al. 2005), are capable of forming discrete biosilicated structures (Kaufman et al. 1981).
Silicon uptake by higher plants has been considered a passive process (Barber and Shone 1966; Hartley and Jones 1972; Mitani and Ma 2005). However, Liang et al. (2005b) reported that silicon uptake and transport in Cucumis sativus L. is active. Ma et al. (2006, 2007) identified that radial silicon transport in roots is controlled by genes Lsi1 which encodes influx transporter and Lsi2 which encodes efflux transporter. Yamaji and Ma (2008) described the Lsi6 gene in the shoots of rice, which is involved in silicon distribution. After uptake by plants, some silicon is deposited in the root endodermis and the Casparian strip (Sangster and Parry 1976; Sangster 1978; Lux et al. 2003; Shi et al. 2005), but most of it is transported to the shoot (Lux et al. 1999). Silicon is likely deposited in cell walls of shoots, the intercellular region, and silica cells (Kaufman et al. 1970; Ma and Takahashi 2002b). A mature silica cell with observable silicon deposition is referred as a silica body, phytolith, or “plant opal”. As the shape of the silica body is determined inherently and varies among species, it is typically used to identify plant species in archeological research (Ball et al. 1993; Zhang and Wang 1998; Ball et al. 1999; Piperno et al. 1999). However, the mechanism controlling silicon deposition in higher plants has not been defined clearly.
Rice is a typical silicon-accumulating plant and it benefits from silicon supplementation. Silicon enhances the resistance of rice plants to various abiotic and biotic stresses and increases grain yields (Epstein 1999; Korndörfer and Lepsch 2001; Ma and Takahashi 2002a). Silicon is deposited in two types of silica cells in the rice leaf to form two corresponding silica bodies, one dumbbell-shaped and the other bulliform-shaped (Zhang and Wang 1998; Ma and Takahashi 2002b). The number of silica bodies in shoots correlates positively with silicon content in shoots. On average, the abaxial epidermis of rice leaves has 1.5 times more silicon than does the adaxial epidermis (Zhang and Wang 1998; Ma and Takahashi 2002b).
An increasing number of studies have been published on the development of silica bodies in higher plants (Blackman 1968; Sangster and Parry 1976; Sangster and Parry 1981; Kaufman et al. 1985; Parry et al. 1985; Hodson and Sangster 1988; Sugimura et al. 1999; Kauss et al. 2003; Lux et al. 2003; Motomura et al 2004, 2006; Currie and Perry 2007). Kaufman et al. (1985) suggested that silica body originates from the silica cell of the cork-silica cell pairs. Hodson et al. (1985) speculated that silicification in the silica cell follows cell cytoplasm degeneration and secondary cell walls thickening. Furthermore, the silica cell wall of rice contains phenol or lignin-carbohydrate complexes and calcium, which might combine with silicon (Inanaga and Okasaka 1995). Kauss et al. (2003) proposed that the synthesis of strongly cationic proline-rich proteins (PRPs) in cucumber as a preparation for enhanced silicon deposition in cell wall. Thus, cell wall could be a matrix for self-assembly of biosilica, similar to its role in biosilica formation in diatoms.
In rice, the young leaf blade is enveloped partially or completely by a sheath. The leaf blade matures basipetally during expansion from the sheath. This gradual maturation process provides an ideal system for studying silicon deposition in the dumbbell-shaped silica cells during leaf ageing. The present study provides details on variation in the silica cell structure and silicon deposition process. Using scanning electron microscopy (SEM), energy dispersive X-ray microanalysis (EDX), transmission electron microscopy (TEM) and cytochemical staining, we investigated differentiation of silica cell and provided insight into the mechanism of silica deposition in the leaf epidermis of rice.
Materials and methods
Plant culture
The surface of rice seeds (Oryza sativa. L., cultivar ‘Yuefu’) was sterilized for 20 min with 0.3 % hydrogen peroxide and rinsed with double-distilled water. Then the sterilized seeds were germinated without light at 25 °C on a tray. After 3 days, seedlings were transferred to nutrient solution in a 1 L plastic pot. There were two treatments: with or without Si in the nutrient solution. Nutrient solutions with Si were modified from Yoshida et al. (1976) and contained 1.0 × 10−3 M K2SiO3 at pH 5.5. Rice plants were grown under controlled conditions (35 °C during the day and 18 °C at night with a 14 h photoperiod) and harvested 30 days after transfer.
Sample collection
Segments (1 × 1 cm2) from the middle of leaf blades were collected from leaves in various developmental stages (Fig. 1). The D0 sample was collected from the tip of the shoot and the D1 sample was collected from the middle of the first leaf, which is the youngest leaf that is fully wrapped by developing leaves or sheaths; samples D2, D3, and D4 were collected at the lower, middle, and upper parts of the second leaf, respectively, which had protruded slightly from the sheath. A fully expanded mature leaf, the third leaf, was identified as D5. The D4 samples had completely stuck out from the sheath, whereas the D2 and D3 samples were embraced by the third leaf. The lengths of the leaf, sheath, and portion of blades that had protruded from the sheath were measured to indicate the leaf age (Table 1).
Sample processing for SEM and EDX microanalyses
To examine the development of dumbbell-shaped silica cell, we investigated silica deposition by SEM and EDX microanalysis. The leaves were prefixed for 2 h with 2.5 % glutaraldehyde in a pH 7.4 phosphate buffer, fixed for 2 h with 1 % osmium tetroxide in the same buffer, and dehydrated through an ethanol series, and then dried using critical point dryer. The samples were then attached to copper stubs and coated with gold for SEM (JSM 6301F) and EDX (Oxford Link-300 EDS) analyses at 20 kV.
Sample processing for histochemistry
The samples were cut into 2 × 10 mm cross-sections and fixed for 24 h in formalin:acetic acid:alcohol (5:5:90 in volume), rinsed with distilled water, dehydrated in an ethanol series, and then rinsed twice in xylene. Samples with a 60° angle to the horizon were embedded in paraffin. After sectioning with a microtome, samples were stained with Safranin-O and Fast-Green as described previously (Berlyn and Mickshe 1976). The wedged paraffin sections were further analyzed by optical microscopy (Olympus, Tokyo Japan).
Isolation of biosilica from rice leaves
Biosilica was isolated as described previously (Harrison 1996). Mature rice leaves were dried and ground using a pestle and mortar before being treated with concentrated acids. The acid treatment involved heating samples at 60–70 °C for 24 h in a 4:1 mixture of concentrated nitric-sulphuric acids, centrifugation, washing with deionized water eight times and lyophilizing. The isolated biosilica was observed with SEM and optical microscopy, respectively, and used for Raman spectrum analysis.
Raman biosilica spectra
Raman experiments were performed using a Renishaw 2000 spectrometer. Excitation was set at 514.5 nm at a power setting <5 mW to minimize heat effects on the biosilica samples. Three spots per sample were evaluated to obtain representative spectra.
Sample processing for TEM and EDX microanalyses
Mature leaves were collected from rice plants grown in solutions with or without added Si. Samples were cut into 2 × 10 mm cross-sections and fixed using the same protocol as mentioned above for SEM. Then, the samples were dehydrated through a graded acetone series and then in 100 % acetone. They were embedded in Epone812 resin. The ultrathin slices were contrasted with 2 % uranyl acetate (w/v) and then with lead citrate, according to a previous study (Reynolds 1963). The ultrathin samples were examined for silica cell ultrastructure using transmission electron microscopy (TEM, Philips EM-400T) at 80 kV and for silicon distribution using energy dispersive X-ray microanalysis (EDX).
Statistical analysis
All treatments were performed in triplicate. Statistical significance was evaluated by analyzing variance (p ≤ 0.05) using SAS version 6.12 (SAS Institute, Cary, NC, USA 1989).
Results
Variation in silica cells with leaf age
The lengths of the leaf, sheath, and protruded blade (Table 1) indicated the different maturity stages of the leaves (Fig. 1). The shoot tip of sample D0, which was 5 mm in length, was composed of undifferentiated cells (Fig. 2a). The leaf blade was 93 mm in length and the sheath length was 2 mm. Stomata had developed in the middle of the first leaf (sample D1), at which stage the silica cells had already become dumbbell-shaped although their outlines were variable (Fig. 2b). The second leaf blade was 228 mm in length, including a part which had protruded 16 mm from the 9-mm-long sheath. Silica cells were observable in sample D2 (the lower part of the second leaf), but appeared to be unexpanded. Stomata could not be seen at this stage (Fig. 2c). In contrast to sample D2, samples D3 and D4 exhibited dumbbell-shaped silica cells, stomata, and trichomes (Fig. 2d, e). Sample D5, collected from the mature leaf blade that had expanded completely, displayed the dumbbell-shaped silica cells clearly (Fig. 2f). In addition, the silica cells were typically distributed between two rows of stomata (Fig. 2b, d and e). The detailed changes of the silica cells in three samples were observed with SEM under high magnification (Fig. 3). Silica cells in sample D2 were unexpanded (Fig. 3b), although those cells in both samples D1 and D3 displayed dumbbell shape (Fig. 3a, c). In sample D3, these silica cells had bulged notably compared with those in sample D1 under high magnification (Fig. 3a, c).
Sequential SE images of dumb-bell-shaped silica cells and/or silica body in rice leaf 4 epidermis. (a) Tip of stem, the sample D0; (b) the first leaf, the sample D1; (c) Lower of the 5 second leaf, the sample D2; (d) Middle of the second leaf, the sample D3; (e) Upper of the second 6 leaf, the sample D4; (f) the third leaf, the sample D5. Scale bars in A = 75 μm, in B-F = 30μm. 7 Arrows in B-E identify silica cells. The arrow in F identifies dumb-bell-shaped silica body. *, 8 trichomes; S, stomata
The scanning electron-microscopic images of silica cells outline in different stages. Arrow shows the silica cells or silica body. a the first leaf (the sample D1); b The lower of second leaf (the sample D2); c the middle of second leaf (the sample D3). Magnification: ×3,000 (bar = 10.0 μm). *, trichome
Localization of silicon and other elements in the leaf epidermis
Silicon and other elements in the leaf epidermis were analyzed by SEM equipped with an EDX apparatus (Fig. 4). The secondary electron images (SEI) showed where carbon, oxygen, and silicon were detected in the developing silica cells by EDX (Fig. 4). Silica cells exhibited lower carbon but higher oxygen content compared with those in the background (Fig. 4). In contrast, silicon was distributed homogeneously in the epidermis of the developing leaves that had not expanded completely (samples D1–D4 in Fig. 4a–c). However, in completely expanded leaves, Si was concentrated in a dumbbell-shaped in the epidermis (sample D5 in Fig. 4d).
The morphological and structural analyses of isolated dumb-bell-shaped silica from rice leaves. a SEM images of the dumb-bell-shaped silica deposited onto the copper grid. b Optical microscopy image of the dumb-bell-shaped silica. c Raman spectrum of the dumb-bell-shaped silica showing peak intensities at 493, 792, and 1,092 cm−1 Raman shift, consistent with the known mineralogy of amorphous silica. There is an unknown peak at 566 cm−1, possibly related to the existence of an organosilicon complex
Raman microspectroscopy was used to study the structure of extracted silica cells (Fig. 5a, b), which was almost consistent with the known mineralogy of amorphous silica. Four prominent bands were observed at 1,092, 792, 566, and 493 cm−1 at room temperature (Fig. 5c). Bands at 1,092, 792, and 493 cm−1 were assigned to the Si-O-Si asymmetric stretch, Si-O-Si symmetric stretch, and Si O3OH tetrahedral vibration mode, respectively. There is an unknown peak at 566 cm−1 which might indicate the existence of an organosilicon complex.
The morphological and structural analyses of isolated dumb-bell-shaped silica from rice leaves. a, b SEM images of the dumb-bell-shaped silica deposited onto the copper grid. c Optical microscopy image of the dumb-bell-shaped silica. d Raman spectrum of the dumb-bell-shaped silica showing peak intensities at 493, 792, and 1,092 cm−1 Raman shift, consistent with the known mineralogy of amorphous silica. There is an unknown peak at 566 cm−1, possibly related to the existence of an organosilicon complex
Lignification in the silica cell wall with leaf ageing
Leaf samples counterstained with the Safranin-O-Fast Green double-dyeing technique were observed with an optical microscope (Fig. 6). Typically, lignified tissues were stained red by the Safranin-O and the other tissues were stained green by the Fast-Green. The cells in the D1 sample were almost all green; therefore, they had not lignified at this stage (Fig. 6a). However, the surroundings of silica cells in the D3 and D5 samples were red (Fig. 6b, c). As mentioned above, silicon was deposited throughout the silica cells in the fully expanded leaves (sample D5) (Fig. 4c), which made silica cells opaque under optical microscope (Fig. 6c). In addition, silica cells were found to be next to stomata (Fig. 6b, c). Intriguingly, silica cells at earlier stages (sample D2) had already formed a sheet of dumbbell-shaped silica bodies. This could be seen clearly even when cells were broken or moved due to sample sectioning (Fig. 6d). However, silicon deposition had not occurred to a large degree and the margin of the silica cells rather than the other cells were stained red (Fig. 4c).
Optical microscopic images of rice leaves counterstained with the safranin-fast green. a the longitudinal section of the sample D1, the first leaf (×20). b the wedged section of the sample D3, the middle of second leaf (×40). c the wedged section of the sample D5, the third leaf (×40). d Zoom in the image B to show the moved (solid arrow) and broken (dot arrow) dumb-bell-shaped silica cell. Vb, vascular bundle; Ad, adaxial; Ab, abaxial; m, mesophyll; S, stomata
Silicification in silica cell walls
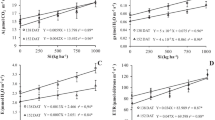
Exogenous silicon increased shoot and root biomass in rice significantly. Plants supplied silicon had more than twice the shoot biomass of plants that were not supplied silicon (Table 2). The leaves of plants grown without silicon became droopy and their surfaces were smooth, in contrast to the leaves of plants grown with silicon, which grew upright and their surfaces were coarse.
Silica cell ultrastructure was examined by TEM, and silicon distribution analysed by EDX (Fig. 7). When rice was supplied with exogenous silicon, the silica cells were filled with needle-like units and these structures were continuous to the inner cell walls (Fig. 7a). In plants not supplied with silicon, no such needle-like structure could be detected (Fig. 7b). A very high silicon content (93.8 wt %) was measured in the needle-shaped structures of silica cells (Fig. 7c, Table 3). Silicon content of the cell walls in the epidermis of leaves supplied with silicon were much higher (91.4 wt %) (Fig. 7e, Table 3) than that of the cell walls of leaf epidermis not supplied with silicon (5.7 wt %) (Fig. 7d). Further, cell walls contained significantly more silicon than the intracellular region (12.4 wt %) in plants supplemented with silicon (Fig. 7f, Table 3).
A transmission electron micrograph of rice leaf seen in cross section and the EDS (energy dispersive spectroscopy) spectrum in certain site. The microanalysis was performed in different aged leaf provided with or without exogenous silicon. Panels a and b, the silica cell ultrastructure of the cross section of rice leaf supplied with Si (a) and without Si (b), ×1,200. Panels c~f, the elemental composition in the needle-shaped structure of silica cell (c), or in the silica cell wall of rice leaf without Si supply (d) or with Si supply (e), or in the lumen of one cortex cell of rice leaf with Si supply (f). The copper peak is from the copper grid used for supporting the ultra-thin section and the osmium peak is from sample processing in which OsO4 was used for post-fixing samples. Ab, abaxial; Ad, adaxial; CC, cortex cell; SC, silica cell; Vb, vascular bundle. * represents for the place where the microanalysis localized
In one silica cell, half was found to be filled with needle-shaped structures and the other was not (Fig. 8). The needle-shaped structures started from the inner layer of the silica cell wall, extended along the identical direction under TEM with high magnification (Fig. 8a). They were stacked tightly and in order (Fig. 8b). Cell walls of matured silica cells showed a sandwich-like structure and the middle layer was brighter than the other two (Fig. 8c).
TEM images of cross-sections of silica cell’s ultrastructure and its cell wall. a a silica cell in which silicon was deposited in half, ×4,000; b the needles in the silica cell, ×20,000; c the ultrastructure of the silica cell wall, ×40,000. The solid-lined rectangle of the image A was zoomed in and showed in the image b; the broke-lined rectangle of the image a was zoomed in and showed in the image c. Ad, adaxial; CW, cell wall; SC, silica cell
Discussion and conclusion
As a typical silicon-accumulating plant, rice accumulates much more silicon than other nutrients (Ma and Takahashi 2002b). In the present study, silicon deficiency reduced shoot and root biomass of rice (Table 2) and silicon deposition in silica cells (Fig. 7b). The underlying mechanism of silicon deposition in silica cells and formation of dumbbell-shaped silica bodies is not yet clear.
Traditionally, silicon deposition in silica cells is considered as a passive process (Yoshida et al. 1962). However, this opinion is currently regarded as being controversial (Kaufman et al. 1981; Kauss et al. 2003; Kröger 2007). Our results demonstrated that silicon deposition increased (Figs. 2 and 4) and silica cells kept expanding (Fig. 3a, b and c) as leaves aged, and finally the typical structures of dumbbell-shaped silica bodies formed in the fully expanded leaf (Figs. 2f and 4c). Thus, significant silicon deposition and silica body formation occurred much later than silica cell differentiation (Fig. 4), confirming some early reports (Parry and Smithson 1958; Blackman 1968; Kaufman et al. 1970; Sakai and Sanford 1984). Hodson and Sangster (1988) indicated that the cytoplasm of the idioblasts or silicified cells degenerated first before their lumens were used as silicon deposition sites but this process did not occur in other cells in the epidermis, such as cork cells and long cells.
An increasing number of studies have focused on silica cell differentiation before the dumbbell-shaped silica body is formed. The silica cells changed shape gradually as the leaves matured, and this change reflected the plasticity of the silica cell wall at earlier stages and the rigid sandwich-like structure at later stages of development (Fig. 8c). Silica cell walls were lignified prior to obvious silicon deposition (Fig. 6b). The silicon content was much higher in the cell wall of epidermis cells of leaves supplemented with silicon than that in the intracellular region of the same leaves, or that in the silica cell wall of leaf epidermis that was not supplemented with silicon (Fig. 7, Table 3). Thus, the silica cell walls were silicified. The lignification and silicification might change the flexibility of the silica cell wall (Figs. 1, 2 and 3).
Lignified and silicified cell walls may function as matrixes for silicon deposition (Waterkeyn et al. 1982; Hodson et al. 1985), similar to the silicon deposition in diatoms and sponges (Kröger et al. 1999, 2000; Perry and Keeling-Tucker 2000; Milligan and Morel 2002). Silicon combines with phenol or lignin-carbohydrate complexes in cell walls of rice shoots (Inanaga and Okasaka 1995; Perry et al. 1987; Perry and Keeling-Tucker 1998), probably through a dihydrogen bond (Dayananda et al. 1983; Crabtree 1998) but not through C-Si or C-O-Si bonds that have not been found in nature so far (Currie and Perry 2007). The Raman microspectroscopy results of extracted silica cells indicated that different intensities existed, such as small shifts in the peak positions between biogenic silica and chemical synthetic (Fig. 5c). This possibly reflected the structural differences in the cell-mediated biomineralization processes. Further analyses are needed to demonstrate whether or not the unidentified peak at 566 cm−1 relates to the existence of an organosilicon complex.
The ultrastructure of the silica cell showed that the needle-shaped structures started from the inner layer of the cell walls (Fig. 8a, b). Some studies reported that each needle-shaped structure is composed of silica particles (Dayananda et al. 1983; Kaufman et al. 1985; Kröger et al. 1999). The intracellular needle-shaped structure could be a real structure, although we could not exclude that it is just artifact. The microanalysis showed that the needle-shaped structure of silicon-added samples was of high silicon concentration (Fig. 7c), and the needle-shaped structure did not present in silicon-depleted samples although both samples were processed exactly similarly (Fig. 7b). Thus, the silica cell in epidermis of leaf of higher plant might consist of structured biosilica but it needs more evidences.
Silica polymerization yields either an ordered ultrastructure or disordered flocculent precipitate depending on many factors, such as pH, silicon concentration. When pH is less than 7, which is common inside the cell, silica particles are thought to aggregate and form chained oligomers due to weak electrostatic repulsion. But when the pH is greater than 7, they form condensed disordered polymers (Coradin and Lopez 2003). Disordered silica polymers also would be yielded under conditions of higher silicon concentration (>2.0–2.3 mM at 25 °C) (Iler 1979). The structure of biosilica in silica cells was almost consistent with the known mineralogy of amorphous silica (Fig. 5c). We assumed that amorphous silica transported to the epidermis might first being polymerized into chained oligomers, which consist of amorphous silica, and then assembled into needle-shaped structure in an orderly stack via unknown mechanisms. The silica cells that were half-filled with needle-shaped structures might be at the primary stage (Fig. 8a, b).
Silicon deposition in higher plants might not be a completely passive process but a controlled biological process. Our hypothesis is that the wall of the silica cell is lignified and silicified at earlier stages and then functions as a matrix for forming the silica body; subsequently some macromolecules, similar to the silaffin proteins from diatoms (Kröger et al. 1997, 2000, 2001; Poulsen et al. 2003) or the novel PRP1 from cucumber plants (Kauss et al. 2003), might control the growth of the silicified needle-shaped structures from the inner layer of the cell wall and gradually fill the lumen of the silica cell by first forming a sheet-like silica body and then a dumbbell-shaped silica body. This will be confirmed in the future.
In conclusion, two processes are involved in silica cell development in the leaf epidermis of rice. First, the silica cell wall is lignified and silicified and its structure becomes dumbbell-shaped; second, the needle-like biosilica is assembled by unknown mechanisms that initiate the structure from the inner wall of the silica cell, extend it in a unique direction, and then gradually stack needles in the lumens of dumbbell cells, filling up the lumens of silica cells when the leaf is fully expanded. However, the detailed mechanisms controlling silicon biomineralization in higher plants is still unknown and additional experiments are required.
Silicon deposition in cell walls and inside dumbbell-shaped silica cells of leaves could play important roles in defending against biotic challenges (Massey and Hartley 2009), such herbivorous insects (Keeping and Kvedaras 2008) and powdery mildew (Shetty et al. 2012). Silica in plants can also alleviate abiotic stress, such as Mn (Horst and Marshner 1978) and Cd toxicity (Rogalla and Römheld 2003; Liang et al. 2005a; Shi et al. 2005) and salt stress (Yeo et al. 1999). Consequently silicon can increase plant fitness in many fundamental aspects of ecology (Cooke and Leishman 2011). Thus, silicon-based defense systems in plants might be more effective than those that are carbon-based (Cooke and Leishman 2012).
References
Ball TB, Brotherson JD, Garder JS (1993) A topologic and morphometric study of variation in idioblasts from inkhorn wheat (Triticum monococcum L.). Can J Bot 71:1182–1192
Ball TB, Gardner JS, Anderson N (1999) Identifying inflorescence idioblasts from selected species of wheat (Triticum Monococcum, T. Dicoccon, T. Dicoccoides, and T. Aestivum) and Barley (Hordecum Vulgare and H. Spontaneum) (Gramineae). Am J Bot 86:1615–1623
Barber DA, Shone MT (1966) The absorption of silica from aqueous solution by plant. J Exp Bot 17:569–578
Berlyn GP, Mickshe JP (1976) Botanical microtechnique and cytochemistry. Iowa State University Press, Ames
Blackman E (1968) The pattern and sequence of opaline silica deposition in Rye (Secale cereale L.). Ann Bot 32:207–218
Cooke J, Leishman MR (2011) Is plant ecology more siliceous than we realise? Trends Plant Sci 16:61–68
Cooke J, Leishman MR (2012) Tradeoffs between foliar silicon and carbon-based defenses: evidence from vegetation communities of contrasting soil types. Oikos 121:2052–2060
Coradin T, Lopez PJ (2003) Biogenic silica patterning: simple chemistry or subtle biology. ChemBioChem 4:251–259
Crabtree RH (1998) A new type of hydrogen bond. Science 282:2000–2001
Currie HA, Perry CC (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot 100:1383–1389
Dayananda P, Kaufman PB, Franklin CI (1983) Detection of silica in plants. Am J Bot 70:1079–1084
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Harrison CC (1996) Evidence for intramineral macromolecules containing protein from plant silicas. Phytochemistry 41:37–42
Hartley RD, Jones LHP (1972) Silicon compounds in xylem exudates of plants. J Exp Bot 23:637–640
Hodson MJ, Sangster AG (1988) Observation on the distribution of mineral elements in the leaf of wheat (Triticum aestivum L.), with particular reference to silicon. Ann Bot 62:463–471
Hodson MJ, Sangster AG, Parry DW (1985) An ultrastructural study on the developmental phases and silicification of the glume of Phalaris canariensis L. Ann Bot 55:649–655
Hodson MJ, White PJ, Mead A, Broadley MR (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot 96:1027–1046
Horst WJ, Marshner H (1978) Effect of silicon on manganese tolerance of beans plants (Phaseolus vulgaris L.). Plant Soil 50:287–304
Iler RK (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. JohnWiley&Sons, NewYork
Inanaga S, Okasaka A (1995) Calcium and silicon binding compounds in cell walls of rice shoots. Soil Sci Plant Nutr 41:103–110
Kaufman PB, Petering LB, Smith JG (1970) Ultrastructural development of cork-silica cell pairs in Avena internodal epidermis. Bot Gaz 131:173–185
Kaufman PB, Dayanandan P, Takeoka Y, Bigelow WC, Jones JD, Iler R (1981) Silica in shoots of higher plants. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York, pp 409–449
Kaufman PB, Dayanandan P, Franklin CI, Takeoka Y (1985) Structure and function of silica bodies in the epidermal system of grass shoots. Ann Bot 55:487–507
Kauss H, Seehaus K, Franke R, Gilbert S, Robert A, Kröger N (2003) Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J 33:87–96
Keeping MG, Kvedaras OL (2008) Silicon as a plant defence against insect herbivory: response to Massey, Ennos and Hartley. J Anim Ecol 77:631–633
Korndörfer GH, Lepsch I (2001) Effect of silicon on plant growth and crop yield. In: Datnoff LE, Snyder GH, Korndörfer GH (eds) Silicon in agriculture. Elsevier Press, Amsterdam, pp 133–147
Kröger N (2007) Prescribing diatom morphology: toward genetic engineering of biological nanomaterials. Curr Opin Chem Biol 11:662–669
Kröger N, Lenhmann G, Rachel R, Sumper M (1997) Characterization of a 200-kDa diatom protein that is specifically associated with a silica-based substructure of the cell wall. Eur J Biochem 250:99–105
Kröger N, Deutzmann R, Sumper M (1999) Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 286:1129–1132
Kröger N, Deutzmann R, Bergsdorf C, Sumper M (2000) Species-specific polyamines from diatoms control silicamorphology. Proc Natl Acad Sci USA 97:14133–14138
Kröger N, Deutzmann R, Sumper M (2001) Silica-precipitating peptides from diatoms. The chemical structure of silaffin-A from Cylindrotheca fusiformis. J Biol Chem 276:26066–26070
Liang YC, Wong JWC, Wei L (2005a) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Liang YC, Si J, Römheld V (2005b) Silicon uptake and transport is an active process in Cucumis sativus. New Phytol 167:797–804
Lopez PJ, Desclés J, Allen AE, Bowler C (2005) Prospects in diatom research. Curr Opin Biotechnol 16:180–186
Lux A, Luxová M, Morita S, Abe J, Inanaga S (1999) Endodermal silicification in developing seminal roots of lowland and upland cultivars of rice (Oryza sativa L.). Can J Bot 77:955–960
Lux A, Luxova M, Abe J, Tanimoto E, Hattori T, Inanaga S (2003) The dynamics of silicon deposition in the sorghum root endodermis. New Phytol 158:437–441
Ma JF, Takahashi E (2002a) Functions of silicon in plant growth. In: Soil, fertilizer, and plant silicon research in Japan. Elsevier Press, Amsterdam pp 5–26
Ma JF, Takahashi E (2002b) Silicon uptake and accumulation in plants. In: Soil, fertilizer, and plant silicon research in Japan. Elsevier Press, Amsterdam pp 73–106
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Murata Y, Yano M, Ishiguro M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448:209–213
Massey FP, Hartley SE (2009) Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores. J Anim Ecol 78:281–291
Milligan AJ, Morel FM (2002) A proton buffering role for silica in diatoms. Science 297:1848–1850
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Bot 56:1255–1261
Motomura H, Fuji T, Suzuki M (2004) Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carrière) rehder (Poaceae: Bambusoideae). Ann Bot 93:235–248
Motomura H, Fuji T, Suzuki M (2006) Silica deposition in abaxial epidermis before the opening of leaf blades of Pleioblastus chino (Poaceae, Bambusoideae). Ann Bot 97:513–519
Parry DW, Smithson F (1958) Techniques for studying opaline silica in grass leaves. Ann Bot 22:543–549
Parry DW, Hodson MJ, Newman RH (1985) The distribution of silicon deposits in the fronds of pteridium aquilinum L. Ann Bot 55:77–83
Perry CC, Keeling-Tucker T (1998) Crystalline silica prepared at room temperature from aqueous solution in the presence of intrasilica bioextracts. Chem Commun 23:2587–2588
Perry CC, Keeling-Tucker T (2000) Biosilicification: the role of the organic matrix in structure control. J Biol Inorg Chem 5:537–550
Perry CC, Willeams RJP, Fry SC (1987) Cell wall biosynthesis during silicifation of grass hairs. J Plant Physiol 126:437–448
Piperno DR (2006) The production, deposition, and dissolution of phytoliths. In: Phytoliths: A comprehensive guide for archaeologists and paleoecologists. AltaMira Press, Lanham pp 1–37
Piperno DR, Pearsall DM, Benfer RA Jr, Kealhofer L, Zhao ZJ, Jiang QH (1999) Idioblast morphology. Science 283:S1265–S1266
Poulsen N, Sumper M, Kröger N (2003) Biosilica formation in diatoms: characterization of native silaffin-2 and its role in silica morphogenesis. Proc Natl Acad Sci USA 100:12075–12080
Reynolds EW (1963) The use of lead citrate at high pH as an electron-opaque stain electron microscopy. J Cell Biol 17:208–212
Rogalla H, Römheld V (2003) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Sakai WS, Sanford WG (1984) A developmental study of silicification in the abaxial epidermal cells of sugarcane leaf blades using scanning electron microscope and energy dispersive X-ray analysis. Am J Bot 71:1315–1322
Sangster AG (1978) Silicon in the roots of higher plants. Am J Bot 65:929–935
Sangster AG, Parry DW (1976) Endodermal silicon deposits and their linear distribution in developing roots of Sorghum bicolor (L) Moench. Ann Bot 40:361–371
Sangster AG, Parry DW (1981) Ultrastructure of silica deposits in higher plants. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York, pp 383–407
SAS Institute Inc (1989) Introductory guide for personal computers, version 6.12 edition. SAS Institute Inc, USA
Shetty R, Jensen B, Shetty NP, Hansen M, Hansen CW, Starkey KR, Jorgensen HJL (2012) Silicon induced resistance against powdery mildew of roses caused by Podeosphaera Pannosa. Plant Pathol 61:120–131
Shi XH, Zhang CC, Wang H, Zhang FS (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Sposito G (1989) The chemistry of soils. Oxford Univ. Press, New York, p 277
Sugimura Y, Mori T, Nitta I, Kotani E, Furusawa T, Tatsumi M, Kusakari S, Wada M, Morita Y (1999) Calcium deposition in idioblasts of mulberry leaves. Ann Bot 83:543–550
Waterkeyn L, Bienfait A, Peeters A (1982) Callose silica epidermiques: rapports avec la transpiration cuticularie. Cellule 73:267–287
Yamaji N, Ma J (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20:1381–1389
Yeo AR, Flowers SA, Rao G, Welfare N, Senanayake N, Flowers TJ (1999) Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transportational bypass flow. Plant Cell Environ 22:559–565
Yoshida S, Ohinshi Y, Kitagishi K (1962) Chemical forms, mobility and deposition of silicon in rice plant. Soil Sci Plant Nutr 8:15–21
Yoshida S, Forno DA, Cock JH, Cock JH, Gomez KA (1976) Routine procedure for growing rice plants in culture solution. In: Laboratory manual for physiological studies of rice, 3th edn. The International Rice Research Institute, Philippines. pp 61–66
Zhang WX, Wang LL (1998) Idioblast in leaves of 7 Oryza species. J China Agric Univ 3:21–25 (in Chinese)
Acknowledgment
This study was funded by Innovative Group Grant 31121062 from the National Natural Science Foundation of China, and the National Natural Science Foundation of China (G30170550). We thank Dr. D. Zhang, Ms. H. Zhou, Ms. F. Hao and Mr. Y. Yan for their kind helps in preparing the samples and conducting the microanalysis with the SEM-EDX-ray and TEM-EDX-ray. We also thank Dr. Y. Lu and Dr. A. Henry for their help in correcting English writing. The English in this document has been checked by at least two professional editors, both are native English speakers. We also thank the two anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philip John White.
Rights and permissions
About this article
Cite this article
Zhang, C., Wang, L., Zhang, W. et al. Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis?. Plant Soil 372, 137–149 (2013). https://doi.org/10.1007/s11104-013-1723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1723-z